
Factors Affecting the Introduction of New Vaccines to
Poor Nations: A Comparative Study of the
Haemophilus
influenzae
Type B and Hepatitis B Vaccines
Aharona Glatman-Freedman
1,2,3
*, Mary-Louise Cohen
1
, Katherine A. Nichols
1
, Robert F. Porges
1,4
, Ivy
Rayos Saludes
1
, Kevin Steffens
1
, Victor G. Rodwin
5
, David W. Britt
6
1 Global Public Health Master’s Program, New York University, New York, New York, United States of America, 2 Department of Family and Community Medicine, New
York Medical College, Valhalla, New York, United States of America, 3 Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, United States of
America, 4 Department of Obstetrics and Gynecology, New York University, New York, New York, United States of America, 5 Robert F. Wagner School of Public Service,
New York University, New York, New York, United States of America, 6 Department of Health and Sports Sciences, University of Louisville, Louisville, Kentucky, United
States of America
Abstract
Background:
A major effort to introduce new vaccines into poor nations of the world was initiated in recent years with the
help of the GAVI alliance. The first vaccines introduced have been the Haemophilus influenzae type B (Hib) and the hepatitis
B (Hep B) vaccines. The introduction of these vaccines during the first phase of GAVI’s operations demonstrated
considerable variability. We set out to study the factors affecting the introduction of these vaccines. The African Region
(AFRO), where new vaccines were introduced to a substantial number of countries during the first phase of GAVI’s funding,
was selected for this study.
Methodology/Principal Findings:
GAVI-eligible AFRO countries with a population of 0.5 million or more were included in
the study. Countries were analyzed and compared for new vaccine introduction, healthcare indicators, financial indicators
related to healthcare and country-level Governance Indicators, using One Way ANOVA, correlation analysis and Qualitative
Comparative Analysis (QCA). Introduction of new vaccines into AFRO nations was associated primarily with high country-
level Governance Indicator scores. The use of individual Governance Indicator scores, as well as a combined Governance
Indicator score we developed, demonstrated similar results.
Conclusions/Significance:
Our study results indicate that good country-level governance is an imperative pre-requisite for
the successful early introduction of new vaccines into poor African nations. Enhanced support measures may be required to
effectively introduce new vaccines to countries with low governance scores. The combined governance score we developed
may thus constitute a useful tool for helping philanthropic organizations make decisions regarding the type of support
needed by different countries to achieve success.
Citation: Glatman-Freedman A, Cohen M-L, Nichols KA, Porges RF, Saludes IR, et al. (2010) Factors Affecting the Introduction of New Vaccines to Poor Nations: A
Comparative Study of the Haemophilus influenzae Type B and Hepatitis B Vaccines. PLoS ONE 5(11): e13802. doi:10.1371/journal.pone.0013802
Editor: David Joseph Diemert, The George Washington University Medical Center, United States of America
Received March 23, 2010; Accepted September 24, 2010; Published November 2, 2010
Copyright: ß 2010 Glatman-Freedman et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: The authors have no support or funding to report.
Competing Interests: The authors have declared that no competing interests exist.
Introduction
Childhood mortality continues to be a major global public
health problem. In 2005, approximately 9.7 million children
under the age of 5 died worldwide [1]. The United Nations
Millennium Developmental Goals (MDGs) delineated in 2002,
express a need for action through goal number 4, aiming for a
two-third reduction in childhood mortality from 1990 to 2015
(www.un.org/millenniumgoals/pdf/mdg2007.pdf). Many child-
hood deaths occurring in poor nations result from diseases and
conditions which are easily preventable by vaccines that are
readily available in developed nations. Of the worldwide estimated
2.5 million deaths from vaccine preventable diseases among
children younger than 5 years of age, 87% occur in poor regions of
the world [2]. Furthermore, it is estimated that approximately 162
million Disability-Adjusted Life Years (DALYs) are lost worldwide
from vaccine-preventable diseases, more than 90% of them in low
income countries [3]. Until recently, funds for supply and
administration of vaccines in poor nations have been scarce and
inconsistent [4–6].
In 2000, a new global alliance for vaccine and immunization,
the GAVI Alliance (formerly known as the Global Alliance for
Vaccines and Immunizations), was established in an effort to end
vaccine inequity between rich and poor nations [5]. The GAVI
Alliance is a public-private partnership whose partners include UN
agencies, the World Health Organization (WHO), the World
Bank, public health institutions, donor and recipient countries, the
Bill and Melinda Gates Foundation, pharmaceutical manufactur-
ers, and other members of the philanthropic and financial
community (www.gavialliance.org/about/in_partnership/index.
PLoS ONE | www.plosone.org 1 November 2010 | Volume 5 | Issue 11 | e13802

php). GAVI’s initial objectives were to provide the basic vaccines
(Polio, Diphtheria, Pertussis, Tetanus, Measles and BCG) as well
as new and underused vaccines – particularly Haemophilus influenzae
type b (Hib), Hepatitis B (HepB) and Yellow Fever, to children
in developing nations (www.unicef.org/chinese/immunization/
files/immunize_every_child.pdf; http://www.gavialliance.org/
performance/global_results/index.php). GAVI’s funding has been
available to any nation with a Gross National Income (GNI) per
capita under $1,000 (www.gavialliance.org/support/who/index.
php) following an application review by independent experts
(www.gavialliance.org/support/how/index.php).
During its first phase of operation (2000–2005), GAVI provided
support for two new vaccines that have been readily available to
children in developing countries, the Hib and HepB vaccines (a
third vaccine against Yellow Fever is used only in Africa and South
America).
Hib is the most common cause of meningitis and a leading cause
of pneumonia in un-immunized infants and children under the age
of 5 years [reviewed in [7;8]. HepB virus can cause acute and
chronic Hepatitis, cirrhosis of the liver and hepatocellular
carcinoma; it can be transmitted at birth, through intimate or
sexual contacts and via needle sticks [9]. Vaccines are the most
effective preventive measure against both pathogens [9;10].
Despite the proven effectiveness of the Hib vaccine, its
introduction into immunization programs of developing nations
has been slow as compared with the HepB vaccine. By the end of
2005, fewer than 20 million children worldwide received a full 3
doses series of Hib vaccine as compared to more than 95 million
children that had received all 3 required doses of HepB (www.
gavialliance.org/performance/index.php). During the first phase
of GAVI’s operation, the number of GAVI-eligible countries using
Hib vaccine increased from 3 to 19, while HepB vaccine usage
increased from 17 to 57. (www.gavialliance.org/resources/15brd_
HepBHibYF_zuber_28Apr05.ppt); [11].
The purpose of this study was to investigate the conditions
which have been conducive to the successful introduction of the
Hib and HepB vaccines by GAVI into poor nations during its first
phase of operations. Recent experiences suggest that for healthcare
projects to succeed in poor countries, governments are central to
the delivery of services on a regional, national or global level, even
in the context of operations led by non-governmental organiza-
tions or the business sector [12]. Furthermore, governments in
poor countries were in some cases chief funders of public
healthcare efforts [12]. We thus examined systematically the
association of new vaccine introduction with different elements of
the healthcare, finance and governance context, characterizing the
nations in our sample of GAVI-eligible African Nations.
Methods
Countries
GAVI-eligible nations of the WHO African region (AFRO) with
a population size of 0.5 million or more were included in the study
(www.who.int/about/regions/afro/en/index.html). Only the is-
land of Sao Tome and Principe (population size 157,000) was
excluded (www.who.int/about/regions/afro/en/index.html). A
total of 35 countries were studied.
Data Collection
Vaccination data were obtained from WHO/UNICEF reports
(www.who.int/vaccines/globalsummary/immunization/country-
profileresult.cfm). Years of HepB and Hib vaccine initiation were
recorded for each country.
Country population sizes and Healthcare Indicators statistics
were obtained from the WHO database (www.who.int/whosis/
en/index.html). The healthcare indicators evaluated for each
country were: Life expectancy for males, life expectancy for
females, number of doctors per 1,000 people and number of
nurses per 1,000 people. The financial indicators related to
healthcare evaluated were: Total Healthcare Expenditure per
Capita (THECAP), Government Healthcare Expenditure per
Capita (GHECAP) and Total Healthcare Expenditure as percent
of the GDP (HEGDP).
Country-level Governance Indicators scores for each country
were obtained from the World Bank database (http://info.
worldbank.org/governance/wgi/index.asp). These included: Po-
litical Stability (PS), Government Effectiveness (GE), Rule of Law
(Law), Regulatory Quality (Reg), Control of Corruption (Corr),
and Voice and Accountability (VA). The scores were provided as
percentile rank with higher values indicating higher performance.
The indicator values for 2005 were collected first, and if
statistically significant differences were found between the country
groups, indicator values were then collected and evaluated for the
years 1995 to 2005.
Statistical Analysis
Statistical analysis was done using SPSS version 15.0 for PC.
Mean, median and standard error were determined for each of the
continuous variables. Comparisons between different groups of
countries were done using One Way ANOVA. A p – value of
,0.05 was considered statistically significant.
Correlation coefficients were calculated to evaluate the
relationship between variables using Pearson’s and Point Biserial
tests. Reliability was determined using Chronbach’s alpha.
Qualitative Comparative Analysis (QCA) was used to examine
the alternative combinations of factors that are conducive to the
success or failure of new vaccine introduction. QCA was
performed using fsQCA software.
Results
New vaccine programs in AFRO nations
New vaccine introduction data revealed that GAVI-eligible
African countries could be divided into 3 distinct groups based on
the status of new vaccine introduction or use during the first phase
of GAVI’s operation (2000–2005). The groups were defined as
follows (Table 1):
I. Countries in which both Hib and Hepatitis B vaccine were
introduced.
II. Countries in which Hepatitis B vaccine, but not Hib
vaccine, was introduced.
III. Countries in which neither Hib nor Hepatitis B vaccine
were introduced.
Country population size
The mean country population size was calculated for each
country group. No statistical differences were found between the
mean country sizes of the 3 groups.
Healthcare Indicators
The 3 country groups were compared for differences and
similarities in male and female life expectancy and number of
doctors and nurses per 1,000 people available by 2005, using One
Way ANOVA. No statistically significant differences were found
between the 3 vaccination country groups.
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 2 November 2010 | Volume 5 | Issue 11 | e13802

Financial Indicators related to Healthcare
The 3 county groups were compared for differences and
similarities in Total Healthcare Expenditure per Capita (THE-
CAP), Government Healthcare Expenditure per Capita (GHE-
CAP) and Total Healthcare Expenditure as percent of the GDP
(HEGDP) using One Way ANOVA. Mean, standard deviation
and standard error of the mean of country group expenditure
values were first evaluated for 2005, and statistically significant
differences were found between the groups for Total Healthcare
Expenditure as percent of the GDP (HEGDP) and Government
Healthcare Expenditure per Capita (GHECAP) (Figures 1A, 1C).
Data were then collected for each country for the years 1995–
2005. Data for Total Healthcare Expenditure per Capita (THE-
CAP) were collected as well to evaluate pattern of expenditure.
The data summary is presented in Figure 1. For all 3 indicators the
means for group III were the lowest throughout the period studied.
The mean values for group II was higher and remained relatively
consistent throughout the period studied for all 3 indicators. The
mean values for group I of all indicators increased gradually with
the highest expenditure growth rate occurring after 2000 (the
beginning of GAVI’s funding period). For group I, GHECAP and
THECAP expenditure values were similar to those of group III in
the early pre-GAVI years, and by the end of the GAVI’s first
phase of funding, their values were the highest among the 3 groups
(Figures 1A, 1B).
For GHECAP, statistically significant differences were found
between group I and III for the years 2002, 2003 (p value,0.05),
2004 and 2005 (p value,0.01) (Figure 1A). For HEGDP, the
differences between groups I and III reached statistical significance
in the years 2004 and 2005 (p value,0.05) (Figure 1C). For
THECAP, the pattern of expenditure was similar to that of
GHECAP, but no statistically significant differences were
demonstrated between the groups (Figure 1B).
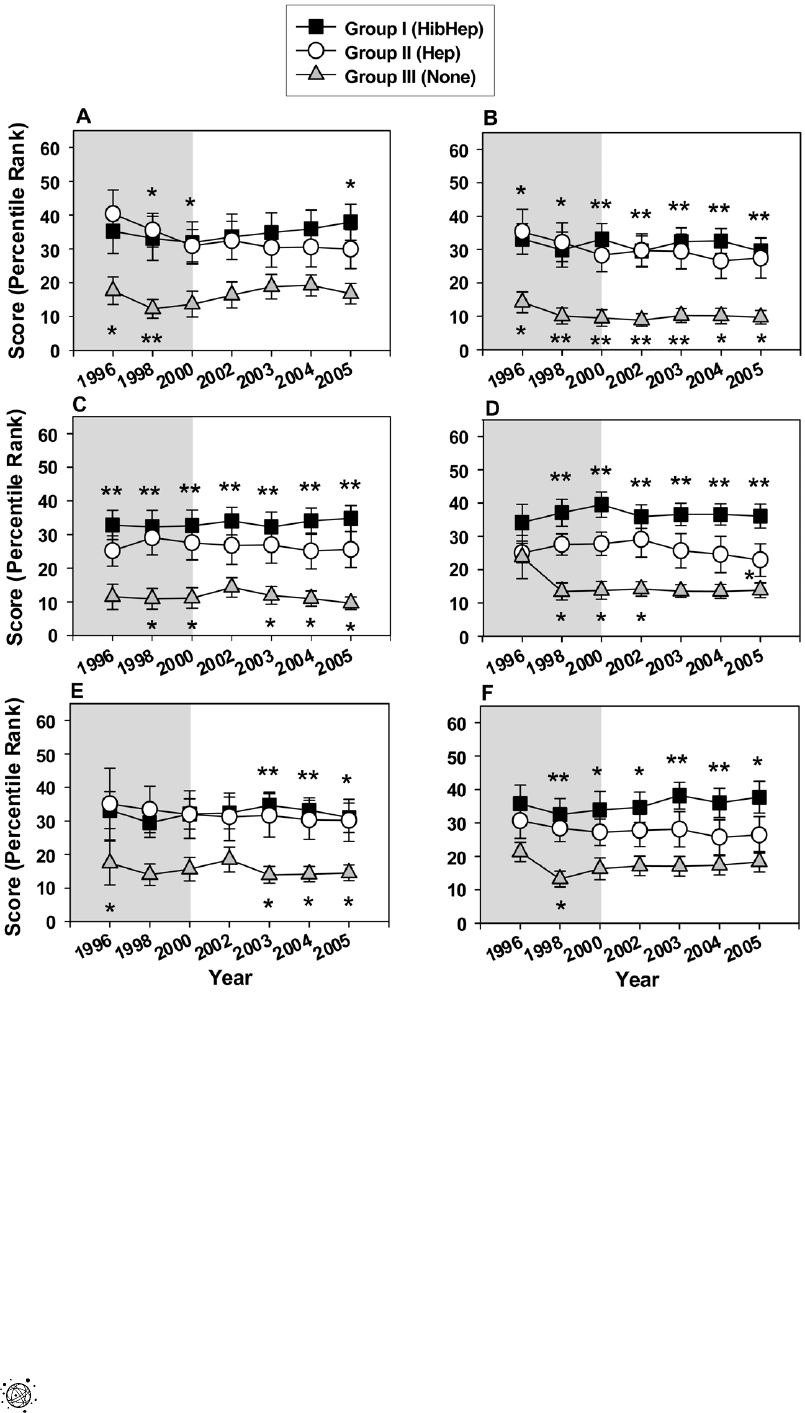
Country-level Governance Indicators
Political Stability, Government Effectiveness, Regulatory Qual-
ity, Rule of Law, Control of Corruption and Voice and
Accountability scores (expressed as percentile rank) were collected
for each country. Country group means were then calculated for
each of the indicators. After determining that the country group
mean for 2005 for each indicator showed statistically significant
differences, scores were evaluated for the years 1996–2005 for
each country (with the exception of 1999 and 2001 for which
scores were not available).
Overall, the mean scores were highest for group I and lowest for
group III (Figure 2). Multiple comparisons analysis demonstrated
that statistically significant differences were found between groups
I and III for all indicators (Figure 2A–F). Statistically significant
differences between groups II and III were also found for all
indicators, however, less frequently (Figure 2). Statistically
significant differences were found between group I and group II
only for Regulatory Control in 2005 (Figure 2D).
Correlation and reliability studies
Given the similar patterns of the various Governance Indicators
scores for the country groups (figure 2), we examined the
correlation between them for the years 1996 to 2005. A strong
positive correlation was found among all the Governance
Indicators (Table 2), with a statistically significant correlation
coefficient (p-value,0.01) for all of them. Analysis of correlation
between Governance Indicator means and country groups
(Table 2) demonstrated a statistically significant relationship (p
value#0.01). Reliability analysis performed using all the Gover-
nance Indicators for all the countries, demonstrated a Cronbach’s
Alpha score of 0.902.
Development of a combined governance scoring system
Given the high correlation and reliability scores for the
Governance Indicators, we set out to develop a single governance
score that will reflect the contribution of each indicator. A
Combined Governance Score, consisting of the average score for
all the Governance Indicators of each country for each year, was
calculated. Combined Governance Score means were then
calculated for each country group for each year in the period
studied. These scores, shown in Figure 3, demonstrated similar
pattern to that of the individual Governance Indicators, with the
highest scores for group I and the lowest for group III. Differences
between group I and III, were statistically significant with a p value
of ,0.01 for each year evaluated, except for 1996 when p value was
found to be ,0.05 (Figure 3). The differences between group II
and III were statistically significant with a p value of ,0.05 for all
the years studied. No statistically significant differences were found
between group I and II (Figure 3).
Prediction of success in the introduction of new vaccines
To complement the group comparisons across time, we used
QCA. Introduced by Ragin in 1987 [13], QCA is an analytic
technique which utilizes Boolean algebra for the purpose of making
multiple comparisons of various combinations of conditions, to
determine which combinations of conditions are most favorable to a
certain outcome. Thus, it is especially adept at examining the
alternative combinations of contextual elements that are conducive
Table 1. New vaccine introduction to GAVI-eligible AFRO
countries.*
Group I
Hib and
HepB
Group II
HepB
Group III
Neither
vaccine
Country Year Year Country Year Country
HepB Hib HepB
Benin 2002 2005 Cameroon 2005 Angola
Burkina
Faso
#
2006 2006 Comoros 2001 Chad
Burundi 2004 2004 Cote D’Ivoire 2001 CAR
Gambia
&
1990 1998 Eritrea 2002 Congo
Ghana 2002 2002 Lesotho 2001 Congo DR
Kenya 2002 2002 Madagascar 2003 Ethiopia
Malawi 2002 2002 Mauritania 2005 Guinea
Mali 2003 2005 Mozambique 2001 Guinea-Bissau
Rwanda 2002 2002 Nigeria 2005 Liberia
Senegal 2004 2005 Tanzania 2002 Niger
Uganda 2002 2002 Zimbabwe 1999 Sierra-Leone
Zambia 2005 2004 Togo
*Countries divided according to new vaccine (Hib and Hepatitis B) introduction
by 2005 and WHO/Unicef coverage reporting by 2006 (first vaccine coverage
reporting year is provided).
#
The application for HepB and Hib vaccines submitted by Burkina Faso was
approved in October 2004 (www.gavialliance.org/resources/
Info_Update_December2004.pdf).
&
Gambia started HepB and Hib vaccination program on its own. It was
approved for GAVI’s support for these vaccines in 2001 (www.gavialliance.org/
performance/country_results/index.php?countID = 23).
doi:10.1371/journal.pone.0013802.t001
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 3 November 2010 | Volume 5 | Issue 11 | e13802

to programs’ success or failure. QCA is intended for use in studies
with small to intermediate N [13]. In the present study, QCA helps
draw attention to the alternative combinations of healthcare finance
and governance conditions that are associated with the successful
introductions of new vaccines.
The variables used for this analysis were the Combined
Governance Score, HEGDP and GHECAP, for the pre-GAVI
period, for each country (thus, the pre-GAVI means for the
Combined Governance Score were obtained by using the values
for 1996 and 1998 for each country, and the pre-GAVI mean for
HEGDP and GHECAP were obtained by using the values for
1995–1999 for each country). The median value of each mean
indicator score was then used to define high and low values. High
values were assigned the number 1, low values were assigned the
number 0, and these were then termed ‘conditions’ following
standard QCA practice. Table 3 demonstrates all the possible
combinations for the tested conditions. In addition, it demon-
strates the number of countries with each of the specific condition
combination, the number of countries in which a specific
combination of conditions was associated with the introduction
Figure 1. Financial healthcare indicators during the pre-GAVI and the first phase of GAVI’s funding. (A) GHECAP; (B) THECAP; (C)
HEGDP. Symbols represent means and error bars represent standard error of the mean. Grey plot background highlights the Pre-GAVI years, white
plot background highlights the GAVI funding years. * p value,0.05, ** p value,0.01. Placement of (*) above square symbols denotes a statistically
significant difference between country groups I and III.
doi:10.1371/journal.pone.0013802.g001
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 4 November 2010 | Volume 5 | Issue 11 | e13802
of at least one new vaccine, and the proportion of the countries
that the specific combination of conditions was associated with the
introduction of at least one new vaccine (defined as Consistency).
If a combination of conditions was associated with a
proportion of at least 0.75 of the countries being successful in
having at least one new vaccine introduced, that combination
was coded as a 1. If a proportion of less than 0.75 of the countries
was associated with that outcome, the combination was
considered as not successful in having at least one new vaccine
introduced and was code d as 0.
Of the 18 countries with condition combinations that included
high Combined Governance Score, 17 introduced at least one new
vaccine (Table 3). In comparison, of 18 countries with condition
combination that included either a high HEGDP or GHECAP
score, 13 and 14 countries respectively introduced at least one new
vaccine (Table 3). Thus, these results demonstrate that countries
Figure 2. Governance Indicators for the pre-GAVI and the first phase of GAVI’s funding. (A) Political stability; (B) Government
effectiveness; (C) Rule of Law; (D) Quality control; (E) Control of corruption; (F) Voice and accountability. Symbols represent means and error bars
represent standard error of the mean. Grey plot background highlights the Pre-GAVI years, white plot background highlights the GAVI funding years.
* p value,0.05, ** p value,0.01. Placement of (*) above square symbols denotes a statistically significant difference between country groups I and III.
Placement of (*) under triangle symbols denotes a statistically significant difference between country groups II and III. Placement of (*) under circle
symbols denotes a statistically significant difference between country groups I and II.
doi:10.1371/journal.pone.0013802.g002
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 5 November 2010 | Volume 5 | Issue 11 | e13802

with high Combined Governance Score (alone or in combination with high HEGDP or GHECAP) for the pre-GAVI years were
more likely to introduce at least one new vaccine than countries
with high HEGDP and/or GHECAP score (alone or in
combination with Combined Governance Score).
Overall, high Combined Governance Score of a country was
found to be the only condition associated with most cases of
success in terms of introducing at least one new vaccine.
Furthermore, this association with success occurred independently
of the presence of high HEGDP or GHECAP score in the
combination. As a result, the Combined Governance Score can be
declared both necessary and sufficient for predicting the
introduction of at least one new vaccine, with raw and unique
solution coverage of 0.74 and solution consistency of 0.94. Thus,
these results indicate that the Combined Governance Score by
itself correctly predicted 74% of the countries that were successful
in introducing at least one new vaccine (17 of the 23 countries
belonging to groups I and II). The results further indicate that the
Combined Governance Score demonstrated an average of 94%
consistency (17 of the 18 countries with high Combined
Governance Score). No good QCA solutions were found for
predicting the introduction of two new vaccines or distinguishing
between the introduction of one or two new vaccines.
Discussion
The GAVI Alliance’s initiative to introduce new vaccines into
developing countries is of utmost importance for the health of
children worldwide. The Alliance’s role in providing the financial
resources for this purpose is crucial, especially given the high costs
Table 2. Correlation between Governance indicator means for the years 1996–2005 and country groups.
CountryGroup PSmean GEmean LawMean RegMean CorrMean VAmean
Country Group Pearson Correlation 1 2.434(**) 2.570(**) 2.595(**) 2.651(**) 2.425(*) 2.517(**)
Sig. (2-tailed) .009 .000 .000 .000 .011 .001
N 35 353535 35 35 35
PSmean
#
Pearson Correlation 2 .434(**) 1 .662(**) .798(**) .608(**) .621(**) .783(**)
Sig. (2-tailed) .009 .000 .000 .000 .000 .000
N 35 353535 35 35 35
GEmean
#
Pearson Correlation 2 .570(**) .662(**) 1 .831(**) .823(**) .697(**) .720(**)
Sig. (2-tailed) .000 .000 .000 .000 .000 .000
N 35 353535 35 35 35
LawMean
#
Pearson Correlation 2 .595(**) .798(**) .831(**) 1 .742(**) .791(**) .704(**)
Sig. (2-tailed) .000 .000 .000 .000 .000 .000
N 35 353535 35 35 35
RegMean
#
Pearson Correlation 2 .651(**) .608(**) .823(**) .742(**) 1 .566(**) .699(**)
Sig. (2-tailed) .000 .000 .000 .000 .000 .000
N 35 353535 35 35 35
CorrMean
#
Pearson Correlation 2 .425(*) .621(**) .697(**) .791(**) .566(**) 1 .443(**)
Sig. (2-tailed) .011 .000 .000 .000 .000 .008
N 35 353535 35 35 35
VAmeanV Pearson Correlation 2 .517(**) .783(**) .720(**) .704(**) .699(**) .443(**) 1
Sig. (2-tailed) .001 .000 .000 .000 .000 .008
N 35 353535 35 35 35
**Correlation is significant at the 0.01 level (2-tailed).
*Correlation is significant at the 0.05 level (2-tailed).
#
PS – Political Stability, GE – Government Effectiveness, Law – Rule of LAW, Reg – Regulatory Quality, Corr – Control of Corruption, VA – Voice and accoun tability.
doi:10.1371/journal.pone.0013802.t002
Figure 3. Combined Governance Indicator scores for the pre-
GAVI and the first phase of GAVI’s funding. Symbols represent
means and error bars represent standard error of the mean. Grey plot
background highlights the Pre-GAVI years, white plot background
highlights the GAVI funding years. * p value,0.05, ** p value,0.01.
Placement of (*) above square symbols denotes a statistically significant
difference between country groups I and III. Placement of (*) under
triangle symbols denotes a statistically significant difference between
country groups II and III.
doi:10.1371/journal.pone.0013802.g003
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 6 November 2010 | Volume 5 | Issue 11 | e13802

of such vaccines. The full Hib and HepB vaccine series, for
example, costs substantially more per child for a series of 3
vaccines, as compared to the battery of basic vaccines (Diphtheria,
Tetanus, Pertussis, Polio, Measles, Tetanus and BCG) [14]. While
the cost for the basic battery of vaccines has been US $1 per child,
the addition of Hib and HepB vaccines has raised the cost to US
$7–13 per child (www.who.int/mediacentre/factsheets/fs288/en/
index.html).
The GAVI alliance’s approach is unique in that it seeks to
provide financial support for vaccines while empowering nations
to become eventually self-sufficient in supporting their vaccination
programs (www.who.int/immunization_financing/analyses/fsp/
process/en/). To do so, GAVI initially required that countries
receiving GAVI’s support, outline in their applications their plans
to finance the vaccines costs in the future, and commit to prepare a
comprehensive Financial Sustainability Plan (www.who.int/im-
munization_financing/analyses/fsp/process/en/). However, to
achieve sustainable success, it is crucial to identify the factors that
contribute or hinder progress. Examining the first phase of GAVI’s
funding, we found that GAVI-eligible AFRO countries differed
with respect to the introduction of new vaccines into their
immunization programs. While some countries introduced both
the Hib and HepB vaccines (Group I), other countries introduced
only the Hep B vaccine (Group II). A third group of countries did
not introduce either vaccine (Table 1).
An initial analysis looking at the end of GAVI’s first phase of
operation (2005) suggested that higher financial indicator scores of
GHECAP and HEGDP and higher Governance Indicator scores
were associated with the introduction of both the Hib and Hep B
vaccines into countries’ immunization programs (Figure 1). A
more detailed analysis of the period from 1995 to 2005 (Figure 1)
demonstrated that while means of Governance Indicators scores
for group I (both Hib and Hep B vaccines introduced) remained
high and relatively stable throughout the pre-GAVI and the first
phase of GAVI’s funding periods (Figure 2), the mean GHECAP
and HEGDP values for country group I changed throughout these
years. Starting at low values in 1995, followed by a gradual
increase (Figure 1), they surpassed the values for both group II and
III by 2002. It is important to note that the largest increase in the
financial healthcare scores of group I occurred between the years
2000 and 2005 (Figure 1), coinciding with the first phase of
GAVI’s operations. This sharp increase in the financial healthcare
values of group I countries also coincided with the overall rapid
increase in developmental assistance for health (DAH) for low
income countries during these years [15]. Additional research is
required to understand the role and effect of GAVI’s funding in
these financial indicator increases of group I countries.
The stable pattern of governance scores for the 3 country
groups before and during GAVI’s first phase of funding, and the
association of higher governance scores with the introduction of
both HepB and Hib vaccines (country group I), solidly support the
strength of governance as an important factor in the ability of
countries to support healthcare initiatives, including the introduc-
tion of new vaccines. These patterns, coupled with the QCA
analysis results, strongly indicate that governance is a stronger
predictor for the introduction of new vaccines as compared to
healthcare financial expenditure.
It is interesting to note that overall, the countries that belong to
group II, which have similar governance scores to those of group I,
did not demonstrate increase in financial healthcare indicator
values during the first phase of GAVI’s funding. This observation
is particularly interesting given the fact that the differences in
governance scores between group I and II were not statistically
significant. Although it is possible that these small differences in
governance scores between groups I and II (with group II scores
being slightly lower than those of group I) were associated with the
inability of countries belonging to group II to increase the financial
healthcare expenditure and to introduce the Hib vaccine, our data
do not support such a conclusion. Furthermore, according to the
QCA analysis, no good solutions were found to distinguish
between countries that introduced one or two new vaccines. Thus,
these results suggest that the lack of introduction of a second new
vaccine (Hib) may be due to governmental decisions, lack of funds,
specific infrastructural issues or indefensible grant application for
GAVI’s Hib funds, rather than lack of governmental execution
abilities. It is also possible that these differences are due to lack of
sufficient country awareness for the role of Hib in causing
mortality and morbidity in countries belonging to group II.
However, recent global estimates demonstrating that most Hib-
related deaths have occurred in developing nations in Africa and
Asia [8], justify the need to introduce and expand the Hib vaccine
usage in these countries.
Overall, our results indicate that country-level governance is the
single most important factor in determining the ability of poor
African nations to introduce new vaccines. Good governance
offers an obvious advantage for a country’s ability to move forward
with new healthcare initiatives like the introduction of a new
vaccine. New efforts require the commitment and attention of
leadership at the top governmental levels who must obtain and
commit funds over multiple years. In nations that are struggling
Table 3. Prediction of introduction of at least one new vaccine based on indicator combination.
Indicator
Indicator
Combination
Combined
Governance
Score HEGDP GHECAP
Number of
countries with
condition
combination
No. of countries with the
condition combination
that introduced at least
one new vaccine
Condition
Combination
Consistency
Success in the
introduction of
at least one
new vaccine
1 1119 8 0.89 1
2 0 0 1 6 3 0.5 0
3 0106 2 0.33 0
4 0 0 0 5 1 0.2 0
5 1 0 0 3 3 1.0 1
6 1 0 1 3 3 1.0 1
7 1 1 0 3 3 1.0 1
8 0110 0 0 0
doi:10.1371/journal.pone.0013802.t003
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 7 November 2010 | Volume 5 | Issue 11 | e13802

financially, political stability and good governance are often
important factors in attracting foreign aid [16]. In this regard,
good country-level governance was previously shown to have a
considerable impact on investments in developing countries made
by for-profit healthcare service providers and large multinational
pharmaceutical and biotechnology corporations [17].
In addition to funding, the introduction of new vaccines
requires adequate infrastructure elements such as sufficient cold
chain capacity, ability to reach remote locations, safe disposal of
needles and syringes, as well as sufficient numbers of adequately
trained personnel [18]. Lack or scarcity of these elements
constitute significant barriers to the adoption of new vaccines
[18]. Country-level governance may have a crucial effect on both
the initiation and mobilization of these important elements of
vaccine programs.
Combining Governance Indicators into a single index has the
advantage of providing one measure of governance that will take
into account all the indicators. Although overall, we found a
correlation between the various indicators of governance, some
countries received substantially different scores for different
Governance Indicators. Thus, a combined Governance score
which takes into account all the components of governance,
without the need to evaluate each one of them separately, provides
a user-friendly measure of governance.
Our results indicate that a scoring system that takes into account
all Governance Indicators (Figure 3) may constitute an effective
quantitative method to predict the ability of poor nations in Africa
to introduce new vaccines. There is a great need for quality
quantitative tools to support decision making in healthcare
philanthropy, and efforts to develop such tools are thus far at
their infancy (http://aspe.hhs.gov/hsp/09/philnpart/chapter5.
shtml). During its first phase of operations, GAVI strongly
encouraged nations to apply for new vaccines. GAVI has been
using a rigorous approach to evaluating, awarding and monitoring
its grants, while attempting to consider individual countries’
priorities, and promoting planning and country ownership.
However, an independent evaluation of the first phase of GAVI
found that it did not use a formal framework in directing its decision
making process regarding approval of funding (www.gavialliance.
org/resources/5._GAVI_Phase_1_Evaluation_Executive_Summary.
pdf). Thus, the association of new vaccines introduction with high
country-level governance scores reflects a phenomenon that is
independent of a specific framework. Our findings could provide a
basis for designing a framework and/or criteria that will guide the
evaluation process and the support required for introducing new
vaccines into nations with different levels of governance scores.
Overall, our findings suggest that for a new vaccine programs to
succeed, special considerations and criteria should be applied to
different countries. Countries with higher governance scores can
be expected to respond faster to GAVI and other international
vaccine initiatives. Although concerns regarding the financial
sustainability of these immunization programs are expected, it is
reasonable to assume that if financial resources are provided to
nations with higher governance scores, vaccination programs that
are in place will continue. However, in the absence of good
country-level governance, such as in the case of country group III,
the ability to respond to international vaccine initiatives will likely
be slower and the optimal use of financial assistance may be at risk.
Although the countries that pioneered the Hib program in sub-
Saharan Africa had overall good governance scores, a real
question remains whether, absent some significant price conces-
sion, even they can sustain this expensive vaccine once GAVI
funding ends. This question has become more relevant since
GAVI has continued to approve Hib vaccine funding to additional
African countries (www.gavialliance.org/media_centre/press_
releases/2007_11_29_en_pr_hib_boost.php). While some of these
newly approved countries had already introduced the HepB
vaccine beforehand, and they belong to an overall more
governmentally resourceful group of countries, most of them had
not used Hib or HepB vaccine before, and they belong to an
overall weaker group of countries with less effective governmental
systems.
GAVI has already begun modifying its criteria for financial
support for fragile countries. These include providing funds to
strengthen health systems (www.gavialliance.org/performance/
evaluation/index.php) and requiring lower co-pays for vaccines
(http://hibaction.org/resources/hibfocus/061117_alert/) [14]. The
need for additional support and different rules of engagement with
those nations (many of which are post-conflict) have been discussed
during GAVI’s meetings (www.gavialliance.org/resources/19brd_
FragileStates.pdf). Analysis of the second phase of GAVI’s funding
(from 2007 to 2010) will be required to evaluate the success of the
new changes in affecting new vaccine introduction. Ultimately,
established criteria, such as those used by the Center for Global
Development to assess the long-term success of global health
initiatives, namely: scale, importance, impact, duration and cost-
effectiveness [12], will probably be most suitable for this purpose.
The issues discussed in this paper are of paramount importance
for the continued introduction of new vaccines into developing
countries as well as maintaining and sustaining immunization
programs. In this regard, additional new licensed vaccines such as
Pneumococcal, Rotavirus, and Human papillomavirus vaccines are
planned to follow HepB and Hib vaccines (www.gavialliance.org/
vision/policies/new_vaccines/adips/index.php; www.gavialliance.
org/resources/FS_HPV_EN.pdf). If these vaccines are successfully
introduced into poor nations, they will likely facilitate the
introduction of novel vaccines to prevent tuberculosis, malaria
and HIV/AIDS when available.
Acknowledgments
We are grateful to Dr. D. Modan-Moses for critical reading of the
manuscript and helpful suggestions.
Author Contributions
Conceived and designed the experiments: AGF VGR DWB. Performed
the experiments: AGF DWB. Analyzed the data: AGF DWB. Wrote the
paper: AGF MLC KAN RFP IRS KS. Data acquisition, data
interpretation: AGF. Data acquisition, drafting the article, final approval
of submitted version: MLC KAN RFP IRS KS. Critical revision of the
article for important intellectual content, final approval of the submitted
version: VGR DWB.
References
1. Murray CJ, Laakso T, Shibuya K, Hill K, Lopez AD (2007) Can we achieve
Millennium Development Goal 4? New analysis of country trends and forecasts
of under-5 mortality to 2015. Lancet 370: 1040–1054.
2. Anonymous (2006) Vaccine preventable deaths and the Global Immunization
Vision and Strategy, 2006–2015. MMWR 55: 511–515.
3. Mathers CD, Lopez AD, Murray CJL (2006) The burden of disease and
mortality by condition: Data, methods, and results by 2001. In: Lopez AD,
Mathers CD, Ezzati M, Jamison DT, Mu rray CJL, eds. Glob al burden of
disease and risk factors. New York: Oxford University Press. pp 45–
240.
4. Aylward RB, Birmingham M, Lloyd J, Eggers R, Bilous J, et al. (2010) Reaching
every child: Achieving equity in global immunization. In: Levine MM, et al. (2010)
New Generation Vaccines, 4th edn. New York: Informa Healthcare. pp 91–
100.
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 8 November 2010 | Volume 5 | Issue 11 | e13802

5. Jacobs L (2010) A paradigm for international cooperation: The GAVI Alliance.
In: Levine MM, et al. (2010) New Generation Vaccines, 4th edn. New York:
Informa Healthcare. pp 101–106.
6. Mahoney RT, Ramachandran S, Xu Z (2000) The introduction of new vaccines
into developing countries II. Vaccine financing. Vac cine 18: 2625–2635.
7. Watt JP, Levine OS, Santosham M (2003) Global reduction of Hib disease:
What are the next steps? Proceeding of the meeting Scottsadle, Arizona,
September 22–25, 2002. J Pediatr 143: S164–S187.
8. Watt JP, Wolfson LJ, Henkle E, O’Brien KL, Deloria-Knoll M, et al. (2009)
Burden of disease caused by Haemophilus influenzae type b in children younger
than 5 years: global estimates. Lancet 374: 903–911.
9. Zanetti AR, Van Damme P, Shouval D (2008) The global impact of vaccination
against hepatitis B: A historical overview. Vaccine 26: 6266–6273.
10. Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group
(2007) Historical comparisons of morbidity and mortality for vaccine-
preventable disease in the United States. JAMA 298: 2155–2163.
11. Rossi IA, Zuber PL, Dumolard L, Walker DG, Watt J (2007) Introduction of
Hib vaccine into national immunization programmes: a descriptive analysis of
global trends. Vaccine 25: 7075–7080.
12. Levine R, What works working group (2004) Millions saved: Proven success in
global health. Washington DC: Center for Global Development.
13. Ragin CC (1987) The comparative method: Moving beyond qualitative and
quantitative methods. Berkeley, Los Angeles, London: University of California
Press.
14. Akumu AO, English M, Scott JA, Griffiths UK (2007) Economic evaluation of
delivering Haemophilus influenzae type b vaccine in routine immunization services
in Kenya. Bull WHO 85: 511–518.
15. Ravishankar N, Gubbins P, Cooley RJ, Leach-Kemon K, Michaud CM, et al.
(2009) Financing of global health: Tracking development assistance for global
health from 1990 to 2007. Lancet 373: 2113–2124.
16. Berthelemy JC (2006) Bilateral donors’ interest vs. recipients’ development
motives in aid allocation: Do all donors behave the same? Rev Develop Econ 10:
179–194.
17. Outerville JF (2007) Foreign direct investment in healthcare sector and most
favoured locations in developing countries. Eur J Health Econ 8: 305–312.
18. Duclos P, Okwo-Bel e JM, Gacic-Dobo M, Cheri an T (2009 ) Global
immunization: Status, progress, challenges and future. BMC Int.Health
Hum.Rights 9(Suppl 1): S2.
New Vaccines for Poor Nations
PLoS ONE | www.plosone.org 9 November 2010 | Volume 5 | Issue 11 | e13802
