
fNTP2016T
Toxicological Profile for
Hexachlorocyclohexane (HCH)
March 2024
HEXACHLOROCYCLOHEXANE (HCH) ii
DISCLAIMER
Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic
Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human
Services.

HEXACHLOROCYCLOHEXANE (HCH) iii
FOREWORD
This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic
Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The
original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised
and republished as necessary.
The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects
information for these toxic substances described therein. Each peer-reviewed profile identifies and
reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is
also presented, but is described in less detail than the key studies. The profile is not intended to be an
exhaustive document; however, more comprehensive sources of specialty information are referenced.
The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile
begins with a relevance to public health discussion which would allow a public health professional to
make a real-time determination of whether the presence of a particular substance in the environment
poses a potential threat to human health. The adequacy of information to determine a substance's health
effects is described in a health effects summary. Data needs that are of significance to the protection of
public health are identified by ATSDR.
Each profile includes the following:
(A) The examination, summary, and interpretation of available toxicologic information and
epidemiologic evaluations on a toxic substance to ascertain the levels of significant
human exposure for the substance due to associated acute-, intermediate-, and chronic-
duration exposures;
(B) A determination of whether adequate information on the health effects of each substance
is available or in the process of development to determine levels of exposure that present
a significant risk to human health of acute, intermediate, and chronic health effects; and
(C) Where appropriate, identification of toxicologic testing needed to identify the types or
levels of exposure that may present significant risk of adverse health effects in humans.
The principal audiences for the toxicological profiles are health professionals at the Federal, State, and
local levels; interested private sector organizations and groups; and members of the public.
This profile reflects ATSDR’s assessment of all relevant toxicologic testing and information that has been
peer-reviewed. Staffs of the Centers for Disease Control and Prevention and other Federal scientists have
also reviewed the profile. In addition, this profile has been peer-reviewed by a nongovernmental panel
and was made available for public review. Final responsibility for the contents and views expressed in
this toxicological profile resides with ATSDR.
Christopher M. Reh, Ph.D.
Associate Director
Agency for Toxic Substances and Disease Registry
Centers for Disease Control and Prevention

HEXACHLOROCYCLOHEXANE (HCH) iv
VERSION HISTORY
Date
Description
March 2024
Final toxicological profile released
January 2023
Draft for public comment toxicological profile released
August 2005
Final toxicological profile released
July 1999
Final toxicological profile released
May 1994
Final toxicological profile released
December 1989
Final toxicological profile released
HEXACHLOROCYCLOHEXANE (HCH) v
CONTRIBUTORS & REVIEWERS
CHEMICAL MANAGER TEAM
Malcolm Williams, D.V.M., Ph.D. (Lead)
Heather Carlson-Lynch, M.S., D.A.B.T.
Melanie Buser, M.P.H.
Claire Heit, Ph.D.
Gaston Casillas, Ph.D.
Julie Melia, Ph.D., D.A.B.T.
Jennifer Rhoades, B.A.
Savannah Sierco, M.S.
ATSDR, Office of Innovation and Analytics,
Toxicology Section, Atlanta, GA
SRC, Inc., North Syracuse, NY
REVIEWERS
Interagency Minimal Risk Level Workgroup:
Includes ATSDR; National Center for Environmental Health (NCEH); National Institute for
Occupational Safety and Health (NIOSH); U.S. Environmental Protection Agency (EPA); National
Toxicology Program (NTP).
Additional reviews for science and/or policy:
ATSDR, Office of Community Health Hazard Assessment; ATSDR,
Office of Capacity Development
and Applied Prevention Science; ATSDR, Office of Science; NCEH, Division of Laboratory Sciences;
NCEH, Division of Environmental Health Science and Practice; EPA, Office of Research and
Development; EPA, Office of Water.
PEER REVIEWERS
1. Lisa Kamendulis, Ph.D.; Department of Environmental and Occupational Health; Indiana
University Bloomington; Bloomington, Indiana
2. Russell C. Cattley, VMD, Ph.D., Dipl. ACVP, Fellow IATP; Tyler & Frances Young Professor of
Pathology; Greene Hall; College of Veterinary Medicine; Auburn University, Alabama
3. Stephen M. Roberts, Ph.D.; Director, Center for Environmental & Human Toxicology; Professor,
College of Veterinary Medicine; College of Medicine, and College of Public Health and Health
Professions; University of Florida; Gainesville, Florida
These experts collectively have knowledge of toxicology, chemistry, and/or health effects. All reviewers
were selected in conformity with Section 104(I)(13) of the Comprehensive Environmental Response,
Compensation, and Liability Act, as amended.
ATSDR scientists review peer reviewers’ comments and determine whether changes will be made to the
profile based on comments. The peer reviewers’ comments and responses to these comments are part of
the administrative record for this compound.
The listing of peer reviewers should not be understood to imply their approval of the profile's final
content. The responsibility for the content of this profile lies with ATSDR.
HEXACHLOROCYCLOHEXANE (HCH) vi
CONTENTS
D
ISCLAIMER .............................................................................................................................................. ii
FOREWORD ............................................................................................................................................... iii
VERSION HISTORY .................................................................................................................................. iv
CONTRIBUTORS & REVIEWERS ............................................................................................................ v
CONTENTS ................................................................................................................................................. vi
LIST OF FIGURES ................................................................................................................................... viii
LIST OF TABLES ........................................................................................................................................ x
CHAPTER 1. RELEVANCE TO PUBLIC HEALTH ................................................................................. 1
1.1 OVERVIEW AND U.S. EXPOSURES ......................................................................................... 1
1.2 SUMMARY OF HEALTH EFFECTS ........................................................................................... 1
1.3 MINIMAL RISK LEVELS (MRLs) ............................................................................................ 11
CHAPTER 2. HEALTH EFFECTS ............................................................................................................ 19
2.1 INTRODUCTION ........................................................................................................................ 19
2.2 DEATH ........................................................................................................................................ 99
2.3 BODY WEIGHT ........................................................................................................................ 102
2.4 RESPIRATORY......................................................................................................................... 105
2.5 CARDIOVASCULAR ............................................................................................................... 107
2.6 GASTROINTESTINAL ............................................................................................................. 109
2.7 HEMATOLOGICAL ................................................................................................................. 110
2.8 MUSCULOSKELETAL ............................................................................................................ 113
2.9 HEPATIC ................................................................................................................................... 114
2.10 RENAL ...................................................................................................................................... 123
2.11 DERMAL ................................................................................................................................... 130
2.12 OCULAR ................................................................................................................................... 131
2.13 ENDOCRINE ............................................................................................................................. 131
2.14 IMMUNOLOGICAL ................................................................................................................. 136
2.15 NE
UROLOGICAL ..................................................................................................................... 140
2.16 REPRODUCTIVE ..................................................................................................................... 150
2.17 DEVELOPMENTAL ................................................................................................................. 163
2.18 OTHER NONCANCER ............................................................................................................. 190
2.19 CANCER .................................................................................................................................... 195
2.20 GENOTOXICITY ...................................................................................................................... 202
CHAPTER 3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS,
CHEMICAL INTERACTIONS ............................................................................................ 209
3.1 TOXICOKINETICS ................................................................................................................... 209
3.1.1 Absorption ........................................................................................................................... 210
3.1.2 Distribution ......................................................................................................................... 212
3.1.3 Metabolism .......................................................................................................................... 215
3.1.4 Excretion ............................................................................................................................. 217
3.1.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models ........... 219
3.1.6 Animal-to-Human Extrapolations ....................................................................................... 221
3.2 CHILDREN AND OTHER POPULATIONS THAT ARE UNUSUALLY
SUSCEPTIBLE .......................................................................................................................... 222
3.3 BIOMARKERS OF EXPOSURE AND EFFECT ..................................................................... 225
HEXACHLOROCYCLOHEXANE (HCH) vii
3.3.1 Biomarkers of Exposure ...................................................................................................... 226
3.3.2 Biomarkers of Effect ........................................................................................................... 228
3.4 INTERACTIONS WITH OTHER CHEMICALS ..................................................................... 228
CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION ........................................................... 232
4.1 CHEMICAL IDENTITY ........................................................................................................... 232
4.2 PHYSICAL AND CHEMICAL PROPERTIES ........................................................................ 233
CHAPTER 5. POTENTIAL FOR HUMAN EXPOSURE ....................................................................... 236
5.1 OVERVIEW .............................................................................................................................. 236
5.2 PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL ................................................ 237
5.2.1 Production ........................................................................................................................... 237
5.2.2 Import/Export ...................................................................................................................... 238
5.2.3 Use ...................................................................................................................................... 239
5.2.4 Disposal ............................................................................................................................... 240
5.3 RELEASES TO THE ENVIRONMENT ................................................................................... 241
5.3.1 Air ....................................................................................................................................... 241
5.3.2 Water ................................................................................................................................... 243
5.3.3 Soil ...................................................................................................................................... 245
5.4 ENVIRONMENTAL FATE ...................................................................................................... 245
5.4.1 Transport and Partitioning ................................................................................................... 245
5.4.2 Transformation and Degradation ........................................................................................ 251
5.5 LEVELS IN THE ENVIRONMENT ......................................................................................... 256
5.5.1 Air ....................................................................................................................................... 259
5.5.2 Water ................................................................................................................................... 259
5.5.3 Sediment and Soil ............................................................................................................... 270
5.5.4 Other Media ........................................................................................................................ 270
5.6 GENERAL POPULATION EXPOSURE .................................................................................. 281
5.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES .............................................. 289
CHAP
TER 6. ADEQUACY OF THE DATABASE ................................................................................ 291
6.1 EXISTING INFORMATION ON HEALTH EFFECTS ........................................................... 291
6.2 IDENTIFICATION OF DATA NEEDS .................................................................................... 291
6.3 ONGOING STUDIES ................................................................................................................ 302
CHAPTER 7. REGULATIONS AND GUIDELINES ............................................................................. 303
CHAPTER 8. REFERENCES .................................................................................................................. 306
APPENDICES
APPENDIX A. ATSDR MINIMAL RISK LEVEL WORKSHEETS .................................................... A-1
APPENDIX B. LITERATURE SEARCH FRAMEWORK FOR HCH .................................................. B-1
APPENDIX C. FRAMEWORK FOR ATSDR’S SYSTEMATIC REVIEW OF HEALTH EFFECTS
DATA FOR HCH ........................................................................................................... C-1
APPENDIX D. USER’S GUIDE ............................................................................................................. D-1
APPENDIX E. QUICK REFERENCE FOR HEALTH CARE PROVIDERS ....................................... E-1
APPENDIX F. GLOSSARY .................................................................................................................... F-1
APPENDIX G. ACRONYMS, ABBREVIATIONS, AND SYMBOLS ................................................. G-1
HEXACHLOROCYCLOHEXANE (HCH) viii
LIST OF FIGURES
1-1. Health Effects Found in Animals Following Inhalation Exposure to γ-Hexachlorocyclohexane ........ 3
1-2. Health Effects Found in Animals Following Oral Exposure to α-Hexachlorocyclohexane ................. 3
1-3. Health Effects Found in Animals Following Oral Exposure to β-Hexachlorocyclohexane ................. 4
1-4. Health Effects Found in Animals Following Oral Exposure to γ-Hexachlorocyclohexane ................. 5
1-5. Health Effects Found in Animals Following Oral Exposure to Technical
Hexachlorocyclohexane ........................................................................................................................ 6
1-6. Summary of Sensitive Targets of α-Hexachlorocyclohexane (α-HCH) – Oral .................................. 12
1-7. Summary of Sensitive Targets of β-Hexachlorocyclohexane (β-HCH) – Oral .................................. 13
1-8. Summary of Sensitive Targets of γ-Hexachlorocyclohexane (γ-HCH) – Inhalation .......................... 14
1-9. Summary of Sensitive Targets of γ-Hexachlorocyclohexane (γ-HCH) – Oral ................................... 15
1-10. Summary of Sensitive Targets of Technical-Hexachlorocyclohexane (technical-HCH) – Oral ...... 16
2-1. Overview of the Number of Studies Examining α-Hexachlorocyclohexane (α-HCH) Health
Effects ................................................................................................................................................. 23
2-2. Overview of the Number of Studies Examining β-Hexachlorocyclohexane (β-HCH) Health
Effects ................................................................................................................................................. 24
2-3. Overview of the Number of Studies Examining γ-Hexachlorocyclohexane (γ-HCH) Health
Effects ................................................................................................................................................. 25
2-4. Overview of the Number of Studies Examining δ-Hexachlorocyclohexane (δ-HCH) and
Unspecified Hexachlorocyclohexanes Health Effects ........................................................................ 26
2-5. Levels of Significant Exposure to γ-Hexachlorocyclohexane (Lindane) – Inhalation ....................... 29
2-6. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral ................................................. 36
2-7. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral ................................................. 46
2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral ................................................. 74
2-9. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane (HCH) – Oral ............ 90
3-1. The Proposed Metabolism of Hexachlorocyclohexane .................................................................... 216
5-1. Number of NPL Sites with Hexachlorocyclohexane Contamination ............................................... 236
6-1.
Summary of Existing Health Effects Studies on α-Hexachlorocyclohexane by Route and
Endpoint ........................................................................................................................................... 292
HEXACHLOROCYCLOHEXANE (HCH) ix
6-2. Summary of Existing Health Effects Studies on β-Hexachlorocyclohexane by Route and
Endpoint ........................................................................................................................................... 293
6-3. Summary of Existing Health Effects Studies on γ-Hexachlorocyclohexane by Route and
Endpoint ........................................................................................................................................... 294
6-4. Summary of Existing Health Effects Studies on δ-Hexachlorocyclohexane and Unspecified
Hexachlorocyclohexanes by Route and Endpoint ............................................................................ 295
HEXACHLOROCYCLOHEXANE (HCH) x
LIST OF TABLES
1-
1. Minimal Risk Levels (MRLs) for α-Hexachlorocyclohexane ............................................................ 17
1-2. Minimal Risk Levels (MRLs) for β-Hexachlorocyclohexane ............................................................ 17
1-3. Minimal Risk Levels (MRLs) for γ-Hexachlorocyclohexane ............................................................ 18
2-1. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Inhalation ........................................ 27
2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral ................................................. 31
2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral ................................................. 40
2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral ................................................. 50
2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral ........................ 81
2-6. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Dermal ............................................ 94
2-7. Levels of Significant Exposure to Technical Hexachlorocyclohexane – Dermal .............................. 96
2-8. Summary of Epidemiological Studies of β-Hexachlorocyclohexane (β-HCH) Exposure and
Body Weight Effects ........................................................................................................................ 103
2-9. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and
Cardiovascular Effects ...................................................................................................................... 107
2-10. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Hepatic
Effects ............................................................................................................................................. 114
2-11. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Renal Effects .................................................................................................................................. 124
2-12. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Endocrine Effects ........................................................................................................................... 132
2-13. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Immune Effects .............................................................................................................................. 137
2-14. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Neurological Effects ....................................................................................................................... 142
2-15. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Reproductive Effects ...................................................................................................................... 151
2-16. Summary of Epidemiological Studies of α-Hexachlorocyclohexane (HCH) Exposure and
Developmental Effects ................................................................................................................... 164
2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and
Developmental Effects ................................................................................................................... 167
HEXACHLOROCYCLOHEXANE (HCH) xi
2-18. Summary of Epidemiological Studies of γ-Hexachlorocyclohexane Exposure and
Developmental Effects ................................................................................................................... 178
2-19. Summary of Epidemiological Studies of δ-HCH and Total HCH Exposure and
Developmental Effects ................................................................................................................... 189
2-20. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and
Other Noncancer Effects ................................................................................................................ 191
2-21. Summary of Epidemiological Studies Evaluating Possible Associations between
Hexachlorocyclohexane Exposure and Risk of Selected Cancer Types......................................... 195
2-22. Genotoxicity of Hexachlorocyclohexane Isomers In Vivo ............................................................. 202
2-23. Genotoxicity of Hexachlorocyclohexane Isomers In Vitro ............................................................ 203
4-1. Chemical Identity of Hexachlorocyclohexane Isomers .................................................................... 232
4-2. Physical and Chemical Properties of Hexachlorocyclohexane Isomers ........................................... 234
5-1. Facilities that Produce, Process, or Use γ-Hexachlorocyclohexane ................................................. 237
5-2. Releases to the Environment from Facilities that Produce, Process, or Use
Hexachlorobenzenes ......................................................................................................................... 242
5-3. γ-HCH Emissions as Reported by the 2017 National Emission Inventory ...................................... 243
5-4. Results of Experimental Bioaccumulation Studies with γ-Hexachlorocyclohexane ........................ 249
5-5. Lowest Limit of Detection Based on Standards ............................................................................... 256
5-6. Summary of Ambient Environmental Levels of HCH ..................................................................... 257
5-7. Hexachlorocyclohexanes Levels in Water, Soil, and Air of National Priorities List (NPL)
Sites .................................................................................................................................................. 258
5-8. Outdoor Air Monitoring Data for Hexachlorocyclohexanes (HCHs) ............................................... 260
5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs) ........................................................ 264
5-10. Soil and Sediment Monitoring Data for Hexachlorocyclohexanes (HCHs) ................................... 271
5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs) ................................................ 275
5-12. Geometric Mean of the Serum Concentration (ng/g) of β-Hexachlorocyclohexane (β-HCH)
(2015–2016) and γ-Hexachlorocyclohexane (γ-HCH) (2011–2012) in the U.S. Population ......... 285
7-1. Regulations and Guidelines Applicable to Hexachlorocyclohexane (HCH) .................................... 303
HEXACHLOROCYCLOHEXANE (HCH) 1
CHAPTER 1. RELEVANCE TO PUBLIC HEALTH
1.1 OVERVIEW AND U.S. EXPOSURES
Hexachlorocyclohexane (HCH) is a mixture of eight isomers, four of which are of commercial
significance: alpha (α)-HCH (Chemical Abstracts Service [CAS] Registry Number 319-84-6),
beta (β)-HCH (CAS Registry Number 319-85-7), gamma (γ)-HCH (CAS Registry Number 58-89-9), and
delta (δ)-HCH (CAS Registry Number 319-86-8). Technical (or technical-grade) HCH (CAS Registry
Number 608-73-1) is not an isomer of HCH, but rather a mixture of several isomers; it consists of
approximately 60–70% α-HCH, 5–12% β-HCH, 10–15% γ-HCH, 6–10% δ-HCH, and 3–4% ε-HCH
(Kutz et al. 1991). The most well-studied isomer is γ-HCH (lindane), an organochlorine insecticide that
was used for a broad range of agricultural applications in the United States and worldwide beginning in
the 1940s. Its agricultural use began to be limited in the 1970s by the U.S. Environmental Protection
Agency (EPA), citing human health concerns, and final registrations for products containing γ-HCH were
cancelled in late 2006. Today, 1% γ-HCH prescription products, regulated by the U.S. Food and Drug
Administration (FDA), are available for lice and scabies treatment. HCH isomers exist as white solids
that can volatilize to the gas or particulate phase. HCH released to the environment can volatilize from,
or partition to, soil and can leach to groundwater. The general population may be exposed to low
amounts of HCH through inhalation of contaminated ambient air and ingestion of contaminated water
(exposure in the range of parts per trillion) or contact with contaminated soils (exposure in the range of
parts per billion). The highest exposures result from the use of γ-HCH pharmaceutical treatments.
Workers who work at facilities that use or process γ-HCH and people who live near HCH-contaminated
sites may have increased exposure.
1.2 SUMMARY OF HEALTH EFFECTS
The toxicological database for HCH includes human observational studies of pesticide workers and the
general population and studies of animals exposed by inhalation, oral administration, and dermal
application. In general, the studies of pesticide applicators with exposure to γ-HCH or technical HCH
used qualitative measures of exposure. Most general population studies used blood or tissue
concentrations of HCH isomers to assess exposure, and the samples were typically collected
simultaneously with or after outcome assessment. As such, the temporal relationship between exposure
and outcome is uncertain.
HEXACHLOROCYCLOHEXANE (HCH) 2
1. RELEVANCE TO PUBLIC HEALTH
Data pertaining to the effects in animals after inhalation or dermal exposure are limited to the γ-HCH
isomer and technical HCH. In addition, the available data on effects in animals exposed by oral
administration to α-, β-, or δ-HCH are relatively limited, compared to the information available for
γ-HCH. Figures 1-1 through 1-5 show the most sensitive effects in animals after inhalation exposure to
γ-HCH, oral exposure to α-HCH, oral exposure to β-HCH, oral exposure to γ-HCH, and oral exposure to
technical-grade HCH, respectively. The available data on δ-HCH are not adequate to identify sensitive
effects by any exposure route. As Figure 1-2 shows, the most sensitive effect of oral exposure to α-HCH
is liver toxicity. A systematic review of this endpoint resulted in the following hazard identification
conclusion:
• Hepatic effects are a presumed health effect for humans.
Figure 1-3 shows that the most sensitive effects of β-HCH in animals exposed orally are liver toxicity and
neurological effects. A systematic review of these endpoints resulted in the following hazard
identification conclusions:
• Hepatic effects are a presumed health effect for humans.
• Neurological effects are a presumed health effect for humans.
Figures 1-1 and 1-4 show that the most sensitive effects of γ-HCH in animals are developmental toxicity
and immune system effects. A systematic review of these endpoints resulted in the following hazard
identification conclusions:
• Developmental effects are a presumed health effect for humans.
• Immune system effects are a presumed health effect for humans.
Figure 1-5 shows the most sensitive effects of technical-grade HCH (a mixture of isomers) or in studies
that did not specify the HCH isomer(s). A systematic review was not conducted for the mixture.
Hepatic Effects. Data on hepatic effects of HCH isomers in humans are inadequate for hazard
identification, but studies in animals show similar liver effects induced by all the subject isomers of HCH
after inhalation, oral, and dermal exposure. Hepatic effects consisting of increased absolute and/or
relative liver weights, hepatocellular hypertrophy, necrosis, fatty degeneration, bile duct proliferation, and
nodular hyperplasia have been observed in rats, mice, and hamsters exposed by oral administration of
α-HCH for intermediate and chronic durations (Fitzhugh et al. 1950; Ito et al. 1975; Nagasaki et al. 1975;
Sumida et al. 2007; Tryphonas and Iverson 1983). Dietary administration of β-HCH for intermediate and
chronic durations has resulted in similar liver toxicity in rats and mice (Fitzhugh et al. 1950; Hanada et al.

HEXACHLOROCYCLOHEXANE (HCH) 3
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-1. Health Effects Found in Animals Following Inhalation Exposure to
γ-Hexachlorocyclohexane
Concentration (ppm)
Effects in Animals
101-603
Acute: Decreased body weight and clinical signs of neurotoxicity in rats
5-10
0.5-1
Intermediate: Death in mice; histological changes in kidneys in rats
Acute: Death in mice; diarrhea in rats
Intermediate:
Diarrhea in rats; bone marrow myelogram changes in rats
Figure 1-2. Health Effects Found in Animals Following Oral Exposure to
α-Hexachlorocyclohexane
Dose (mg/kg/day) Effects in Animals
18-20
4
0.002 mg/kg/day Intermediate MRL
45-70
0.0009 mg/kg/day Chronic MRL
Intermediate: Histological changes in kidneys in rats; hepatoma in
female mice; decreased body weight in rats
Chronic: Hepatocellular carcinoma in rats
Intermediate: Increased liver weight and histological changes in liver in
mice; hepatoma in male mice
Chronic: Increased liver weight and histological changes in liver in rats

HEXACHLOROCYCLOHEXANE (HCH) 4
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-3. Health Effects Found in Animals Following Oral Exposure to
β-Hexachlorocyclohexane
Dose (mg/kg/day) Effects in Animals
5-9
0.18-0.7
0.08 mg/kg/day Acute MRL
20-38
0.0006 mg/kg/day Intermediate MRL
60-200
Acute: Lateral recumbency and death
Acute: Ataxia and hypoactivity in rats
Intermediate: Reduced tail nerve conduction velocity in rats; immune
suppression and hepatic histopathology changes in mice
Intermediate: Death; hematology changes; histological changes in
kidneys, adrenal glands, spleen, thymus, ovaries, and testes in rats; pup
mortality in rats
Chronic: Liver tumors in mice
Intermediate: Decreased body weight gain in rats; increased liver
weight in rat pups
Intermediate: Histological changes in liver in rats
Chronic: Increased liver weight and histological changes in liver in rats

HEXACHLOROCYCLOHEXANE (HCH) 5
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-4. Health Effects Found in Animals Following Oral Exposure to
γ-Hexachlorocyclohexane
Dose (mg/kg/day) Effects in Animals
0.25-1.7
0.00015-0.07
0.003 mg/kg/day Acute MRL
3-5
0.0000008 mg/kg/day Intermediate MRL
7-10
13.6-20
Acute: Death in rats; impaired development of male and female
reproductive tracts in mice
Chronic: Hepatocellular carcinoma in mice
Acute: Reduced delayed-type hypersensitivity in rats; increased
spontaneous activity in rats; hematological effects in mice
Intermediate: Female reproductive effects in rats; suppressed body
weight gain in dogs
Chronic: Increased liver weight and histological changes in liver in rats;
histological changes in kidney in rats
Acute: Histological changes in liver in rats; changes to serotonin levels
and seizures in rats
Intermediate: Decreased sperm count and motility in rats; immune
suppression in rats; cardiotoxicity in rats
Acute: Impaired development of male reproductive tract in rats
Intermediate: Histological changes in liver in rats; reproductive effects
in mink; Reduced ovulation rate in rabbits; persistent hyperactivity and
ultrastructural changes in the brains of rat pups
Intermediate: Histological changes in kidney in male rats; immune
suppression in mice; decreased body weight and cardiac histopathology
in rat pups; altered ventricular electrophysiology in rat pups

HEXACHLOROCYCLOHEXANE (HCH) 6
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-5. Health Effects Found in Animals Following Oral Exposure to
Technical Hexachlorocyclohexane
Dose (mg/kg/day) Effects in Animals
2-4
0.4-0.8
10
50-144
Acute: Death in mice; histological changes in liver in mice
Intermediate: Liver tumors in mice
Acute: Reduced enzyme activity in the brain in rats; reproductive effects
in male rats
Intermediate: Changes in neurotransmitter levels in rat pups
Chronic:
Convulsions in mice; hepatocellular carcinoma in mice
Intermediate: Decreased body weight gain in rats; decreased vas
deferens weight and degeneration in rats; altered behavior,
ultrastructural changes in brain in rats; increased liver weight in rats
Chronic: Histological changes in liver in rats
Intermediate: Increased kidney weight in pigs; tremors, convulsions,
paralysis of limbs in rats
HEXACHLOROCYCLOHEXANE (HCH) 7
1. RELEVANCE TO PUBLIC HEALTH
1973; Ito et al. 1973, 1975; Van Velsen et al. 1986). In intermediate-duration studies of rats exposed to
γ-HCH aerosol, increased liver weights were seen without histology changes (Oldiges et al. 1983). After
oral exposure to γ-HCH for acute, intermediate, and chronic durations, liver effects in rats, mice, and
rabbits have included increased serum enzymes indicative of hepatocellular injury, increased serum lipids,
increased liver weight, hepatocellular hypertrophy, vacuolar degeneration, necrosis, and congestion (Ali
and Shakoori 1998; Amyes 1990; Attia et al. 2011; Boll et al. 1995; Cerón et al. 1995; EPA 1991a,
2000a; Fatih Fidan et al. 2008; Fitzhugh et al. 1950; Grabarczyk et al. 1990; Hfaiedh et al. 2012; Kamal
El-Dein et al. 2016; Kopec-Szlezak et al. 1989; Matsuura et al. 2005; Parmar et al. 2003; Singh and
Sharma 2011; Sumida et al. 2007; Suter 1983; Vijaya Padma et al. 2011). Centrilobular hepatocellular
hypertrophy was also reported in rats exposed to γ-HCH for 13 weeks by dermal application (EPA
1988a). In intermediate-duration studies of rats and mice exposed to δ-HCH, increased liver weight
and/or centrilobular hypertrophy were reported (Ito et al. 1973, 1975). Studies of animals exposed to
technical-grade HCH by oral or dermal administration (e.g., Dikshith et al. 1978, 1989b, 1991a, 1991c;
Fitzhugh et al. 1950; Philip et al. 1989; Trivedi et al. 2007, 2009) provide supporting evidence for hepatic
effects of HCH isomers.
Developmental Effects. Epidemiological studies examining relationships between birth outcomes and
maternal or fetal blood or tissue levels of β-HCH have reported associations with decreased birth weight
(Anand and Taneja 2020; Callan et al. 2016; Fang et al. 2019a, 2019b; Guo et al. 2014; Lopez-Espinosa et
al. 2011; Yang et al. 2020) and fetal growth restriction (Sharma et al. 2012). Studies using α- or γ-HCH
levels in maternal or fetal tissues to assess the relationship between HCH exposure and developmental
outcomes in humans have not shown consistent results and are limited by the relatively short half-life of
these isomers in the human body (see details in Section 3.1.4). No developmental toxicity studies of
animals exposed to α-HCH were located. Developmental toxicity data for β-HCH are very limited but
show increased perinatal mortality and increased liver weight of pups after exposure during gestation and
lactation or lactation only (Srinivasan et al. 1991). After oral administration of technical-grade HCH
during gestation, mice exhibited increased fetal resorptions (Dikshith et al. 1990; Srivastava and Raizada
2000) and rats have shown altered neurotransmitter levels in the brain (Nagaraja and Desiraju 1994).
Studies in a variety of species exposed to γ-HCH for acute or intermediate durations during gestation or
postnatal development have demonstrated effects on a wide range of endpoints, including birth outcomes
and development of the male and female reproductive tracts, central nervous system, heart, liver, thymus,
and spleen. Increased stillbirths, reduced neonatal viability, and decreased pup weights have been
reported in rats, mice, and mink (Beard et al. 1997; EPA 1991a, 1999c; Hassoun and Stohs 1996a;
HEXACHLOROCYCLOHEXANE (HCH) 8
1. RELEVANCE TO PUBLIC HEALTH
Matsuura et al. 2005; Sauviat et al. 2005). In male offspring of rats and mice exposed to γ-HCH via oral
administration during gestation and/or postnatal development, effects on preputial separation, serum
hormone levels, spermatogenesis, reproductive organ weights, and testicular histopathology have been
reported (Agrahari et al. 2019; Dalsenter et al. 1997a, 1997b; Di Consiglio et al. 2009; La Sala et al. 2009;
Traina et al. 2003). Female offspring of rats and mice exposed similarly exhibited effects on vaginal
opening, oogenesis, and uterine weight (La Sala et al. 2009; Maranghi et al. 2007; Matsuura et al. 2005).
Oral exposure of maternal rats and mice to γ-HCH has resulted in significant decreases in thymus and
spleen weights in the offspring (Hassoun et al. 1996; Matsuura et al. 2005), increases in pup liver weight
(Srinivasan et al. 1991), and cardiac electrophysiology and histopathology changes in pups (Sauviat et al.
2005). Developmental neurotoxicity findings in animals orally exposed to γ-HCH in utero or during
development included seizures and convulsions (Albertson et al. 1985; Johri et al. 2008); effects on motor
activity, learning, and memory (EPA 1999c; Johri et al. 2007; Rivera et al. 1998; Srivastava et al. 2019);
changes in neurotransmitter levels (Rivera et al. 1991, 1998); altered brain wave activity (Breton et al.
2005); and ultrastructural changes in the brain (Srivastava et al. 2019).
Immune System Effects. There are inadequate data on effects of HCH isomers on the immune system of
humans. No studies of immune endpoints in animals exposed to α-HCH by inhalation, oral, or dermal
routes were located. Information on immune effects of β-HCH includes a report of decreased lympho-
proliferative responses to mitogens in mice exposed via diet for 30 days (Cornacoff et al. 1988) and a
report of thymic and splenic histopathology changes (atrophy of the thymus and depletion of splenic
lymphoid tissue) in rats at doses associated with humane sacrifice due to moribund condition (Van Velsen
et al. 1986). Suppression of the immune system has been demonstrated in a small number of acute- and
intermediate-duration studies of γ-HCH administered orally to rats, mice, rabbits, and sheep. Effects seen
in these studies include reduced delayed-type hypersensitivity response (Khurana et al. 1999; Mediratta et
al. 2008) and decreased antibody titers in response to antigens (Banerjee et al. 1996; Desi et al. 1978;
Dewan et al. 1980; Koner et al. 1998; Meera et al. 1992). Decreased spleen and thymus weights and
histopathology changes in the thymus, lymph nodes, and spleen have also been seen in animals exposed
to γ-HCH (Hong and Boorman 1993; Meera et al. 1992).
Neurological Effects. The available epidemiological data on neurological effects of HCH isomers are
generally inadequate for hazard identification, but case reports support a relationship between oral and
dermal exposure to γ-HCH and seizures or convulsions in humans of all ages (Aks et al. 1995; Boffa et al.
1995; CDC 2005; Davies et al. 1983; Fischer 1994; Forrester et al. 2004; Hall and Hall 1999; Harris et al.
1969; Lee and Groth 1977; Lifshitz and Gavrilov 2002; Matsuoka 1981; Munk and Nantel 1977; Nordt
HEXACHLOROCYCLOHEXANE (HCH) 9
1. RELEVANCE TO PUBLIC HEALTH
and Chew 2000; Powell 1980; Ramabhatta et al. 2014; Ramchander et al. 1991; Solomon et al. 1995;
Starr and Clifford 1972; Storen 1955; Sudakin 2007; Wheeler 1977; Telch and Jarvis 1982; Tenenbein
1991; Wiles et al. 2015). Information on neurotoxicity of α-HCH in animals is limited to a single study
showing no effect on nerve conduction velocity in rats exposed for 30 days (Muller et al. 1981). In
addition, few data on this endpoint are available for β-HCH. Studies include reports of clinical signs of
neurotoxicity after acute durations (ataxia and hypoactivity progressing in some cases to coma)
(Cornacoff et al. 1988; Van Velsen et al. 1986) and reduced nerve conduction velocity in the tail of rats in
the isomer comparison study by Muller et al. (1981).
Neurological effects have been observed in rats and/or mice exposed to γ-HCH by inhalation, oral, and
dermal exposure routes. Inhalation exposure of rats for acute durations resulted in central nervous system
depression or restlessness, excitation, and ataxia, with spasms observed at higher concentrations (Oldiges
et al. 1980; Ullmann 1986b). In rats exposed by gavage or dietary administration of γ-HCH, seizures and
convulsions have been observed (Amyes 1990; EPA 1999a; Fitzhugh et al. 1950; Gilbert and Mack 1995;
Johri et al. 2008; Joy et al. 1982; Martinez and Martinez-Conde 1995; Martinez et al. 1991; Matsuura et
al. 2005; Parmar et al. 2003; Tusell et al. 1988; Vendrell et al. 1992a, 1992b; Woolley and Griffith 1989).
Altered neurotransmitter levels in the brain were noted in rats exposed orally for acute or intermediate
durations (Attia et al. 1991; Martinez and Martinez-Conde 1995). Clinical signs of toxicity in orally-
dosed rats have included decreased motor activity, decreased grooming behavior, increased rearing,
altered gait, and hypersensitivity to touch (EPA 1999a, 1999b). Effects on motor activity, anxiety,
cognition, and memory were demonstrated in neurobehavioral testing of rats after acute- and
intermediate-duration oral exposures to γ-HCH (Desi 1974; EPA 1999a; Llorens et al. 1990; Sahaya et al.
2007; Srivastava et al. 2019; Tilson et al. 1987); in one study, the behavioral changes were accompanied
by ultrastructural changes in the hippocampus and substantia nigra of the rats (Srivastava et al. 2019).
Clinical signs of neurotoxicity, including seizures, convulsions, hyperactivity, ataxia, and/or sedation
were reported in rats and rabbits after single or repeated dermal applications of γ-HCH (EPA 1988a;
Hanig et al. 1976; Ullmann 1986a).
Cancer. Human epidemiological data provide evidence for an association between exposure to HCH
isomers and non-Hodgkin’s lymphoma (NHL). The strongest evidence is derived from a prospective
cohort study of pesticide applicators in Iowa and North Carolina, which showed that NHL incidence
increased with duration and intensity of exposure to γ-HCH (Alavanja et al. 2014). A large, pooled case-
control study reported similar findings. Kachuri et al. (2020) pooled data across three population-based,
case-control studies in the United States and Canada (North American Pooled Project). The odds of NHL
HEXACHLOROCYCLOHEXANE (HCH) 10
1. RELEVANCE TO PUBLIC HEALTH
were increased with self-reported exposure to γ-HCH in analyses of 1,690 cases and 5,131 controls
(Kachuri et al. 2020). Additional support for the association with NHL comes from a case-control study
nested within three large prospective cohorts in Shanghai and Singapore. Bassig et al. (2020) observed a
positive association between incident NHL and blood levels of β-HCH measured approximately 7 years
prior to diagnosis. Nested case-control studies that reported no association between NHL and blood or
tissue levels of β-HCH (Brauner et al. 2012; Cantor et al. 2003) generally reported lower exposure levels
than the study by Bassig et al. (2020).
Other epidemiological studies reported positive associations between β- or γ-HCH in blood or qualitative
exposure to γ-HCH and multiple myeloma, leukemia, colorectal cancer, female breast cancer, prostate
cancer, lung cancer, thyroid cancer, brain cancer, and hepatocellular carcinoma (Arrebola et al. 2015a;
Band et al. 2011; Lee et al. 2018a; Lerro et al. 2021; Ibarluzea et al. 2004; Kumar et al. 2010; Miao et al.
2021; Purdue et al. 2007; Salimi et al. 2023; Waliszewski et al. 2005; Weber et al. 2018; Xu et al. 2010;
Yousefi et al. 2022; Zhao et al. 2012). However, the evidence for an association between HCH isomer
exposure and these cancer types is much weaker than that for NHL.
Studies in rats and mice exposed to α-, β-, γ-, and technical HCH by dietary administration have shown
increased incidences of liver tumors (Bhatt and Bano 2009; Bhatt and Nagda 2012; Hanada et al. 1973;
Ito et al. 1973, 1975, 1976; Karnik et al. 1981; Kashyap et al. 1979; Munir et al. 1983; Nagasaki et al.
1975; NCI 1977; Thakore et al. 1981; Thorpe and Walker 1973; Trivedi et al. 2007, 2009; Tryphonas and
Iverson 1983; Tsukada et al. 1979; Wolff et al. 1987). In addition, chronic dermal exposure to technical-
grade HCH resulted in liver tumors in mice (Kashyap et al. 1979). γ-HCH has been reported to induce
increased incidences of bronchiolar-alveolar adenomas and carcinomas in female mice exposed via diet
(EPA 2000a; Wolff et al. 1987).
The EPA (IRIS 1987a) listed α-HCH as a probable human carcinogen based on sufficient evidence of
carcinogenicity in animals and inadequate data in humans. The Integrated Risk Information System (IRIS
1987b) listed β-HCH as a possible human carcinogen based on evidence for benign liver tumors in
exposed mice and inadequate data in humans. Data on δ-HCH were considered inadequate to classify the
potential human carcinogenicity (IRIS 1987d). Although the IRIS (1987c) program did not evaluate the
carcinogenicity of γ-HCH, EPA’s Office of Pesticide Programs (EPA 2001, 2002) classified γ-HCH into
the category “suggestive evidence of carcinogenicity, but not sufficient to assess human carcinogenic
potential.” The Department of Health and Human Services (HHS) National Toxicology Program (NTP)
determined that γ-HCH and other HCH isomers may reasonably be anticipated to cause cancer in humans
HEXACHLOROCYCLOHEXANE (HCH) 11
1. RELEVANCE TO PUBLIC HEALTH
(NTP 2021). In 2018, the International Agency for Research on Cancer (IARC) determined that there
was sufficient evidence in both humans and animals for the carcinogenicity of γ-HCH, assigning it to
Group 1 (carcinogenic to humans). IARC (2018) concluded that γ-HCH causes NHL in humans.
1.3 MINIMAL RISK LEVELS (MRLs)
α-HCH. The inhalation database was considered inadequate for derivation of acute-, intermediate-, or
chronic-duration inhalation MRLs for α-HCH. The oral database for α-HCH was considered inadequate
for derivation of an acute-duration oral MRL, but data were adequate for derivation of intermediate- and
chronic-duration oral MRLs. As shown in Figure 1-6, hepatic effects are the most sensitive targets of
toxicity in animals exposed orally to α-HCH.
β-HCH. The inhalation database was considered inadequate for derivation of acute-, intermediate-, or
chronic-duration inhalation MRLs for β-HCH. The oral database for β-HCH was considered adequate for
derivation of acute- and intermediate-duration oral MRLs, but not for a chronic-duration oral MRL. As
shown in Figure 1-7, neurological and hepatic effects are the most sensitive targets of toxicity in animals
exposed orally to β-HCH.
γ-HCH (Lindane). The inhalation database was considered inadequate for derivation of acute-,
intermediate-, or chronic-duration inhalation MRLs for γ-HCH. Figure 1-8 shows that death and renal
and gastrointestinal effects were seen at the lowest concentrations of γ-HCH in available inhalation
studies. The oral database for γ-HCH was considered adequate for derivation of acute- and intermediate-
duration oral MRLs, but not for a chronic-duration oral MRL. As shown in Figure 1-9, developmental
and immune system effects are the most sensitive targets of toxicity in animals exposed orally to γ-HCH.
δ-HCH. The inhalation and oral databases were considered inadequate for derivation of acute-,
intermediate-, or chronic-duration inhalation or oral MRLs for δ-HCH.
Technical HCH or Unspecified Isomers of HCH. MRLs were not derived for technical-grade HCH due
to the wide variation in isomer composition of technical HCH. Figure 1-10 shows the sensitive targets in
studies of technical-grade HCH or unspecified HCH isomers.

HEXACHLOROCYCLOHEXANE (HCH) 12
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-6. Summary of Sensitive Targets of α-Hexachlorocyclohexane (α-HCH) –
Oral
Available data indicate that the liver, and liver cancers, are the most sensitive targets of α-HCH
oral exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals; no human data were identified.
18
18
45
60
4
70
Cancer
Hepatic
Body weight
Renal
Hepatic
Cancer
Intermediate (mg/kg/day)
Chronic (mg/kg/day)

HEXACHLOROCYCLOHEXANE (HCH) 13
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-7. Summary of Sensitive Targets of β-Hexachlorocyclohexane (β-HCH) –
Oral
Available data indicate that the liver is the most sensitive target of β-HCH oral exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals.
No reliable dose-response data were available for humans.
38
72
200
0.2
5
9
23
23
23
66.3
0.7
34
Acute (mg/kg/day)
Neurological
Renal
Death
Hepatic
Developmental
Body weight
Immunological
Renal
Reproductive
Neurological
Hepatic
Cancer
Intermediate (mg/kg/day)
Chronic (mg/kg/day)

HEXACHLOROCYCLOHEXANE (HCH) 14
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-8. Summary of Sensitive Targets of γ-Hexachlorocyclohexane (γ-HCH) –
Inhalation
Available data indicate that the kidney is the most sensitive target of γ-HCH inhalation exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals; no human data were identified.
5
10
101
603
0.5
1
5
5
Acute (ppm)
Intermediate (ppm)
Gastrointestinal
Death
Neurological
Body weight
Renal
Death
Gastrointestinal
Hematological

HEXACHLOROCYCLOHEXANE (HCH) 15
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-9. Summary of Sensitive Targets of γ-Hexachlorocyclohexane (γ-HCH) –
Oral
Available data indicate that the developing organism is the most sensitive target of γ-HCH oral
exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals; no human data were identified.
1
3
5
6
10
10
20
0.00015
0.012
0.07
0.8
1.7
2.5
3
7
7
7
Acute (mg/kg/day)
Developmental
Neurological
Hepatic
Reproductive
Hematological
Immunological
Death
Developmental
Immunological
Renal
Reproductive
Hepatic
Neurological
Cardiovascular
Body weight
Hepatic
Renal
Intermediate (mg/kg/day)
Chronic (mg/kg/day)

HEXACHLOROCYCLOHEXANE (HCH) 16
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-10. Summary of Sensitive Targets of Technical-Hexachlorocyclohexane
(technical-HCH) – Oral
Available data indicate that the central nervous system is the most sensitive target of technical-
HCH oral exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals; no human data were identified.
1
3
5
6
10
10
20
0.00015
0.012
0.07
0.8
1.7
2.5
3
7
7
7
Acute (mg/kg/day)
Developmental
Neurological
Hepatic
Reproductive
Hematological
Immunological
Death
Developmental
Immunological
Renal
Reproductive
Hepatic
Neurological
Cardiovascular
Body weight
Hepatic
Renal
Intermediate (mg/kg/day)
Chronic (mg/kg/day)
The MRL values for α-HCH, β-HCH, and γ-HCH are summarized in Tables 1-1, 1-2, and 1-3,
respectively, and discussed in greater detail in Appendix A.

HEXACHLOROCYCLOHEXANE (HCH) 17
1. RELEVANCE TO PUBLIC HEALTH
Table 1-1. Minimal Risk Levels (MRLs) for α-Hexachlorocyclohexane
a
Exposure
route
Exposure
duration
MRL
Critical effect
POD type
POD value
Uncertainty/
modifying
factor
Reference
Inhalation
No inhalation MRLs were derived for any duration.
Oral
Acute
None
–
–
–
–
–
Intermediate
0.002 mg/kg/day
Increased liver weight and
histopathology
NOAEL
2 mg/kg/day
UF: 100
MF: 10
Sumida et al.
2007
Chronic
9x10
-4
mg/kg/day
Increased liver weight and
histopathology
NOAEL
0.9 mg/kg/day
UF: 100
MF: 10
Fitzhugh et al.
1950
a
See Appendix A for additional information.
MF = modifying factor; NOAEL = no-observed-adverse-effect level; POD = point of departure; UF = uncertainty factor
Table 1-2. Minimal Risk Levels (MRLs) for β-Hexachlorocyclohexane
a
Exposure
route
Exposure
duration
MRL
Critical effect
POD type
POD value
Uncertainty/
modifying factor
Reference
Inhalation
No inhalation MRLs were derived for any duration.
Oral
Acute
0.08 mg/kg/day
Clinical signs of neurotoxicity
(ataxia, inactivity) at higher doses
NOAEL
8 mg/kg/day
UF: 100
Van Velsen
et al. 1986
Intermediate
6x10
-4
mg/kg/day
Hyalinization of centrilobular liver
cells
LOAEL
0.18 mg/kg/day
UF: 300
Van Velsen
et al. 1986
Chronic
None
–
–
–
–
–
a
See Appendix A for additional information.
LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; UF = uncertainty factor

HEXACHLOROCYCLOHEXANE (HCH) 18
1. RELEVANCE TO PUBLIC HEALTH
Table 1-3. Minimal Risk Levels (MRLs) for γ-Hexachlorocyclohexane
a
Exposure
route
Exposure
duration
MRL
Critical effect
POD type
POD value
Uncertainty/
modifying
factor
Reference
Inhalation
No inhalation MRLs were derived for any duration.
Oral
Acute
0.003 mg/kg/day
Reduced reproductive organ
weights, sperm numbers, serum
testosterone, and increased
intromission frequency in male
offspring
LOAEL
1 mg/kg/day
UF: 300
Dalsenter
et al. 1997b
Intermediate
8x10
-7
mg/kg/day
Cardiac effects in offspring
NOAEL
7.6x10
-5
mg/kg/day
UF: 100
Sauviat et
al. 2005
Chronic
None
–
–
–
–
–
a
See Appendix A for additional information.
LOAEL = lowest-observed-adverse-effect level; NOAEL = no-observed-adverse-effect level; POD = point of departure; UF = uncertainty factor
HEXACHLOROCYCLOHEXANE (HCH) 19
CHAPTER 2. HEALTH EFFECTS
2.1 INTRODUCTION
The primary purpose of this chapter is to provide public health officials, physicians, toxicologists, and
other interested individuals and groups with an overall perspective on the toxicology of HCH. It contains
descriptions and evaluations of toxicological studies and epidemiological investigations and provides
conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health. When
available, mechanisms of action are discussed along with the health effects data; toxicokinetic
mechanistic data are discussed in Section 3.1.
A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile.
To help public health professionals and others address the needs of persons living or working near hazardous
waste sites, the information in this section is organized by health effect. These data are discussed in terms of
route of exposure (inhalation, oral, and dermal) and three exposure periods: acute (≤14 days), intermediate
(15–364 days), and chronic (≥365 days).
As discussed in Appendix B, a literature search was conducted to identify relevant studies examining health
effect endpoints. Figures 2-1, 2-2, and 2-3 provide an overview of the database of studies in humans or
experimental animals for α-, β-, and γ-HCH included in this chapter of the profile. These studies evaluate
the potential health effects associated with inhalation, oral, or dermal exposure to HCH, but may not be
inclusive of the entire body of literature. A systematic review of the scientific evidence of the health effects
associated with exposure to HCH was also conducted; the results of this review are presented in Appendix C.
Tabulated human studies of specific health endpoints are presented in the corresponding subsections of
this Chapter. Animal inhalation studies of γ-HCH are presented in Table 2-1 and Figure 2-5. There were
no inhalation studies of other HCH isomers or mixtures of isomers. Animal oral studies are presented in
Table 2-2 and Figure 2-6 (α-HCH), Table 2-3 and Figure 2-7 (β-HCH), Table 2-4 and Figure 2-8
(γ-HCH), and Table 2-5 and Figure 2-9 (δ-HCH and technical-grade HCH or unspecified isomers).
Animal dermal studies are presented in Table 2-6 (γ-HCH) and Table 2-7 (technical-grade or unspecified
isomers). There were no dermal studies of other HCH isomers.
HEXACHLOROCYCLOHEXANE (HCH) 20
2. HEALTH EFFECTS
Levels of significant exposure (LSEs) for each route and duration are presented in tables and illustrated in
figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-
observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies.
Effects have been classified into “less serious LOAELs” or “serious LOAELs (SLOAELs).” "Serious"
effects (SLOAELs) are those that evoke failure in a biological system and can lead to morbidity or
mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected
to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear.
ATSDR acknowledges that a considerable amount of judgment may be required in establishing whether
an endpoint should be classified as a NOAEL, "less serious" LOAEL, or "serious" LOAEL, and that in
some cases, there will be insufficient data to decide whether the effect is indicative of significant
dysfunction. However, the Agency has established guidelines and policies that are used to classify these
endpoints (ATSDR 2018). ATSDR believes that there is sufficient merit in this approach to warrant an
attempt at distinguishing between "less serious" and "serious" effects. The distinction between "less
serious" effects and "serious" effects is considered to be important because it helps the users of the
profiles to identify levels of exposure at which major health effects start to appear. LOAELs or NOAELs
should also help in determining whether or not the effects vary with dose and/or duration, and place into
perspective the possible significance of these effects to human health. Levels of oral exposure associated
with cancer (Cancer Effect Levels, CELs) of HCH are indicated in Tables 2-2 through 2-5 and
Figures 2-6 through 2-9.
A User's Guide has been provided at the end of this profile (see Appendix D). This guide should aid in
the interpretation of the tables and figures for LSEs and MRLs.
The discussion of the available data for health effects in this chapter is organized into human and animal
data, with isomer-specific subsections on the animal data provided in the following order: α-HCH,
β-HCH, γ-HCH, δ-HCH, and technical-grade and mixtures of HCH isomers. Case reports of effects in
humans are limited to γ-HCH and technical-grade HCH and are discussed under the isomer-specific
subsections. If there are no case reports or animal data for a given isomer or for technical grade/mixtures,
there is no corresponding subsection.
Effects of HCH isomers have been evaluated in epidemiological studies and in laboratory animals
exposed under controlled conditions. Most of the human epidemiological studies used measures of HCH
isomers in blood or tissues to assess exposure, so the route is unknown; for the purpose of enumerations,
these studies are considered to reflect oral exposure (e.g., through contaminated food). In addition, there
HEXACHLOROCYCLOHEXANE (HCH) 21
2. HEALTH EFFECTS
are several case reports of health effects in humans exposed by inhalation, oral, or dermal exposure to γ-
HCH. The human data were not considered adequate for identification of sensitive target organs for any
of the HCH isomers or mixtures.
As shown in Figure 2-1 (α-HCH), there were a small number of human studies examining a handful of
endpoints; the largest number of studies were devoted to developmental endpoints. There were no
inhalation or dermal animal studies of α-HCH, and few oral studies. The available animal studies
primarily examined liver effects and cancer. Animal studies suggest that hepatic effects are a sensitive
target of α-HCH toxicity.
• Hepatic endpoints: Hepatic toxicity is a presumed health effect for humans based on a high
evidence level in animals showing increased liver weight and histopathological lesions after oral
exposure to α-HCH. No information was located on hepatic effects in humans exposed to
α-HCH.
Figure 2-2 provides an overview of the health effects data for β-HCH. For this isomer, human studies
examined a wide range of outcomes, with more studies of endocrine endpoints (thyroid hormone levels)
developmental outcomes, other noncancer endpoints (diabetes and metabolic perturbations), and cancer
than other outcomes. Animal studies are limited to oral exposures, and the endpoints examined were
largely focused on liver, kidney, body weight, nervous system, and cancer. Animal studies suggest that
neurological and hepatic effects are sensitive targets of β-HCH toxicity after acute-duration exposures and
intermediate- or chronic-duration exposures, respectively.
• Neurological endpoints: Neurotoxicity is a presumed health effect in humans based on human
and animal studies. There is a moderate level of evidence in humans suggesting associations
between serum β-HCH and risk of Parkinson disease, Alzheimer’s disease, and cognitive deficits.
There is a high level of evidence in animal studies of oral exposure showing clinical signs of
neurotoxicity in rats and mice after acute durations and reduced nerve conduction velocity in rats
after an intermediate duration. Clinical signs showed a dose-related increase in severity.
Hepatic endpoints: Hepatic toxicity is a presumed health effect for humans based on a high
level of evidence in animals showing increased liver weight and histopathology changes in rats
and mice exposed by dietary administration for intermediate and chronic durations. In humans,
there is a very low level of evidence for a minimal liver toxicity based on two cross-sectional
studies reporting no association between serum or adipose levels of β-HCH and hepatic clinical
chemistry endpoints except for increased serum bilirubin.
An overview of health effects data for γ-HCH is presented in Figure 2-3. Most of the human studies
evaluated developmental, reproductive, renal, endocrine, or cancer endpoints. Studies of occupational
exposure via pesticide application are considered to reflect primarily inhalation exposure. Most of the
HEXACHLOROCYCLOHEXANE (HCH) 22
2. HEALTH EFFECTS
animal studies used oral administration, and the available studies examined comprehensive noncancer and
cancer endpoints. The effects seen at the lowest doses in the animal studies were developmental and
immune system effects. Animal studies suggest that developmental and immune system effects are
sensitive targets of γ-HCH toxicity after acute-duration exposures (developmental) and intermediate-
duration exposures (developmental and immune system). Available studies of chronic-duration oral
exposure to γ-HCH were limited and identified effects on other systems (hepatic and renal) at much
higher doses than those associated with developmental and immune system effects in acute- and
intermediate-duration exposure studies.
• Developmental endpoints: Developmental toxicity is a presumed health effect in humans based
on human and animal evidence. There is a low level of evidence in humans based on associations
between γ-HCH in maternal or fetal blood (or tissue) and fetal growth retardation, preterm birth,
and cryptorchidism or hypospadias. There is a high level of evidence in animals based on studies
in a variety of species exposed orally to γ-HCH for acute or intermediate durations during
gestation or postnatal development demonstrating adverse effects on a wide range of
developmental endpoints, including birth outcomes and development of the male and female
reproductive tracts, central nervous system, heart, thymus, and spleen.
• Immune system endpoints: Immunotoxicity is a presumed health effect in humans based
primarily on animal evidence. There is a low level of evidence in humans based on an observed
association between asthma and plasma levels of γ-HCH in children and no evidence for
increased prevalence of monoclonal gammopathy of undetermined significance in male pesticide
applicators. There is a high level of evidence in animals based on acute- and intermediate-
duration studies of γ-HCH administered orally to rats, mice, rabbits, and sheep showing
suppression of the immune system and effects on thymus, spleen, and lymph node weights or
histology.
Figure 2-4 shows the limited health effects data available for δ-HCH and unspecified HCHs. The human
studies primarily evaluated other noncancer, developmental, reproductive, and neurological endpoints.
The small number of animal studies used oral or dermal administration and were focused on hepatic and
cancer endpoints. Data were not adequate to identify sensitive targets of δ-HCH.

HEXACHLOROCYCLOHEXANE (HCH) 23
2. HEALTH EFFECTS
Figure 2-1. Overview of the Number of Studies Examining α-Hexachlorocyclohexane (α-HCH) Health Effects*
Most studies examined the potential body weight, hepatic, and cancer effects of α-HCH
Fewer studies evaluated health effects in humans than animals (counts represent studies examining endpoint)
5
3
1
3
3
2
4
4
8
1
2
1
16
3
1
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
Oral
100%
Exposure Route
Acute
12%
Intermediate
47%
Chronic
41%
Exposure Duration
*Includes studies discussed in Chapter 2. A total of 40 studies (including those finding no effect) have examined toxicity; most studies examined multiple
endpoints. Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of this figure.

HEXACHLOROCYCLOHEXANE (HCH) 24
2. HEALTH EFFECTS
Figure 2-2. Overview of the Number of Studies Examining β-Hexachlorocyclohexane (β-HCH) Health Effects*
Most studies examined the potential developmental, other noncancer, and cancer effects of β-HCH
More studies evaluated health effects in humans than animals (counts represent studies examining endpoint)
31
22
10
11
8
3
10
4
2
4
1
3
2
1
3
4
4
3
8
3
4
3
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
Oral
100%
Exposure Route
Acute
15%
Intermediate
23%
Chronic
63%
Exposure Duration
*Includes studies discussed in Chapter 2. A total of 41 studies (including those finding no effect) have examined toxicity; most studies examined multiple
endpoints. Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of this figure.

HEXACHLOROCYCLOHEXANE (HCH) 25
2. HEALTH EFFECTS
Figure 2-3. Overview of the Number of Studies Examining γ-Hexachlorocyclohexane (γ-HCH) Health Effects*
Most studies examined the potential body weight, hepatic, and neurological effects of γ-HCH
Fewer studies evaluated health effects in humans than animals (counts represent studies examining endpoint)
25
6
7
7
4
4
5
6
1
1
6
2
34
28
38
12
4
2
3
19
43
7
2
6
8
27
22
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
Dermal
5%
Oral
91%
Inhalation
4%
Exposure Route
Acute
27%
Intermediate
34%
Chronic
39%
Exposure Duration
*Includes studies discussed in Chapter 2. A total of 158 studies (including those finding no effect) have examined toxicity; most studies examined multiple
endpoints. Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of this figure.

HEXACHLOROCYCLOHEXANE (HCH) 26
2. HEALTH EFFECTS
Figure 2-4. Overview of the Number of Studies Examining δ-Hexachlorocyclohexane (δ-HCH) and Unspecified
Hexachlorocyclohexanes Health Effects*
Most studies examined the potential hepatic and cancer effects of δ-HCH and/or Unspecified HCHs
Fewer studies evaluated health effects in animals than humans (counts represent studies examining endpoint)
1
3
3
2
2
1
1
1
3
1
1
4
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
Oral
94%
Dermal
6%
Exposure Route
Intermediate
25%
Chronic
75%
Exposure Duration
*Includes studies discussed in Chapter 2. A total of 16 studies (including those finding no effect) have examined toxicity; most studies examined multiple
endpoints. Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of this figure.

HEXACHLOROCYCLOHEXANE (HCH) 27
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Inhalation
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/m
3
)
Parameters
monitored
Endpoint
NOAEL
(mg/m
3
)
Less serious
LOAEL
(mg/m
3
)
Serious
LOAEL
(mg/m
3
)
Effects
ACUTE EXPOSURE
Oldiges et al. 1980
1
Rat
(Wistar)
5 M, 5 F
4 hours
0, 273, 603
LE, CS, GN,
OW
Bd wt
603 F
Body weight loss in females
during first week of observation
Neuro
273
603
LOAEL: clinical signs of
restlessness, hyperactivity
Serious LOAEL: marked
somnolence
Ullmann 1986b
2
Rat
(Wistar)
5 M, 5 F
4 hours
0, 101, 378,
642, 2,104
LE, CS, BW,
GN
Death
378
20% of rats died (LC
50
:
1,560 mg/m
3
)
Neuro
101
Clinical signs (sedation, curved
body position)
Klonne and Kintigh 1988
3
Mouse
(CD-1)
45 M, 45 F
1 week
5 days/week
6 hours/day
0, 0.3, 1, 5,
10
LE, CS
Death
10
12/45 females and 2/45 males
died during first week of 13-
week
study
INTERMEDIATE EXPOSURE
Oldiges et al. 1983
4
Rat
(Wistar)
12 M, 12 F
90 days
7 days/week
6 hours/day
0, 0.02, 0.1,
0.5, 5
LE, CS, BW,
FI, WI, HE,
UR, OW, HP
Bd wt
5
Resp
5
Cardio
5
Gastro
0.5
5
Diarrhea
Hemato
0.5
5
Bone marrow myelogram
changes (increased
reticulocytes, stem cells and
myeloblasts; decreased
lymphocytes)
Hepatic
5
Renal
5 F
0.5 M
Dilated tubules with protein-
containing contents; proliferated
tubules
Endocr
5

HEXACHLOROCYCLOHEXANE (HCH) 28
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Inhalation
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/m
3
)
Parameters
monitored
Endpoint
NOAEL
(mg/m
3
)
Less serious
LOAEL
(mg/m
3
)
Serious
LOAEL
(mg/m
3
)
Effects
Neuro
5
Repro
5
Klonne and Kintigh 1988
5
Mouse
(CD-1)
45 M, 45 F
14 weeks
5 days/week
6 hours/day
0, 0.3, 1.0, 5
LE, CS, BW,
FI, WI, HE,
BC, UR,
GN, OW,
HP
Death
1
1/45 males and 1/45 females
died at 1 mg/m
3
; 5/45 males and
15/45 females died at 5 mg/m
3
Bd wt
5
Resp
5
Cardio
5
Gastro
5
Hemato
5
Hepatic
5
Renal
5
Endocr
5
Repro
5
No histopathology changes in
reproductive organs
a
The number corresponds to entries in Figure 2-5; differences in levels of health effects and cancer effects between male and females are not indicated in
Figure 2-5. Where such differences exist, only the levels of effect for the most sensitive sex are presented.
BC = serum (blood) chemistry; Bd wt or BW = body weight; Cardio = cardiovascular; CS = clinical signs; Endocr = endocrine; F = female(s); FI = food intake;
Gastro = gastrointestinal; GN = gross necropsy; HE = hematology; Hemato = hematological; LE = lethality; LOAEL = lowest-observed-adverse-effect level;
M = male(s); Neuro = neurological; NOAEL = no-observed-adverse-effect level; OW = organ weight; Repro = reproductive; Resp = respiratory; UR = urinalysis;
WI = water intake

HEXACHLOROCYCLOHEXANE (HCH) 29
2. HEALTH EFFECTS
Figure 2-5. Levels of Significant Exposure to γ-Hexachlorocyclohexane (Lindane) – Inhalation
Acute (≤14 days)

HEXACHLOROCYCLOHEXANE (HCH) 30
2. HEALTH EFFECTS
Figure 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane (Lindane) – Inhalation
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 31
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
ACUTE EXPOSURE
Sumida et al. 2007
1
Rat
(Fischer-
344)
4 M
1, 3, 7, or
14 d
(GO)
0, 2, 20
BW, BC, OW
Bd wt
20
Hepatic
2
20
24% increase in relative liver
weight
INTERMEDIATE EXPOSURE
Fitzhugh et al. 1950
2
Rat
(Wistar)
10 F, 10 M
6–9 months
(F)
Males: 0, 60
Females: 0,
70
LE, BW, FI,
GN, OW, HP
Death
60 M
70 F
Mean survival was
35.9 weeks versus
58.3 weeks in controls
Bd wt
60 M
70 F
11–15% decrease in body
weight gain
Resp
60 M
70 F
Cardio
60 M
70 F
Gastro
60 M
70 F
Hemato
60 M
70 F
Musc/skel
60 M
70 F
Hepatic
60 M
70 F
Moderate histopathology
changes (focal necrosis,
fatty degeneration); >2-fold
increase in liver weight
Renal
60 M
70 F
Slight to moderate
histopathology changes
including tubular dilatation,
hyaline tubular casts,
glomerular fibrosis or
atrophy, pigment deposition

HEXACHLOROCYCLOHEXANE (HCH) 32
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Endo
60 M
70 F
Repro
60 M
70 F
Ito et al. 1975
3
Rat
(W strain)
18–24 M
48 weeks
(F)
0, 35, 70
BW, OW, HP
Hepatic
35
Hepatocellular hypertrophy
Cancer
70
CEL: liver tumors after
48 weeks
Muller et al. 1981
4
Rat
(Wistar)
15 M
30 days
(F)
0, 5.1, 54.2,
106.2
NX
Neuro
106.2
No reduction in motor
conduction velocity
Nagasaki et al. 1975
5
Rat
(Wistar)
8 M
24 weeks
(F)
0, 45
BW, OW, HP
Bd wt
45
15% decrease in terminal
body weight
Hepatic
45
Mild hypertrophy; ~2-fold
increase in absolute and
relative liver weight
Sumida et al. 2007
6
Rat
(Fischer-
344)
4 M
28 days
(GO)
0, 2, 20
BW, BC,
OW, HP
Bd wt
20
Hepatic
2
b
20
Increased relative liver
weight (25%); centrilobular
hepatocellular hypertrophy
Hanada et al. 1973
7
Mouse
(dd)
10–11 M,
10–11 F
32 weeks
(F)
M: 0, 18, 54,
108 F: 0, 20,
60, 120
BC, GN, HP
Cancer
18 M
60 F
CEL: hepatoma

HEXACHLOROCYCLOHEXANE (HCH) 33
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Ito et al. 1973
8
Mouse
(dd)
20–40 M
24 weeks
(F)
0, 18, 45, 90
BW, OW,
GN, HP
Bd wt
90
Hepatic
18
Increased relative liver
weight (33%); hepatocellular
hypertrophy
Cancer
45
CEL: hepatocellular
carcinoma
Ito et al. 1976
9
Mouse
(DDY)
13–20 M
16–36 weeks
(F)
0, 90
BW, OW, HP
Cancer
90
CEL: hepatocellular
carcinoma
Nagasaki et al. 1975
10
Mouse
(DDY,
ICR,
DBA/2,
C57BL/6,
C3H/He)
20 M, 20 F
24 weeks
(F)
Males: 0, 90
Females: 0,
100
BW, OW, HP
Bd wt
90 M
17% decrease in terminal
body weight of male
C57BL/6 mice
Hepatic
90 M
100 F
Parenchymal cell
hypertrophy, bile duct
proliferation, oval cells;
nodular hyperplasia; 2-fold
increase in liver weight
Cancer
90 M
100 F
CEL: hepatocellular
carcinomas
Tryphonas and Iverson 1983
11
Mouse
(HPB)
75 M
50 weeks
(F)
0, 90
BW, GN,
OW, HP
Hepatic
90
Hepatomegaly;
megalocytosis
Cancer
90
CEL: neoplastic nodules of
the liver after 21 weeks
Tsukada et al. 1979
12
Mouse
(DD)
6 M
16–36 weeks
(F)
0, 90
GN HP
Cancer
90
CEL: hepatomas after
28 weeks

HEXACHLOROCYCLOHEXANE (HCH) 34
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Nagasaki et al. 1975
13
Hamster
(Golden
Syrian)
6–10 M
24 weeks
(F)
0, 45
BW, OW, HP
Bd wt
45
14% decrease in terminal
body weight
Hepatic
45
20–38% increase in liver
weight; liver cell hypertrophy
CHRONIC EXPOSURE
Fitzhugh et al. 1950
14
Rat
(Wistar)
10 F, 10 M
107 weeks
(F)
M: 0, 0.7, 4, 7
F: 0, 0.9, 4, 9
LE, BW, FI,
GN, OW, HP
Resp
9 F
7 M
Cardio
9 F
7 M
Gastro
9 F
7 M
Hemato
9 F
7 M
Musc/skel
9 F
7 M
Hepatic
0.7 M
0.9
c
F
4
32% increase in relative liver
weight and very slight to
slight microscopic damage
Renal
9 F
7 M
Endo
9 F
7 M
Repro
9 F
7 M

HEXACHLOROCYCLOHEXANE (HCH) 35
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Ito et al. 1975
15
Rat
(W strain)
18–24 M
72 weeks
(F)
0, 70, 105
BW, OW, HP
Cancer
70
CEL: hepatocellular
carcinoma
a
The number corresponds to entries in Figure 2-6; differences in levels of health effects and cancer effects between male and females are not indicated in
Figure 2-6. Where such differences exist, only the levels of effect for the most sensitive sex are presented.
b
Used to derive an intermediate-duration oral minimal risk level (MRL). The NOAEL of 2 mg/kg/day was divided by an uncertainty factor of 100 (10 for human
variability and 10 for animal to human extrapolation) and a modifying factor of 10 (for lack of data on developmental toxicity, immunotoxicity, and neurotoxicity),
resulting in an MRL of 0.002 mg/kg/day (2x10
-3
mg/kg/day).
c
Used to derive a chronic-duration oral MRL. The NOAEL of 0.9 mg/kg/day was divided by an uncertainty factor of 100 (10 for human variability and 10
for animal
to human extrapolation) and a modifying factor of 10 (for lack of data on immunotoxicity and neurotoxicity), resulting in an MRL of 0.0009 mg/kg/day
(9x10
-4
mg/kg/day).
BC = serum (blood) chemistry; Bd wt or BW = body weight; CEL = cancer effect level; (F) = feed; F = female(s); FI = food intake; GN = gross necropsy;
(GO) = gavage in oil; HP = histopathology; LE = lethality; LOAEL = lowest-observed-adverse-effect level; M = male(s); Musc/skel = muscular/skeletal;
Neuro = neurological; NOAEL = no-observed-adverse-effect level; NX = neurotoxicity; OW = organ weight; (W) = drinking water
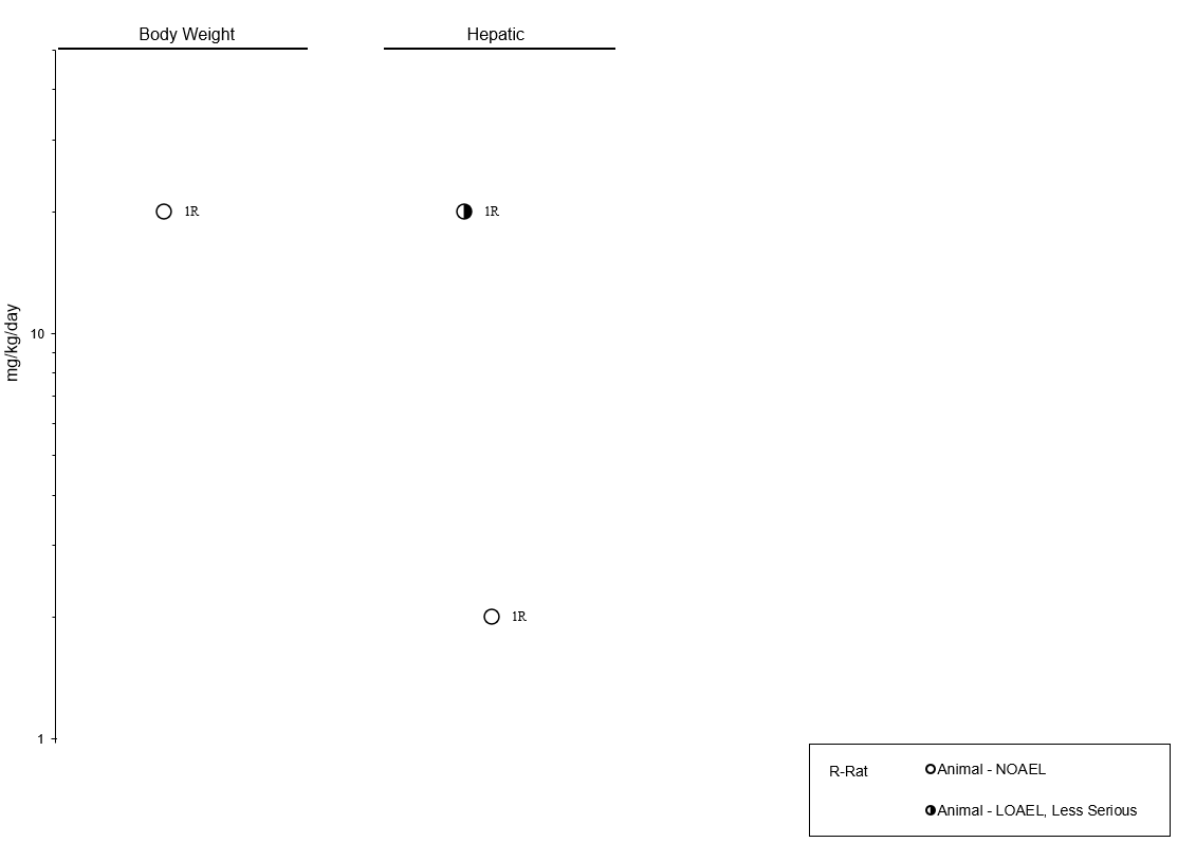
HEXACHLOROCYCLOHEXANE (HCH) 36
2. HEALTH EFFECTS
Figure 2-6. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Acute (≤14 days)

HEXACHLOROCYCLOHEXANE (HCH) 37
2. HEALTH EFFECTS
Figure 2-6. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 38
2. HEALTH EFFECTS
Figure 2-6. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)
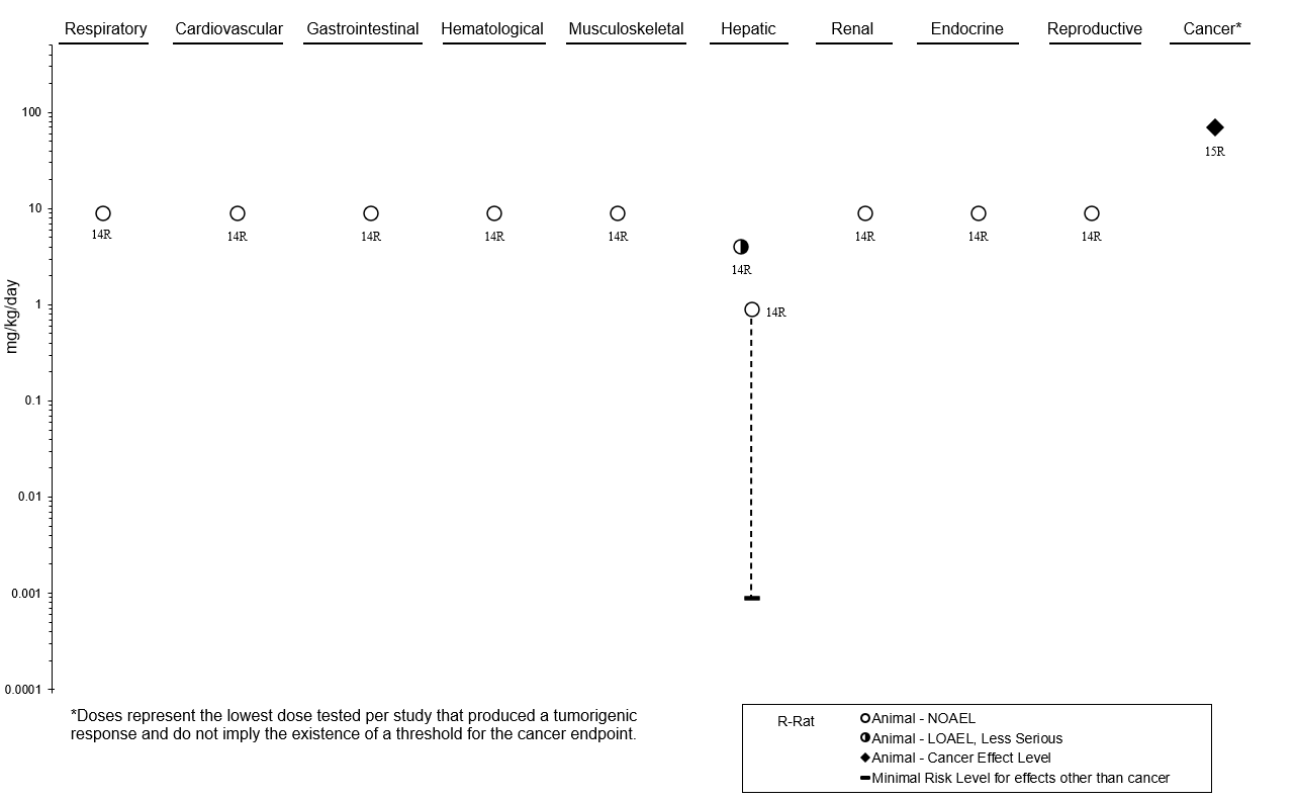
HEXACHLOROCYCLOHEXANE (HCH) 39
2. HEALTH EFFECTS
Figure 2-6. Levels of Significant Exposure to α-Hexachlorocyclohexane – Oral
Chronic (≥365 days)

HEXACHLOROCYCLOHEXANE (HCH) 40
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
ACUTE EXPOSURE
Srinivasan et al. 1984
1
Rat
(Wistar)
6 M
2 weeks
(F)
0, 72
BW, BC, UR,
HP
Renal
72
Tubular degeneration,
distention of glomeruli,
swelling of tubular epithelia,
22% increase in kidney
weight, glucosuria,
increased urinary excretion
of urea and creatinine,
decreased excretion of
protein
Van Velsen et al. 1986
2
Rat
(Wistar)
10 F, 10 M
2 weeks
(F)
0, 8, 38
CS
Neuro
8
b
38
Ataxia, hypoactivity
Cornacoff et al. 1988
3
Mouse
(B6C3F1)
6 F
1 weeks
(F)
0, 20, 60, 200
CS
Death
200
Lateral recumbency leading
to humane sacrifice in 80%
of mice
Neuro
20
60
Ataxia resolving within a few
days
INTERMEDIATE EXPOSURE
Fitzhugh et al. 1950
4
Rat
(Wistar)
10 F, 10 M
10 weeks
(F)
Males: 0, 60
Females: 0,
70
LE, BW, FI,
GN, OW, HP
Death
70 F
60 M
All animals died by
10 weeks of exposure;
mean age at death was
4.4 weeks
Resp
70 F
60 M
Cardio
70 F
60 M
Gastro
70 F
60 M

HEXACHLOROCYCLOHEXANE (HCH) 41
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Hemato
70 F
60 M
Musc/skel
70 F
60 M
Hepatic
70 F
60 M
Moderate to marked liver
damage including fatty
degeneration and focal
necrosis
Renal
70 F
60 M
Very slight nephritis; basal
vacuolation
Endo
70 F
60 M
Repro
70 F
60 M
Fitzhugh et al. 1950
5
Rat
(Wistar)
10 M, 10 F
6 months
(F)
M: 0, 7 F: 0, 9
BW
Bd wt
7 M
9 F
11% decrease in body
weight gain among females
Ito et al. 1975
6
Rat
(W strain)
18–24 M
48 weeks
(F)
0, 35, 70
BW, OW, HP
Hepatic
35
Hepatocellular hypertrophy
Muller et al. 1981
7
Rat
(Wistar)
15 M
30 days
(F)
0, 66.3, 270.6
NX
Neuro
66.3
Reduced tail nerve
conduction velocity
Srinivasan et al. 1991
8
Rat
(Wistar)
6 F
GDs 0–21
(F)
0, 5, 20, 40,
80
DX
Death
80
None of the dams survived
3 weeks of treatment
Develop
5
20
48% pup mortality before
PND 5

HEXACHLOROCYCLOHEXANE (HCH) 42
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Srinivasan et al. 1991
9
Rat
(Wistar)
6 F
GDs 0–21
and LDs 1–
28 or LDs 1–
28 only (F)
0, 5, 25
RX, DX
Develop
5
25
LOAEL: increased liver
weight in pups at 28 days of
age
Serious LOAEL: 100%
mortality before PND 5 in
pups exposed in utero
Van Velsen et al. 1986
10
Rat
(Wistar)
10 F, 10 M
13 weeks
(F)
Males: 0,
0.18, 0.9, 4.
5,
22.5
Females: 0,
0.2, 1.0, 5, 25
CS, BW FI,
HE, BC, BI,
OW, HP
Death
22.5 M
25 F
50% of animals were
moribund and sacrificed
humanely
Bd wt
5 F
4.5 M
25 F
22.5 M
≥10% decrease in body
weight
Hemato
5 F
4.5 M
25 F
22.5 M
Decreased red blood cells,
leukocytes, and hemoglobin
concentrations
Hepatic
ND M
1 F
0.18
c
M
5 F
Hyalinization of centrilobular
cells in males; increased
mitoses in females
Renal
4.5 M
22.5 M
Renal medullary calcinosis
Endo
5 F
4.5 M
25 F
22.5 M
Adrenal cortical hypertrophy
Immuno
5 F
4.5 M
25 F
22.5 M
Depletion of splenic
lymphoid tissue; thymic
cortical atrophy
Repro
5 F
4.5 M
25 F
22.5 M
Atrophy of testes and
ovaries

HEXACHLOROCYCLOHEXANE (HCH) 43
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Cornacoff et al. 1988
11
Mouse
(B6C3F1)
6 F
30 days
(F)
0, 20, 60
CS, BW, HE,
OW, HP, NX
Bd wt
60
Immuno
20
60
Decreased
lymphoproliferative
responses to T-cell
mitogens, decreased natural
killer cell activity
Repro
60
No changes in ovarian or
uterine histology
Hanada et al. 1973
12
Mouse
(dd)
10–11 M,
10–11 F
32 weeks
(F)
Males: 0, 20,
50, 100
Females: 0,
20, 60, 100
GN, HP
Hepatic
20
60 F
50 M
Nuclear irregularities in foci
of enlarged hepatocytes
Ito et al. 1973
13
Mouse
(dd)
20–40 M
24 weeks
(F)
0, 18, 45, 90
BW, OW, HP
Bd wt
90
Hepatic
18
18% increase in relative liver
weight with histopathology
changes (liver cell
hypertrophy) at higher doses
Thorpe and Walker 1973
14
Mouse
CF1
30 M, 30 F
3 months
(F)
0, 34
LE
Death
34
12% of males and 25% of
females died during the first
3 months of a chronic study

HEXACHLOROCYCLOHEXANE (HCH) 44
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
CHRONIC EXPOSURE
Fitzhugh et al. 1950
15
Rat
(Wistar)
10 F, 10 M
107 weeks
(F)
M: 0, 0.7, 7
F: 0, 0.9, 9
LE, BW, FI,
GN, OW, HP
Resp
7 M
9 F
Cardio
7 M
9 F
Gastro
7 M
9 F
Hemato
7 M
9 F
Musc/skel
7 M
9 F
Hepatic
0.7 M
0.9 F
34% increase in relative liver
weight; very slight
histopathology changes
Renal
7 M
9 F
Endo
7 M
9 F
Repro
0.7 M
9 F
7 M
Slight testicular atrophy

HEXACHLOROCYCLOHEXANE (HCH) 45
2. HEALTH EFFECTS
Table 2-3. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Thorpe and Walker 1973
16
Mouse
(CF1)
30 M, 30 F
104 weeks
(F)
0, 34
CS, GN, HP
Cancer
34
CEL: liver tumors in males;
unspecified tumors in
females.
a
The number corresponds to entries in Figure 2-7; differences in levels of health effects and cancer effects between male and females are not indicated in
Figure 2-7. Where such differences exist, only the levels of effect for the most sensitive sex are presented.
b
Used to derive an acute-duration oral minimal risk level (MRL). The NOAEL of 8 mg/kg/day was divided by an uncertainty factor of 100 (10 for human variability and
10 for animal to human extrapolation) resulting in an MRL of 0.08 mg/kg/day (8x10
-2
mg/kg/day).
c
Used to derive an intermediate-duration oral MRL. The LOAEL of 0.18 mg/kg/day was divided by an uncertainty factor of 300 (10 for human variability, 10 for animal
to human extrapolation, and 3 for use of a minimal LOAEL) resulting in an MRL of 0.0006 mg/kg/day (6x10
-4
mg/kg/day).
Bd wt or BW = body weight; CEL = cancer effect level; CS = clinical signs; Develop = developmental; DX = developmental toxicity; Endocr = endocrine; (F) = feed;
F = female(s); FI = food intake; GD = gestation day; GN = gross necropsy; Hemato = hematological; HP = histopathology; Immuno = immunological; LD = lactation
day; LOAEL = lowest-observed-adverse-effect level; M = male(s); Musc/skel = muscular/skeletal; ND = not determined; Neuro = neurological; NOAEL = no-observed-
adverse-effect level; NX = neurotoxicity; OW = organ weight; PND = postnatal day; Repro = reproductive; RX = reproductive toxicity; UR = urinalysis

HEXACHLOROCYCLOHEXANE (HCH) 46
2. HEALTH EFFECTS
Figure 2-7. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Acute (≤14 days)
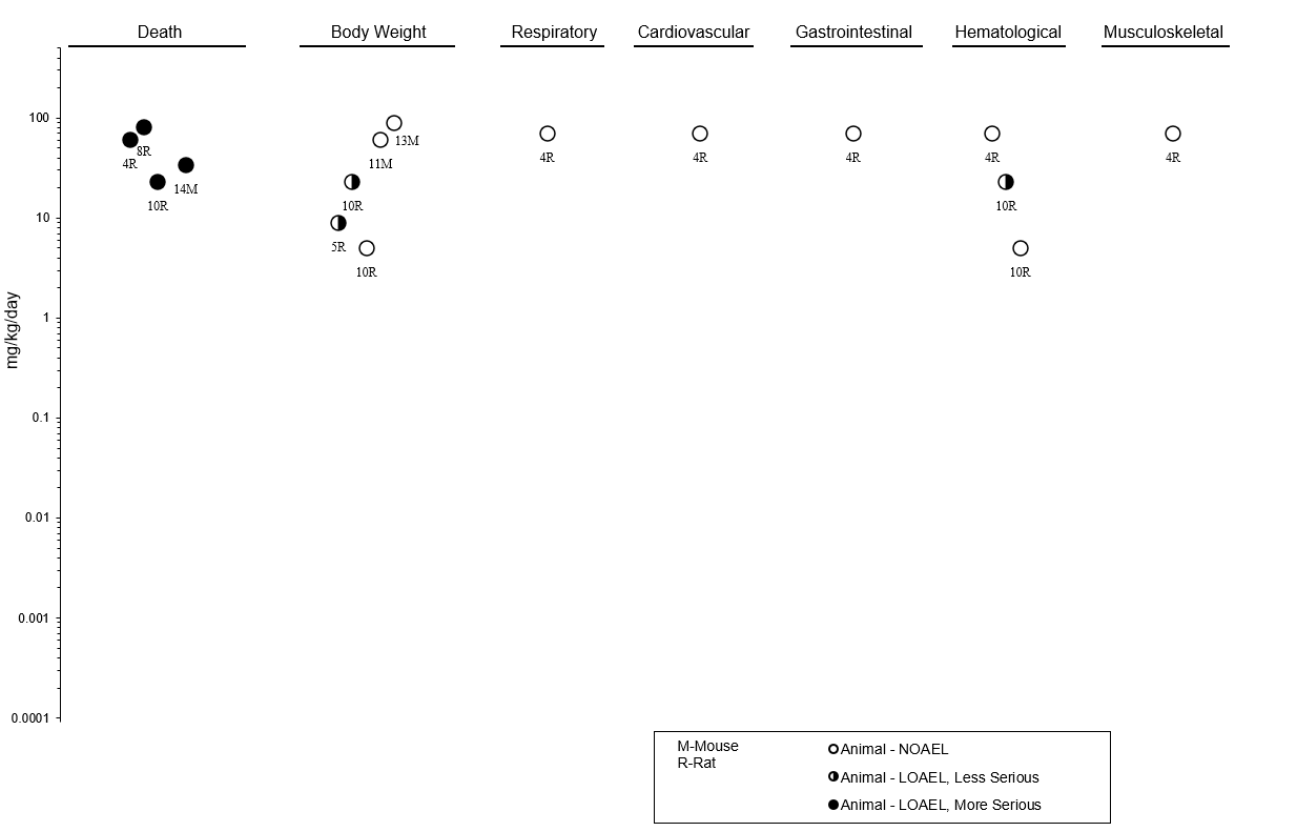
HEXACHLOROCYCLOHEXANE (HCH) 47
2. HEALTH EFFECTS
Figure 2-7. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 48
2. HEALTH EFFECTS
Figure 2-7. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 49
2. HEALTH EFFECTS
Figure 2-7. Levels of Significant Exposure to β-Hexachlorocyclohexane – Oral
Chronic (≥365 days)

HEXACHLOROCYCLOHEXANE (HCH) 50
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
ACUTE EXPOSURE
Ali and Shakoori 1998
1
Rat
(Sprague-
Dawley)
3–5, NS
2 days
(F)
0, 30
HP
Hepatic
30
Reduced number of cells
per field; increased cell,
nucleus, and nucleolus
size; slight cellular
disorganization
Attia et al. 1991
2
Rat
(Sprague-
Dawley)
9 M
6 days
(GO)
0, 3
BI
Neuro
3
Increased pineal N-acetyl-
transferase, decreased
serotonin levels
Dalsenter et al. 1996
3
Rat
(Wistar)
15 M
1–5 days
0, 6, 30
RX
Repro
6
Decreased number of
spermatids per epididymis
Dalsenter et al. 1997a
4
Rat
(Wistar)
15 M
GDs 15
once
(GO)
0, 30
DX
Develop
30
Reduced serum
testosterone in adult
offspring
Dalsenter et al. 1997b
5
Rat
(BOR)
9 F
LD 9 or 14
once
(GO)
0, 6
CS, BI, OW,
HP, NX, RX
Develop
6
In male pups, reduced
relative testicular and
epididymis weight (~10%),
spermatid and sperm
counts (~8–10%),
testosterone levels (~30–
50%), Leydig cell numbers,
and spermatogenesis at
maturity, with no effect on
fertility

HEXACHLOROCYCLOHEXANE (HCH) 51
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Dalsenter et al. 1997b
6
Rat
(BOR)
9 F
LDs 9–14
(GO)
0, 1
CS, BI, OW,
HP, NX, RX
Develop
1
b
In male pups, reduced
relative testicular and
epididymis weight (~10%),
spermatid and sperm
counts (~10%), and
testosterone levels (30–
50%) at maturity, with no
effect on fertility
EPA 1999a
7
Rat
(CD)
10 M, 10 F
Once
(G)
0, 6, 20, 60
Neuro
6 F
20 M
20 F
60 M
LOAEL: decreased motor
activity and grooming
behavior, increased
forelimb grip strength in
females
Serious LOAEL: tremors
and convulsions in one
male
Gaines 1960
8
Rat
(Sherman)
89 M, 69 F
Once
(GO)
NS
LE, CS
Death
91 F
LD
50
88 M
LD
50
Gilbert 1995
9
Rat
(Long-
Evans)
15–16 M
10 days
3 days/week
(GO)
0, 10
CS
Neuro
10
Myoclonic jerks and clonic
seizures
Gilbert and Mack 1995
10
Rat
(Long-
Evans)
14 M
once
(GO)
0, 5, 10, 20
CS
Neuro
5
Myoclonic jerks and single
clonic seizure in naive
animals

HEXACHLOROCYCLOHEXANE (HCH) 52
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Hfaiedh et al. 2012
11
Rat
(Wistar)
6 M
3 days
(GO)
0, 5
BC, BI, HP
Hepatic
5
Fatty degeneration,
vacuolation, and necrosis
of the liver
Johri et al. 2008
12
Rat
(Wistar)
10 M
Once
(GO)
0, 30
CS
Neuro
30
Convulsions in 5/10
animals
Joy et al. 1982
13
Rat
(Sprague-
Dawley)
7–14 M
4 days
(GO)
0, 1, 3, 10
CS, BW,
BC, OW,
HP, NX
Neuro
1
3
10
LOAEL: increased kindling
acquisition
Serious LOAEL: seizures
Khera et al. 1979
14
Rat
(Wistar)
20 F
GDs 6–15
(GO)
0, 6.25, 12.5,
25
DX
Develop
25
No teratogenic effects
Llorens et al. 1989
15
Rat
(Wistar)
9 M
Once
(GO)
0, 10, 15, 30
CS, NX
Neuro
10
Increased spontaneous
motor behavior
Llorens et al. 1990
16
Rat
(Wistar)
9 M
Once
(GO)
0, 20
CS, NX
Neuro
20
Increased anxiety
Martinez and Martinez-Conde 1995
17
Rat
(Wistar)
8 M, 8 F
Once
(GO)
0, 60
CS, NX
Neuro
60
Convulsions
Martinez et al. 1991
18
Rat
(Wistar)
7 M
Once
(GO)
0, 60
LE, CS, NX
Death
60
1/7 died
Neuro
60
Tonic-clonic seizures

HEXACHLOROCYCLOHEXANE (HCH) 53
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Mediratta et al. 2008
19
Rat
(Wistar)
8 M
14 days
(NS)
0, 10
IX
Immuno
10
Reduced delayed-type
hypersensitivity (43%
decrease in foot pad
thickness)
Palmer et al. 1978
20
Rat
(CFY)
20 F
GDs 6–16
(G)
0, 5, 10, 20
DX
Develop
20
Parmar et al. 2003
21
Rat
(Wistar)
10 M
Once
(GO)
0, 35
CS
Neuro
35
Convulsions in 4/10 rats
Parmar et al. 2003
22
Rat
(Wistar)
10 M
5 days
(GO)
0, 2.5, 5, 10,
15
CS, BW, BI,
OW
Bd wt
15
Rivera et al. 1991
23
Rat
(Wistar)
4 M, 4 F
Once
(GO)
0, 20
CS, BI, NX
Develop
20
Regional changes in brain
noradrenaline, serotonin,
and dopamine metabolite
levels in suckling rats
Rivera et al. 1998
24
Rat
(Wistar)
NS M, F
PND 15
once
(G)
0, 20
DX
Develop
20
Altered acquisition of a
passive avoidance task,
decreased motor activity,
altered neurotransmitter
levels in brain
Rivera et al. 1998
25
Rat
(Wistar)
NS M, F
PNDs 8–14
(G)
0, 10
DX
Develop
10
Altered acquisition of a
passive avoidance task,
increased motor activity,
altered neurotransmitter
levels in brain

HEXACHLOROCYCLOHEXANE (HCH) 54
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Serrano et al. 1990
26
Rat
(Wistar)
5 M, 5 F
PNDs 8–10
(GO)
0, 5, 10, 15,
20
BW, BI
Develop
5
Decreased myelin in
developing brain
Sharma and Singh 2010
27
Rat
(Wistar)
6 M
14 days
(GO)
0, 30
RX
Repro
30
Markedly decreased
epididymis (27%) and
testes (68%) weights;
substantial and persistent
reductions (≥85% less than
controls) in sperm head
count, motility, and percent
live sperm; marked and
persistent increases
(4-
fold) in percent abnormal
sperm
Singh and Sharma 2011
28
Rat
(Wistar)
NS M
1 day
(G)
0, 60
BI, HP
Hepatic
60
Marked centrilobular
necrosis
Sinha and Shukla 2003
29
Rat
(Druckrey)
8 M
3 days
(GO)
0, 8.8
BW, OW
Bd wt
8.8
Srinivasan et al. 1984
30
Rat
(Wistar)
6 M
2 weeks
(F)
0, 72
BW, BC,
UR, OW, HP
Renal
72
10% increase in kidney
weight, distention of
glomeruli, swelling of
tubular epithelia,
glucosuria, increased
excretion of urea and
creatinine, decreased
excretion of protein

HEXACHLOROCYCLOHEXANE (HCH) 55
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Sumida et al. 2007
31
Rat
(Fischer-
344)
4 M
1, 3, 7, or
14 days
(GO)
0, 1, 10
BW, BC,
OW
Bd wt
10
Hepatic
10
Tusell et al. 1988
32
Rat
(Wistar)
4 M
3–12 days
0, 5, 12, 20
LE, CS
Death
20
2/18 died on 3
rd
day
Neuro
5
12
Convulsions after 3 days
Uphouse and Williams 1989
33
Rat
(CDF-
F344)
8–11 F
Once
(G)
0, 12.5, 25,
33, 50
RX
Repro
25
Increased length of estrous
cycle
Woolley and Griffith 1989
34
Rat
(Sprague-
Dawley)
7 M
Once
(GO)
0, 30, 40, 50
CS, NX
Neuro
30
Seizures
Di Consiglio et al. 2009
35
Mouse
(CD-1)
2–10 F
GDs 9–16
(GO)
0, 25
LE, CS, BW,
BI, OW, HP,
DX
Bd wt
25
Develop
25
Decreased sperm
concentration (20%) and
count (27%) in F1 males at
PNDs 65–69

HEXACHLOROCYCLOHEXANE (HCH) 56
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Hassoun and Stohs 1996a
36
Mouse
(C57BL/6J
and
DBA/2J)
NS F
GD 12
once
(GO)
0, 30, 45
LE, DX
Death
30
14% of dams died at
30 mg/kg and 25% died at
45 mg/kg
Develop
30
45
LOAEL: decreased fetal,
placental, and thymic
weights in C57BL/6J mice
Serious LOAEL: increased
early resorptions in
C57BL/6J mice
Hong and Boorman 1993
37
Mouse
(B6C3F1)
7 M
3 days
(GO)
0, 10, 20, 40
CS, BW,
HE, OW HP
Bd wt
40
Hemato
20
Transient reductions in
marrow progenitor cell
numbers
Immuno
10
20
40
LOAEL: decreased thymus
weights
Serious LOAEL: atrophy of
thymic cortex
Hong and Boorman 1993
38
Mouse
(B6C3F1)
7 M
10 days
(GO)
0, 10, 20
CS, BW,
HE, OW HP
Bd wt
20
Hemato
10
Transient decrease in
marrow progenitor cell
numbers
Immuno
10
Decreased relative thymus
and spleen weights
La Sala et al. 2009
39
Mouse
(CD-1)
NS F
3 days
(GO)
0, 15, 30
DX
Develop
15
Decreased numbers of
primordial germ cells in
male and female offspring
Liu and Morgan 1986
40
Mouse
(DBA/2)
6 F
10 days
20
LE, CS, BC
Death
20
6/6 died

HEXACHLOROCYCLOHEXANE (HCH) 57
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Maranghi et al. 2007
41
Mouse
(CD)
12 F
GDs 9–16
(GO)
0, 15
BW, FI, BI,
OW, HP, DX
Bd wt
15
Repro
15
Develop
15
Early vaginal patency;
increased absolute (17%)
and relative (13%) uterine
weight at PND 22;
decreased oocyte diameter
(21%) in primary follicles at
PND 60 in F1 females
Scascitelli and Pacchierotti 2003
42
Mouse
(CD-1)
16–29 F
3 days
(GO)
0, 15, 25
RX
Repro
15
25
Increase in degenerating
two-cell embryos following
preovulatory exposure
Sinha and Shukla 2003
43
Mouse
Swiss
8 M
3 days
(GO)
0, 5.9
BW, OW
Bd wt
5.9
Traina et al. 2003
44
Mouse
(CD-1)
10–24 F
GDs 9–16
(GO)
0, 15, 25
BW, DX
Bd wt
25
Develop
15
14% decrease in sperm
count in F1 offspring with
more severe effects
observed at higher dose
Palmer et al. 1978
45
Rabbit
(New
Zealand)
13 F
GDs 6–18
(G)
0, 5, 10, 20
DX
Develop
20

HEXACHLOROCYCLOHEXANE (HCH) 58
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
INTERMEDIATE EXPOSURE
Ahmed et al. 2008
46
Rat
(Wistar)
10 M
4 weeks,
7 days/week
(GO)
0, 30
CS, BW, FI,
BC
Bd wt
30
Ali and Shakoori 1998
47
Rat
(Sprague-
Dawley)
3–5 NS
15 days
(F)
0, 18
HP
Hepatic
18
Reduced number of cells
per field; increased cell,
nucleus, and nucleolus
size; vacuoles in the
cytoplasm and granulation;
apparent fatty degeneration
Amyes 1990
48
Rat
(Wistar)
15 M, 15 F
Up to
52 weeks
(F)
Males: 0,
0.07, 0.7, 7,
28
Females: 0,
0.08, 0.8, 8,
32
LE, CS, BW,
FI, WI, HE,
BC, BI, UR,
GN, OW,
HP, NX
Death
32 F
Statistically significant
decreased survival
Bd wt
32F
28 M
Hemato
32F
28 M
Hepatic
0.8 F
0.7 M
8 F
7 M
Periacinar hepatocytic
hypertrophy
Renal
32 F
0.07 M
Hyaline droplets, interstitial
chronic nephritis, and
regeneration in proximal
tubules
Neuro
32 F
Convulsions in
11/55 females

HEXACHLOROCYCLOHEXANE (HCH) 59
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Anand et al. 1995
49
Rat
(albino)
NS M
6 weeks
(G)
0, 3
BW, BC, OF
Cardio
3
Tachycardia (~30%
increase in heart rate);
increased blood pressure,
plasma calcium levels, and
myocardial calcium influx;
decreased Ca, K-ATPase
activity; ECG changes
Andrews and Gray 1990
50
Rat
(Long-
Evans)
NS M
10 weeks
7 days/week
(G)
0, 10, 20
BW, OW,
BC
Bd wt
20
Renal
10
Increased kidney weight;
hyaline droplet
accumulation and tubular
regeneration
Musc/skel
10
20
Decreased femur medullary
area
Arisi et al. 1994
51
Rat
(Wistar)
4–12 M
90 days
(F)
0, 90
CS
Neuro
90
Tonic convulsions
Chadwick et al. 1988
52
Rat
(Fischer-
344)
6–12 F
15 weeks
(GO)
0, 5, 10, 20,
40
LE, BW, FI,
BI, OW
Death
20
2/12 died at 20 mg/kg/day;
7/12 died at 40 mg/kg/day
Repro
5
10
Delayed vaginal opening,
disrupted ovarian cycling
Desi 1974
53
Rat
(Wistar)
8–10 NS
40 days
(F)
0, 2.5, 5, 10,
50
NX, OW,
OF, HP
Hepatic
50
Neuro
2.5
Significantly altered
Skinner box behavior

HEXACHLOROCYCLOHEXANE (HCH) 60
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Dewan et al. 1980
54
Rat
(Charles
Foster)
5 M, 5 F
35 days
0, 6.25, 25
IX
Immuno
6.25
Immunosuppression
(decreased antibody titers)
EPA 1991a
55
Rat
(CD)
30 M, 30 F
2 generation
s; 70 days
prior to
mating until
sacrifice
0, 0.09, 1.7,
13.1
CS, BW, FI,
HP, RX, DX
Bd Wt
1.7 F
13.1 M
13.1 F
Decreased body weight
gain during gestation in F0
females
Hepatic
0.09 M
13.1 F
1.7 M
In F1 males: hepatocellular
hypertrophy
Renal
0.09 M
13.1 F
1.7 M
In F0 and F1 males:
Increased kidney weights;
nephritis, tubular cell
regeneration, hyaline
droplets, tubular necrosis
Repro
13.1
Develop
13.1
Reduced F1 and F2 pup
weight and viability;
delayed tooth eruption and
hair growth in F2
EPA 1999b
56
Rat
(CD)
10 M, 10 F
13 weeks
(F)
Males: 0,
1.4, 7.1, 28.1
Females: 0,
1.6, 7.9, 30.2
NX
Death
30.2 F
3/10 females died during
the study
Neuro
7.1 M
7.9 F
28.1 M
30.2 F
Increased rearing, walking
on tiptoes, hypersensitivity
to touch, and hunched
posture

HEXACHLOROCYCLOHEXANE (HCH) 61
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
EPA 1999c
57
Rat
(Wistar)
10 M, 10 F
GD 6 to
LD 10
(F)
Gestation:
0.8–0.9, 4.2–
4.6, 8.0–
10.5,
Lactation:1.2
–1.7, 5.6–
8.3, 13.7–
19.1
DX
Develop
1.2
5.6
Up to 18% reduction in pup
body weight and 24% lower
body weight gain during
lactation
Fatih Fidan et al. 2008
58
Rat
(Sprague-
Dawley)
10 M
30 days
(GO)
0, 10, 20, 40
BC, BI, HP
Hepatic
10
20
Megalocytosis; vacuolar
degeneration; severe
venous and sinusoidal
congestion; and
lymphocytic infiltration
Renal
10
20
Severe kidney congestion,
medullary and cortical
hemorrhage, and
degeneration and
vacuolation of proximal
convoluted tubules
Fitzhugh et al. 1950
59
Rat
(Wistar)
10 F, 10 M
10 months
(F)
Males: 0, 30,
60, 120
Females: 0,
30, 70, 140
LE, BW
Death
60 M
70 F
Mean age at death was
39.7 weeks versus
58.3 weeks in controls
Bd wt
60 M
70 F
120 M
140 F
13–17% decrease in body
weight gain at 6 months
Resp
120 M
140 F
Cardio
120 M
140 F
Gastro
120 M
140 F

HEXACHLOROCYCLOHEXANE (HCH) 62
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Hemato
120 M
140 F
Musc/skel
120 M
140 F
Hepatic
60 M
70 F
Slight microscopic damage
Renal
60 M
70 F
Very slight microscopic
damage
Neuro
60 M
70 F
Convulsions
Endo
120 M
140 F
Repro
120 M
140 F
Gilbert 1995
60
Rat
(Long-
Evans)
15–16 M
30 days
(GO)
0, 10
CS
Neuro
10
Myoclonic jerks and clonic
seizures
Hfaiedh et al. 2011
61
Rat
(Wistar)
6 M
30 days
(W)
0, 50
BC, BI, OW,
HP, RX
Endocr
50
85% increase in T4, 74%
decrease in TSH
Repro
50
Decreased testes (52%),
epididymides (42%),
prostate gland (50%), and
seminal vesicles (5%)
weights; 56% reduced
sperm count; 37% reduced
sperm motility; 74%
decrease in FSH
Ito et al. 1975
62
Rat
(W strain)
18–24 M
48 weeks
(F)
0, 35
BW, OW,
HP
Hepatic
35
Hepatocellular hypertrophy

HEXACHLOROCYCLOHEXANE (HCH) 63
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Johri et al. 2007
63
Rat
(Wistar)
13–14 F
GDs 5–21
(GO)
0, 0.0625,
0.125, 0.25
DX, BI
Develop
0.125
0.25
Persistent hyperactivity
Johri et al. 2008
64
Rat
(Wistar)
13 F
GDs 5–21
and PND 45
(GO)
0, 0.25
(prenatal)
and 30
(postnatal)
CS, BI
Develop
30
Convulsions in
8/10 animals
Koner et al. 1998
65
Rat
(Wistar)
8–10 M
8 weeks
(F)
0, 3.6, 7.0
IX
Immuno
3.6
Reduced serum antibody
response to SRBC
Martinez and Martinez-Conde 1995
66
Rat
(Wistar)
8 M, 8 F
30 days,
every 3 days
(GO)
0, 6
CS, NX
Neuro
6
Decreased brain dopamine
levels
Matsuura et al. 2005
67
Rat
(Crj:CD
[SD] IGS)
24 M, 24 F
~10 weeks
(2-generation
; premating
to PND 21)
(F)
Males, F0: 0,
0.56, 3.4,
17.2
Males, F1:
0.74, 4.5,
23.3
Females, F0:
0, 0.88, 5.2,
26.1
Females, F1:
0.95, 5.6,
28.0
LE, CS, BW,
FI, BC, BI,
GN, OW,
HP, RX, DX,
NX
Death
26.1 F
2 F0 females died
Bd wt
26.1 F
17.2 M
Hepatic
0.88 F
0.56 M
5.2 F
3.4 M
Hepatocellular hypertrophy
in F0 and F1 male and
female parents
Renal
26.1 F
0.56 M
Basophilic tubules and
hyaline droplets in the
proximal tubules
Endocr
5.2 F
3.4 M
26.1 F
17.2 M
Decreased absolute and
relative pituitary weights in
F0 and F1 females; altered
serum thyroid hormone
levels; thyroid follicular cell
hypertrophy in F0 females
and F1 males

HEXACHLOROCYCLOHEXANE (HCH) 64
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Neuro
28 F
Convulsions in two F1
females
Repro
26.1 F
17.2 M
Develop
0.95
5.6
26.1
LOAEL: 10% decrease in
F2 female offspring body
weight at PND 4
Serious LOAEL: 49%
decrease in F2 offspring
viability (PNDs 0–4); 8–
29% decreases in male
and female F1 and F2
offspring weights on
PNDs
0, 4, and 21; delayed
sexual maturation
(preputial separation in
males and vaginal opening
in females) in F1
generation
Mediratta et al. 2008
68
Rat
(Wistar)
8 M
21 days
(NS)
0, 10
BC, IX
Immuno
10
Decreased anti-SRBC
antibody titer (32%)
Mudawal et al. 2018
69
Rat
(Wistar)
6 M
3 weeks
7 days/week
(NS)
0, 2.5
BI, NX, DX
Neuro
2.5
Decreased conditioned
avoidance, alternations,
and locomotor activity;
ultrastructural changes in
the hippocampus and
substantia nigra in adult
animals
Muller et al. 1981
70
Rat
(Wistar)
15 M
30 days
(F)
0, 1.3, 12.3,
25.4
NX
Neuro
12.3
25.4
Reduced tail nerve
conduction velocity

HEXACHLOROCYCLOHEXANE (HCH) 65
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Parmar et al. 2003
71
Rat
(Wistar)
10 M
15 or 21
days
(GO)
0, 2.5
CS, BW, BI,
OW
Bd wt
2.5
Prasad et al. 2016
72
Rat
(Wistar)
6 M
15, 30, or
45 days
(GO)
0, 20
BC, BI, GN,
HP
Renal
20
Severe corticomedullary
and glomerular congestion,
intertubular hemorrhage,
severe tubular
degeneration,
desquamation of tubular
epithelium, cystic dilatation,
mononuclear cell infiltrate,
necrosis, and atrophic
glomeruli
Sahaya et al. 2007
73
Rat
(Wistar)
10 M
6 weeks,
7 days/week
(NS)
0, 15
BI, NX
Neuro
15
Impaired neurocognition
(decreased step-down
latency in passive
avoidance test and
prolonged transfer latency
in elevated plus maze)
Saradha and Mathur 2006
74
Rat
(Wistar)
4 M
45 days
(GO)
0, 1, 5, 50
BW, OW,
RX
Repro
1
5
50
LOAEL: decreased sperm
count (~27%) and motility
(~25%)
Serious LOAEL: d
ecreased
sperm count (~40%),
motility (~40%), and
viability (~15%); decreased
epididymal weight (~10%)

HEXACHLOROCYCLOHEXANE (HCH) 66
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Sauviat et al. 2005
75
Rat
(Sprague-
Dawley)
NS F
~13 weeks
(W)
0, 0.000076,
0.00015,
0.00030
GN, OW,
HP
Develop
0.000076
c
0.00015
0.0003
LOAEL: altered ventricular
electrophysiology
Serious LOAEL: 21%
decrease in pup body
weight; altered heart
morphometry and
electrophysiology; cardiac
histopathology
(hypertrophy in left
ventricular area,
unorganized collagen
bundles and layers,
fibroblast destruction)
Sharma and Singh 2010
76
Rat
(Wistar)
6 M
28 days
(GO)
0, 30
RX
Repro
30
Decreased cauda
epididymis (32%) and
testes (70%) weights;
≥89% decreases in sperm
head count, motility, and
percent live sperm; 4-fold
increase in percent
abnormal sperm
Srinivasan et al. 1991
77
Rat
(Wistar)
6 F
GDs 0–21
and
LDs 1–28 or
LDs 1–28
(F)
0, 25
DX
Develop
25
Increased pup relative liver
weights
Srivastava et al. 2019
78
Rat
(Wistar)
25 F
GDs 5–21
(GO)
0, 0.25
DX
Develop
0.25
Ultrastructural changes in
the brain (moderately
distorted mitochondria and
demyelinated neurons)

HEXACHLOROCYCLOHEXANE (HCH) 67
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Srivastava et al. 2019
79
Rat
(Wistar)
6 M
21 days
(GO)
0, 2.5
NX
Neuro
2.5
Reduced locomotor activity
and spatial memory;
ultrastructural changes in
the hippocampus and
substantia nigra (swollen
mitochondria with
disintegrated cristae,
shortened fuzzy synapse,
disintegrated myelin layer,
and autophagosomes)
Sumida et al. 2007
80
Rat
(Fischer-
344)
4 M
28 days
(GO)
0, 1, 10
BW, BC,
OW, HP
Bd wt
10
Hepatic
10
Suter 1983
81
Rat
(Wistar)
15 M, 15 F
12 weeks
(F)
0, 0.02, 0.08,
0.4, 2.0, 10
LE, CS, BW,
FI, HE, UR,
OW, HP
Hemato
10
Hepatic
0.4
2
Centrilobular hypertrophy
Renal
0.4 F
0.4 M
2 F
Basophilic proximal
tubules; proximal tubular
distention, hyaline droplet
formation; and minimal to
moderate interstitial
nephritis in males; slight
epithelial cell necrosis in
proximal convoluted
tubules in both sexes

HEXACHLOROCYCLOHEXANE (HCH) 68
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Vijaya Padma et al. 2011
82
Rat
(Wistar)
6 F
30 days
(GO)
0, 100
BC, BI, HP
Hepatic
100
“Extensive” liver injuries
consisting of vacuolar
degeneration of
hepatocytes and “massive”
degradation of the central
vein
Renal
100
Glomerular degeneration
and shrinkage;
degeneration of proximal
and distal tubules
Vijaya Padma et al. 2013
83
Rat
(Wistar)
6 M
30 days
(GO)
0, 100
BC, BI, OW,
HP
Cardio
100
Histopathology changes
(inflammatory cells and
separated muscle fibers)
Yang et al. 2014; Zhang et al. 2016
84
Rat
(Sprague-
Dawley)
7–9 F
4 weeks,
7 days/week
(GO)
0, 4, 8
BW, BC,
OW, HP
Bd wt
8
Repro
4
8
Low columnar endometrial
glandular epithelial cells in
the uterus
Yuksel et al. 2009
85
Rat
(Sprague-
Dawley)
10 M
30 days
(GO)
0, 10, 20, 40
CS, BC, GN,
OW, HP, RX
Death
20
1/10 rats died at
20 mg/kg/day and
1/10 died at 40 mg/kg/day
Banerjee et al. 1996
86
Mouse
(Hissar)
NS M
3–12 weeks
0, 1.8, 5.4, 9
IX
Immuno
1.8
5.4
Immunosuppression
(decreased splenic plaque-
forming colonies) after
6 weeks; decreased
antibody titers at
9 mg/kg/day

HEXACHLOROCYCLOHEXANE (HCH) 69
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Hanada et al. 1973
87
Mouse
(dd)
10–11 M,
10–11 F
32 weeks
Males: 0, 18,
54, 108
Females: 0,
20, 60, 120
BC, GN, HP
Cancer
120
CEL: hepatoma
Ito et al. 1973
88
Mouse
(dd)
20–40 M
24 weeks
(F)
0, 18, 45, 90
BW, OW,
HP
Bd wt
90
Hepatic
45
90
Relative liver weight
increase of 33% and
centrilobular hypertrophy
Meera et al. 1992
89
Mouse
(Swiss
albino)
6 F
24 weeks
(F)
0, 0.012,
0.12, 1.2
CS, HP, IX
Immuno
0.012
1.2
LOAEL: changes in cell-
and humoral-mediated
immune system
Serious LOAEL:
histopathology changes in
thymus consisting of
marked decrease in cortical
lymphocytes, many
necrosed cells in medulla,
congestion of blood
vessels, and severe loss of
cortex and medulla
distinction
Nagda and Bhatt 2011
90
Mouse
(Swiss)
8–10 M
60 days
(GO)
0, 40
BI, OW, HP
Repro
40
10% decrease testes
weight; histopathology
changes in testes
(shrunken and distorted
seminiferous tubules,
sparse Leydig cells, and
oligospermia)

HEXACHLOROCYCLOHEXANE (HCH) 70
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Thorpe and Walker 1973
91
Mouse
(CF1)
30 M, 30 F
3 months
(F)
0, 68
LE
Death
68
10% of males and 20% of
females died in first
3 months of chronic study
Rivett et al. 1978
92
Dog
(Beagle)
1 M, 1 F
7 weeks
0.9, 2, 4, 7
CS, BW,
HE, BC, UR,
GN, OW,
HP
Bd wt
4
7
Decreased body weight
gain
Desi et al. 1978
93
Rabbit
(NS)
6 M
5–6 weeks
5 days/week
(C)
0, 1.5, 3, 6,
12
LE, OF, IX
Death
12
“Numerous” deaths
Lindenau et al. 1994
94
Rabbit
(hybrid)
5 F
12 weeks
3 days/week
(GO)
0, 0.8
CS, BC, BI
Repro
0.8
Reduced ovulation rate
Seiler et al. 1994
95
Rabbit
(New
Zealand)
5 F
12–15 weeks
3 days/week
(GO)
0, 0.8
DX, RX
Repro
0.8
Develop
0.8
Beard and Rawlings 1998
96
Mink
(NS)
8–10 F
3
generations
(F)
0, 1
CS, BW, FI,
GN, OW,
HP, RX, DX
Repro
1
Reduced litter size in F2
females (~40% lower than
controls); reduced testis
size (11–13% shorter
length and 34–36% lower
mass) in F3 males

HEXACHLOROCYCLOHEXANE (HCH) 71
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Beard et al. 1997
97
Mink
(NS)
10 F
12 weeks
3 weeks
premating to
8 weeks
postpartum
(F)
0, 1
CS, BW, FI,
BC, GN,
OW, HP,
RX, DX
Repro
1
Reduced mating receptivity
and whelping rate
Beard et al. 1997
98
Mink
(NS)
15 F
17 weeks
6 weeks
premating to
10 weeks
postpartum
(F)
0, 1
CS, BW, FI,
BC, GN,
OW, HP,
RX, DX
Repro
1
Reduced whelping rate and
increased post-
implantation
embryo loss
CHRONIC EXPOSURE
Ali and Shakoori 1998
99
Rat
(Sprague-
Dawley)
3–5, NS
18 months
(F)
0, 9
HP
Hepatic
9
Increased cell, nucleus,
and nucleolus size;
extensive cytoplasmolysis;
slight cytoplasmic
degeneration; increasing
nuclear distortion
Fitzhugh et al. 1950
100
Rat
(Wistar)
10 F, 10 M
107 weeks
(F)
Males: 0,
0.4, 0.7, 4, 7,
30
Females: 0,
0.4, 0.9, 4, 9,
30
LE, BW, FI,
GN, OW,
HP
Resp
30
Cardio
30
Gastro
30
Hemato
30
Musc/skel
30
Hepatic
4
9 F
7 M
Increased relative liver
weight (35%); very slight
microscopic liver damage
Renal
4
9 F
7 M
Very slight microscopic
kidney damage

HEXACHLOROCYCLOHEXANE (HCH) 72
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Endo
30
Repro
30
EPA 2000a
101
Mouse
(CD-1)
50 M, 50 F
78 weeks
(F)
Males: 0,
1.3, 5.2, 20.5
Females: 0,
1.8, 7.1, 26.8
LE, BW, FI,
GN, OW,
HP
Bd wt
20.5 M
26.8 F
Resp
20.5 M
26.8 F
Hemato
20.5 M
Hepatic
5.2 M
26.8 F
20.5 M
Centrilobular hepatocyte
hypertrophy
Renal
20.5 M
26.8 F
Endocr
20.5 M
26.8 F
Neuro
20.5 M
26.8 F
Cancer
26.8 F
CEL: bronchiolar-alveolar
adenomas and carcinomas
Herbst et al. 1975; Weisse and Herbst 1977
102
Mouse
(NMRI)
50 M, 50 F
80 weeks
(F)
Males: 0,
2.1, 4.1, 8.2
Females: 0,
2.0, 3.9, 7.8
BW, GN, HP
Bd wt
8.2 M
7.8 F
Hepatic
8.2 M
7.8 F
NCI 1977
103
Mouse
(B6C3F1)
50 M, 50 F
80 weeks
(F)
0, 13.6, 27.2
CS, BW, BI,
HP
Cancer
13.6 M
CEL: hepatocellular
carcinoma
Thorpe and Walker 1973
104
Mouse
(CF1)
30 M, 30 F
104 weeks
(F)
0, 68
CS, GN, HP
Cancer
68
CEL: liver and other
unspecified tumors in both
sexes

HEXACHLOROCYCLOHEXANE (HCH) 73
2. HEALTH EFFECTS
Table 2-4. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Wolff et al. 1987
105
Mouse
(F-1
hybrid)
24–96 F
24 months
(F)
0, 27.2
CS, BW, BI,
HP
Cancer
27.2
CEL: hepatocellular
carcinoma, lung tumors
Rivett et al. 1978
106
Dog
(Beagle)
4 M, 4 F
104 weeks
(F)
0, 0.83, 1.60,
2.92
CS, BW,
HE, BC, UR,
GN, OW,
HP, NX
Bd wt
2.92
Hepatic
2.92
Livers were dark but
without histopathology
changes
Hemato
2.92
Ocular
2.92
a
The number corresponds to entries in Figure 2-8; differences in levels of health effects and cancer effects between male and females are not indicated in
Figure 2-8. Where such differences exist, only the levels of effect for the most sensitive sex are presented.
b
Used to derive an acute-duration oral minimal risk level (MRL). The LOAEL of 1 mg/kg/day was divided by an uncertainty factor of 300 (3 for use of a minimal
LOAEL, 10 for human variability, and 10 for animal to human extrapolation), resulting in an MRL of 0.003 mg/kg/day (3x10
-3
mg/kg/day).
c
Used to derive an intermediate-duration oral MRL. The NOAEL of 0.000076 mg/kg/day was divided by an uncertainty factor of 100 (10 for human variability, an
d
10 for animal to human extrapolation), resulting in an MRL of 0.0000008 mg/kg/day (8x10
-7
mg/kg/day).
BC = serum (blood) chemistry; Bd wt or BW = body weight; BI = biochemical changes; (C) = capsule; Cardio = cardiovascular; CEL = cancer effect level;
CS = clinical signs; Develop = developmental; DX = developmental toxicity; ECG = electrocardiogram; Endocr = endocrine; (F) = feed; F = female(s);
F0 = parental generation; F1 = first generation; F2 = second generation; F3 = third generation; FI = food intake; FSH = follicle stimulating hormone; (G) = gavage;
GD = gestation day; GN = gross necropsy; (GO) = gavage in oil;; HE = hematology; Hemato = hematological; HP = histopathology; Immuno = immunological;
IX = immunotoxicity; LD = lactation day; LD
50
= lethal dose, 50% kill; LE = lethality; LOAEL = lowest-observed-adverse-effect level; M = male(s);
Musc/skel = musculoskeletal; Neuro = neurological; NOAEL = no-observed-adverse-effect level; NS = not specified; NX = neurotoxicity; OF = organ function;
OW = organ weight; PND = postnatal day; Repro = reproductive; Resp = respiratory; RX = reproductive toxicity; SRBC = sheep red blood cell; T4 = thyroxine;
TSH = thyroid stimulating hormone; UR = urinalysis; (W) = drinking water; WI = water intake

HEXACHLOROCYCLOHEXANE (HCH) 74
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Acute (≤14 days)

HEXACHLOROCYCLOHEXANE (HCH) 75
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Acute (≤14 days)

HEXACHLOROCYCLOHEXANE (HCH) 76
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 77
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 78
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 79
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Chronic (≥365 days)

HEXACHLOROCYCLOHEXANE (HCH) 80
2. HEALTH EFFECTS
Figure 2-8. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Oral
Chronic (≥365 days)

HEXACHLOROCYCLOHEXANE (HCH) 81
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
ACUTE EXPOSURE
Joseph et al. 1992a
Technical HCH
1
Rat
(CFT-
Wistar)
6 M
Once
(GO)
0–4,000
LE
Death
2,428
LD
50
Sahoo et al. 1999
Technical HCH
2
Rat
(Wistar)
10 M
7 days
0, 10, 20
BI, NX
Neuro
10
Reduced brain ATPase
activities; 12% reduction in
brain acetylcholinesterase
activity; increased motor
activity at 20 mg/kg/day
Samanta et al. 1999
Technical HCH
3
Rat
(Wistar)
10 M
7 days
(GO)
0, 10, 20
BW, BC, BI,
OW, HP
Repro
10
54% reduction in total sperm
count in adult rats;
increased frequency of
damaged sperm and sperm
with anomalous heads
Dikshith et al. 1990
Technical HCH
4
Mouse
(Swiss
albino)
6 F
GD 9
once
(GO)
0, 5, 25, 50,
100, 200
BW, DX
Repro
25
Increased fetal resorptions
Philip et al. 1989
Technical HCH
5
Mouse
(NS)
NS
1 or 5 days
(GO)
0, 50
HP
Hepatic
50
Marked damage including
severe congestion of portal
vessels and central vein,
severe fatty changes in
periportal cells
Renal
50
A few cases of interstitial
hemorrhaging in medulla,
cystic dilation of tubules,
hyaline casts

HEXACHLOROCYCLOHEXANE (HCH) 82
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Ravinder et al. 1989, 1990
Technical HCH
6
Mouse
(Swiss
albino)
10 M
2 weeks
(F)
0, 72, 144
BI, OW, OF
Death
144
14% mortality
Hepatic
72
>2-fold increase in relative
liver weight, hepatocellular
hypertrophy, mild
centrilobular degeneration,
focal necrosis in a few
specimens
INTERMEDIATE EXPOSURE
Anand et al. 1991
Technical HCH
7
Rat
(NS)
45 M
90 days
6 days/week
(GO)
0, 50
BI, NX
Neuro
50
Increased dopamine and
decreased norepinephrine in
brain; behavioral changes;
increased brain wave
frequency
Dikshith et al. 1989a
Technical HCH
8
Rat
(NS)
10 M
30 days
(GO)
0, 60
CS, HE, BC,
BI, OW, HP
Hemato
60
Hepatic
60
65% increase in liver weight
Renal
60
Repro
60
Dikshith et al. 1991a
Technical HCH
9
Rat
(NS)
20 M
360 days
(F)
0, 0.04, 0.4,
2, 20, 40
BW, FI, BC,
BI, OW, OF,
HP
Death
0.4
4/20 deaths
Hepatic
2
20
Focal necrosis, enlargement
of hepatocytes, nuclear
pyknosis, vacuolation,
margination
Renal
2
20
Debris cells in lumen,
glomerular degeneration
Neuro
0.04
0.4
Tremors, convulsions, hind
limb paralysis
HEXACHLOROCYCLOHEXANE (HCH) 83
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Repro
2
20
Testicular necrosis and
degeneration
Dikshith et al. 1991b
Technical HCH
10
Rat
(NS)
12 M, 12 F
90 days
(GO)
0, 5, 25
LE, BW, BI,
BC, OW, HP,
OF
Death
5
33% mortality in females
and 50% mortality in males
Fitzhugh et al. 1950
Technical HCH
11
Rat
(Wistar)
10 F, 10 M
6 months
(F)
Males: 0, 7,
60
Females: 0,
9, 70
LE, BW
Death
60 M
70 F
Decreased mean age at
death (32.9 versus
58.3 weeks in controls)
Bd wt
7 M
9 F
70 F
60 M
LOAEL: 16% decrease in
body weight of females
Serious LOAEL: 26%
decrease in body weight of
males
Resp
60 M
70 F
Cardio
60 M
70 F
Gastro
60 M
70 F
Hemato
60 M
70 F
Hepatic
60 M
70 F
Moderate liver damage
Renal
60 M
70 F
Slight kidney damage
Musc/skel
60 M
70 F
Endo
60 M
70 F
Repro
70 F
60 M
Moderate testicular atrophy

HEXACHLOROCYCLOHEXANE (HCH) 84
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Gautam et al. 1989
Technical HCH
12
Rat
(Charles
Foster)
10 M
180 days
(GO)
0, 3, 6
BW
Bd wt
3
17% decrease in body
weight gain
Repro
3
6% decrease in vas
deferens weight,
degeneration of inner
muscle and cell layers of
vas deferens
Gopal et al. 1992
Technical HCH
13
Rat
(NS)
50 M
120 days
(GO)
0, 50
CS, NX
Neuro
50
Increased motor activity,
decreased resting
stereotypic time
Joseph et al. 1992c
Technical HCH
14
Rat
(CFT-
Wistar)
4 M
7 weeks
(F)
0, 90
HE, BC
Hemato
90
Decreased white blood cell
counts
Ito et al. 1975
Delta HCH
15
Rat
(W strain)
18–24 M
48 weeks
0, 35, 70
BW, OW, HP
Hepatic
35
70
Hepatocellular hypertrophy
Mudawal et al. 2018
Technical HCH
16
Rat
(Wistar)
6 M
3 weeks
7 days/week
(NS)
0, 2.5
BI, NX, DX
Neuro
2.5
Statistically significant
decreases in conditioned
avoidance, alternations, and
locomotor activity;
ultrastructural changes in
the hippocampus and
substantia nigra in adult
animals
Nagaraja and Desiraju 1994
Technical HCH
17
Rat
(Wistar)
6–8 F
PNDs 2–60
(GO)
0, 10, 20
CS, BW, BI
Develop
10
Alterations in levels of
dopamine, serotonin, and
noradrenaline in pup brains

HEXACHLOROCYCLOHEXANE (HCH) 85
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Nagaraja and Desiraju 1994
Technical HCH
18
Rat
(Wistar)
6–7 F
90 days
(F)
0, 20
CS, BW, BI
Bd wt
20
Significantly decreased
(23%) terminal body weight
Neuro
20
Increased GABA levels,
increased GAD activity,
decreased glutamate levels
Roy Chowdhury and Gautam 1990
Technical HCH
19
Rat
(Charles
Foster)
5–10 M
180 days
(G)
0, 3, 6
BW, OW, HP
Repro
3
6
LOAEL: detachment of
germinal cells from
peritubular membrane of
seminiferous tubules,
atrophy of Leydig cells, and
intertubular edema
Serious LOAEL: “complete
degeneration” of testicular
tissue
Sahoo et al. 1999
Technical HCH
20
Rat
(Wistar)
10 M
15 or 30
days
7 days/week
0, 10, 20
BI, NX
Neuro
10
Reduced brain ATPase
activity; 39% decrease in
acetylcholinesterase activity
after 15 days, with reduced
grooming behavior at
20 mg/kg/day after 30 days
Samanta et al. 1999
Technical HCH
21
Rat
(Wistar)
10 M
15 or 30
days
(GO)
0, 10, 20
BW, BC, BI,
OW, HP
Repro
10
58–65% reduction in total
sperm count in adult rats;
increased frequency of
damaged sperm and sperm
with anomalous heads
Bhatt and Bano 2009
Technical HCH
22
Mouse
(Swiss)
6–18 M
2, 4, or
6 months
(F)
0, 90
BI, HP
Cancer
90
CEL: Liver tumors

HEXACHLOROCYCLOHEXANE (HCH) 86
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Bhatt and Nagda 2012
HCH Not further specified
23
Mouse
(Swiss)
18–20 M
2, 4, or
6 months
(F)
0, 90
BI, HP
Hepatic
90
Hepatocyte degeneration,
vacuolation, fatty changes;
hypertrophy, and
hyperplasia after 4 months
Cancer
90
CEL: liver tumors
Ito et al. 1973
Delta HCH
24
Mouse
(dd)
20–40 M
24 weeks
(F)
0, 18, 45, 90
BW, OW, HP
Bd wt
90
Hepatic
45
90
23% increase in relative liver
weight and centrilobular
hypertrophy
Karnik et al. 1981
Technical HCH
25
Mouse
(Swiss)
6 NS
2–8 months
(F)
0, 90
OW, OF
Hepatic
90
100% increase in liver
weight; glycogen
accumulation, smooth
endoplasmic reticulum
proliferation
Cancer
90
CEL: hepatocellular
carcinoma
Nigam et al. 1979
Technical HCH
26
Mouse
(Swiss)
6 M
3 months
(F)
0, 90
BW, OW, HP
Repro
90
Increased (27%) relative
testis weight, degeneration
of seminiferous tubules,
shrunken and edematous
tubules (some completely
hyalinized); decreased
(sparse) and damaged
spermatocytes
HEXACHLOROCYCLOHEXANE (HCH) 87
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Philip et al. 1989
27
Mouse
(NS)
NS
15 d
(GO)
0, 50
HP
Hepatic
50
Marked damage consisting
of congestion of portal
vessels and central vein,
granular degeneration
Renal
50
Marked damage consisting
of congestion of blood
vessels and glomeruli,
vacuolation of epithelial cells
in glomeruli, fatty changes,
cystic dilation of the tubules,
and interstitial hemorrhaging
Thakore et al. 1981
Technical HCH
28
Mouse
(Swiss)
6 NS
2–8 months
(F)
0, 90
BW, BI, OW
Cancer
90
CEL: hepatocellular
carcinoma
Trivedi et al. 2007, 2009
Technical HCH
29
Mouse
(Swiss)
6 M
1–8 months
(F)
0, 90
BC, BI, GN,
HP
Hepatic
90
“Severe” liver damage after
6 months
Cancer
90
CEL: liver tumors
Wang et al. 2006
Technical HCH
30
Pig
(Duroc X
Landrace
X Large
white)
12 M, 12 F
90 days
(F)
0, 0.4, 0.8
BW, FI, BC,
BI, OW, IX
Bd wt
0.8
Hepatic
0.8
No effect on liver weight;
<30% increases in serum
ALT and ALP
Renal
0.4
0.8
24% increase in relative
kidney weight

HEXACHLOROCYCLOHEXANE (HCH) 88
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
CHRONIC EXPOSURE
Fitzhugh et al. 1950
Technical HCH
31
Rat
(Wistar)
10 F, 10 M
107 weeks
(F)
Males: 0, 0.7,
4, 7
Females: 0,
0.4, 0.9, 4, 9,
LE, BW, FI,
GN, OW, HP
Resp
7 M
9 F
Cardio
7 M
9 F
Gastro
7 M
9 F
Hemato
7 M
9 F
Musc/skel
7 M
9 F
Hepatic
0.7 M
0.9 F
4
Very slight microscopic
damage
Renal
7 M
9 F
Endo
7 M
9 F
Repro
7 M
9 F
Kashyap et al. 1979
Technical HCH
32
Mouse
(Swiss)
30 M, 30 F
80 weeks
(GO)
0, 10
CS, BW, FI,
GN, HP
Neuro
10
Convulsions
Cancer
10
CEL: hepatocellular
carcinoma
Kashyap et al. 1979
Technical HCH
33
Mouse
(Swiss)
30 M, 30 F
80 weeks
(F)
0, 17
CS, BW, FI,
GN, HP
Neuro
17
Convulsions
Cancer
17
CEL: hepatocellular
carcinoma

HEXACHLOROCYCLOHEXANE (HCH) 89
2. HEALTH EFFECTS
Table 2-5. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane – Oral
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Munir et al. 1983
Technical HCH
34
Mouse
(Swiss)
10–37 M
20 months
(F)
0, 21.3, 42.5,
85
BW, OW, HP
Cancer
21.3
CEL: hepatocellular
carcinoma
a
The number corresponds to entries in Figure 2-9; differences in levels of health effects and cancer effects between male and females are not indicated in
Figure 2-9. Where such differences exist, only the levels of effect for the most sensitive sex are presented.
ALP = alkaline phosphatase; ALT= alanine aminotransferase; BC = serum (blood) chemistry; Bd wt or BW = body weight; BI = biochemical changes;
CEL = cancer effect level; CS = clinical signs; Develop = developmental; DX = developmental toxicity; (F) = feed; F = female(s); FI = food intake; (G) = gavage;
GABA = gamma-aminobutyric acid; GAD =glutamate decarboxylase; GD = gestation day; GN = gross necropsy; (GO) = gavage in oil; HCH = hexachlorocyclo-
hexane; HE = hematology; Hemato = hematological; HP = histopathology; IX = immunotoxicity; LD
50
= lethal dose, 50% kill; LE = lethality; LOAEL = lowest-
observed-adverse-effect level; M = male(s); Musc/skel = muscular/skeletal; Neuro = neurological; NOAEL = no-observed-adverse-effect level; NS = not specified;
NX = neurotoxicity; OF = organ function; OW = organ weight; PND = postnatal day; Repro = reproductive; (W) = drinking water

HEXACHLOROCYCLOHEXANE (HCH) 90
2. HEALTH EFFECTS
Figure 2-9. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane (HCH) – Oral
Acute (≤14 days)

HEXACHLOROCYCLOHEXANE (HCH) 91
2. HEALTH EFFECTS
Figure 2-9. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane (HCH) – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 92
2. HEALTH EFFECTS
Figure 2-9. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane (HCH) – Oral
Intermediate (15–364 days)

HEXACHLOROCYCLOHEXANE (HCH) 93
2. HEALTH EFFECTS
Figure 2-9. Levels of Significant Exposure to δ- and Technical Hexachlorocyclohexane (HCH) – Oral
Chronic (≥365 days)
HEXACHLOROCYCLOHEXANE (HCH) 94
2. HEALTH EFFECTS
Table 2-6. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Dermal
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less serious
LOAEL
Serious
LOAEL
Effects
ACUTE EXPOSURE
Gaines 1960
Rat
(Sherman)
100 M, 70 F
Once
(GO)
NS
LE, CS
Death
900 F
LD
50
1,000 M
LD
50
Hanig et al.
1976
Rabbit
(New Zealand)
2–6 M
Once
0, 60 mg/kg
LE, CS
Death
60
Deaths among weanlings
Neuro
60
Convulsions
Ullmann 1986a
Rat
(Wistar)
5 M, 5 F
24 hours
once
0, 250, 600,
1,000,
2,000 mg/kg
LE, CS, BW,
GN
Death
600
2/10 died at 600 mg/kg;
LD
50
=1,000 mg/kg
Neuro
600
1,000
2,000 F
LOAEL: sedation, curved body
position
Serious LOAEL: severe sedation
and spasms in one female
Ullmann 1986c
Rabbit
(New Zealand)
3 M, 3 F
Once
0, 40 mg/kg
LE, CS, BW
Ocular
40
Mild eye irritation
INTERMEDIATE EXPOSURE
Dikshith et al. 1973
Rat (I.T.R.C.)
30 F
25 days
0,
180 mg/kg/day
CS
Dermal
180
Mild dermatitis
HEXACHLOROCYCLOHEXANE (HCH) 95
2. HEALTH EFFECTS
Table 2-6. Levels of Significant Exposure to γ-Hexachlorocyclohexane – Dermal
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less serious
LOAEL
Serious
LOAEL
Effects
EPA 1988a
Rat
(Crl:(WI)BR)
13–23 M, 13–
23 F
13 weeks
5 days/week
6 hours/day
0, 10, 60,
400 mg/kg/day
LE, CS, BW,
FI, HE, BC,
UR, GN, OF,
HP, NX
Death
400 F
23 deaths out of 49
Bd wt
400
Resp
10
Rapid respiration or wheezing
Hemato
400
Hepatic
10
60
Centrilobular hypertrophy,
increased absolute liver weight
(8%) in females
Renal
10 M
60 F
60 M
Basophilic tubules in males
Neuro
10
60 F
LOAEL: hyperactivity
Serious LOAEL: ataxia, tremors,
convulsions
BC = serum (blood) chemistry; Bd wt or BW = body weight; CS = clinical signs; F = female(s); FI = food intake; GN = gross necropsy; HE = hematology;
HP = histopathology; LE = lethality; LD
50
= lethal dose, 50% kill; LOAEL = lowest-observed-adverse-effect level;; M = male(s); Neuro = neurological; NOAEL = no-
observed-adverse-effect level; NR = not reported; NS = not specified; NX = neurotoxicity; OF = organ function; Resp = respiratory; UR = urinalysis

HEXACHLOROCYCLOHEXANE (HCH) 96
2. HEALTH EFFECTS
Table 2-7. Levels of Significant Exposure to Technical Hexachlorocyclohexane – Dermal
Species (strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
ACUTE EXPOSURE
Dikshith et al. 1978
Guinea pig
(NS)
24 M
5–12 days
0, 100, 200,
500
LE
Death
200
24/24 deaths
INTERMEDIATE EXPOSURE
Dikshith et al. 1991c
Rat
(Wistar)
6 F
15–30 days
0, 100
LE, BW, BC,
BI, OW, HP
Death
100
Two deaths by day 15 and 2
by day 30
Hepatic
100
Severe liver injury including
hypertrophy, fatty
degeneration, nuclear
pyknosis of hepatocytes,
diffuse and focal liver
necrosis, and bile duct
proliferation
Renal
100
Mild to severe tubular
epithelial cell necrosis and
glomerular atrophy
Dermal
100
Hyperkeratosis, epidermal cell
vacuolization, thickening of
collagen fibers
Neuro
100
Tremors, degenerative
changes in the cerebellum
Dikshith et al. 1989b
Rabbit
(NS)
8 M
30 days
0, 25
LE, CS, BW,
BC, BI, UR,
OW, HP
Death
25
6/24 deaths
Hepatic
25
Hepatocyte degeneration,
pycnotic nuclei, enlarged liver,
altered ALT, AST, LDH, and
ALP activities

HEXACHLOROCYCLOHEXANE (HCH) 97
2. HEALTH EFFECTS
Table 2-7. Levels of Significant Exposure to Technical Hexachlorocyclohexane – Dermal
Species (strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Renal
25
Altered epithelial lining of
proximal convoluted tubules,
loss of brush borders of
tubules, atrophy of glomerular
capsules
Dermal
25
Thickened epidermis,
hyperkeratinization, and
infiltration of mononuclear
cells
Neuro
25
Convulsions, tremor, and
paralysis; changes in Purkinje
cells of cerebellum including
loss of dendrites and presence
of deciduomatous cell body
Repro
25
Severe effects on germinal
cells of testes, including
vacuolation, cytoplasmic
changes, cell sloughing, and
multinucleated giant cells
Dikshith et al. 1978
Guinea pig
(NS)
24 M
30 days
0, 100, 200,
500
BW, BC, BI,
OW, HP
Hepatic
100
38% increase in liver weight,
hepatic hypertrophy, pyknotic
nuclei in cytoplasm, focal fatty
inclusions, increased ALT and
ALP activity
Renal
100
Repro
100
Degeneration of seminiferous
tubules, necrosed
spermatogenic cells,
multinucleated giant cells, and
no active sperm in lumen

HEXACHLOROCYCLOHEXANE (HCH) 98
2. HEALTH EFFECTS
Table 2-7. Levels of Significant Exposure to Technical Hexachlorocyclohexane – Dermal
Species (strain)
No./group
Exposure
parameters
Doses
(mg/kg/day)
Parameters
monitored
Endpoint
NOAEL
(mg/kg/day)
Less
serious
LOAEL
(mg/kg/day)
Serious
LOAEL
(mg/kg/day)
Effects
Mathur et al. 1992
Guinea pig
(NS)
6 NS
30 days
0, 100
BI, HP
Hepatic
100
Increased enzyme activity and
fatty and degenerative
changes
Renal
100
increased enzyme activity and
histopathological changes
Mathur et al. 1993
Guinea pig
(NS)
6 M
15, 30 days
0, 100
BI, HP
Dermal
100
Dermal histopathology:
hyperkeratinization,
mononuclear infiltration,
sloughing
CHRONIC EXPOSURE
Kashyap et al. 1979
Mouse
(Swiss)
30 M, 30 F
80 weeks
2 days/week
0, 2.4
BW, FI, GN,
HP, CS
Cancer
2.4
CEL: liver tumors
Prasad et al. 1995
Rat
(Wistar)
10 M
120 days
0, 50, 100
CS, BI, OF,
RX
Repro
50
Decreased sperm count (57%
relative to vehicle control) and
motility (38%), increased
percent abnormal sperm
(>5-fold), alterations in
testicular enzyme activity
ALP = alkaline phosphatase; ALT= alanine aminotransferase; AST = aspartate aminotransferase; BC = serum (blood) chemistry; BW = body weight; BI =
biochemical changes; CEL = cancer effect level; CS = clinical signs; F = female(s); FI = food intake; GN = gross necropsy; HP = histopathology; LDH = lactate
dehydrogenase; LE = lethality; LOAEL = lowest-observed-adverse-effect level; M = male(s); Neuro = neurological; NOAEL = no-observed-adverse-effect level;
NS = not specified; OF = organ function; OW = organ weight; Repro = reproductive; RX = reproductive toxicity; UR = urinalysis

HEXACHLOROCYCLOHEXANE (HCH) 99
2. HEALTH EFFECTS
2.2 DEATH
Human fatalities have been reported for γ-HCH but not for other isomers or for mixtures of isomers.
Studies reporting deaths associated with γ-HCH are described below in the subsection on γ-HCH.
α-HCH. In a long-term study where Wistar rats were administered α-HCH in the diet, mean age of death
was significantly decreased to 35.9 weeks in animals administered 60–70 mg/kg/day compared to
58.3 weeks in control animals (Fitzhugh et al. 1950). No inhalation or dermal studies of mortality in
animals were identified for α-HCH.
β-HCH. Mortalities have occurred in rats and mice exposed to β-HCH in the diet for acute and
intermediate exposure durations, often after showing signs of pronounced neurotoxicity. In the first week
of a 30-day study, 80% of female mice receiving 200 mg/kg/day β-HCH in feed exhibited ataxia
progressing to lateral recumbent position and were humanely sacrificed (Cornacoff et al. 1988). No
deaths were observed in male rats exposed to 72 mg/kg/day β-HCH in food for 2 weeks (Srinivasan et al.
1984). However, maternal mortality occurred in a developmental study in rats (Srinivasan et al. 1991), in
which all dams exposed to 80 mg/kg/day β-HCH in feed died within 3 weeks of treatment (during
gestation). Similarly, in the first 2 weeks of a 13-week dietary study, two male and three female rats
exposed to doses of 38 mg/kg/day exhibited ataxia and hypoactivity, progressing within 3 days to coma
and leading to their humane sacrifice
1
(Van Velsen et al. 1986). Subsequently, five males and six females
in this dose group became moribund and were euthanized later in the study (timing not reported) (Van
Velsen et al. 1986). In a dietary study where Wistar rats were administered β-HCH, mean age of death
for both males and females administered 60–70 mg/kg/day was 4.4 weeks compared to 58.3 weeks in
control animals (Fitzhugh et al. 1950). All animals exposed to β-HCH at this dose died by 10 weeks of
exposure (Fitzhugh et al. 1950). Finally, in a chronic study in which CF1 mice were administered
34 mg/kg/day β-HCH for up to 104 weeks, 12% of males and 25% of females died within the first
3 months (Thorpe and Walker 1973). Survival of the remaining animals did not differ from controls. No
inhalation or dermal studies of mortality in animals were identified for β-HCH.
No information on the causes or mechanisms of death in animals exposed to β-HCH was located,
although neurotoxicity preceded death in most instances. There appears to be substantial variability in
susceptibility to the lethal effects of β-HCH, as demonstrated by the chronic study by Thorpe and Walker
1
Because the deaths occurred after the end of 2 weeks, they were considered to occur as a result of intermediate-
duration exposure.
HEXACHLOROCYCLOHEXANE (HCH) 100
2. HEALTH EFFECTS
(1973), in which animals that survived the first 3 months did not have any reductions in subsequent
survival.
γ-HCH (Lindane). γ-HCH was once used in insecticide vaporizer and fumigator devices, resulting in
human inhalation and dermal exposure to unspecified levels. Occasional deaths associated with the use of
this product for several months or years have been reported, but it is not clear that γ-HCH was responsible
for the deaths (Loge 1965). Two cases of pulmonary edema resulting in fatalities were reported in
toddlers inhaling and ingesting unknown quantities of γ-HCH-containing pesticidal powder (McQueen et
al. 1968). Following an accidental chemical spill from storage tanks in India, seven deaths occurred in
the area, with the cause of death reported as asphyxia (Jain et al. 2022). Sampling near the spill revealed
γ-HCH in water and soil at levels that exceeded permissible concentrations. All deaths were in
individuals within 200 m of the site. Sixteen survivors exhibited symptoms including headache, nausea,
and breathlessness.
Case reports of deaths in humans, often in children or suicidal adults, following ingestion of γ-HCH in
tablets (doses unknown) intended for γ-HCH vaporizers have been reported (Storen 1955; Sunder Ram
Rao et al. 1988). A single acute, whole-body dermal application of 1% γ-HCH in lotion to a 2-month-old
infant for scabies treatment resulted in death (Davies et al. 1983). Autopsy identified pulmonary and
epicardial petechiae and a concentration in the brain of 110 ppb γ-HCH. The death of an elderly woman
was reported following a 6-hour dermal application of γ-HCH-containing lotion (approximately 40 mg
total γ-HCH) to the head for the treatment of scabies (Katsumata and Katsumata 2003). No data were
reported for blood or tissue levels of γ-HCH. A 66-year-old man died after being treated for scabies in a
hospital with a 1% γ-HCH lotion applied dermally from neck to toes (Sudakin 2007). Eight hours after
application, the man exhibited worsening of mental status and hypoxemia. Over the next 2 hours, his
neurological symptoms increased in severity to include seizure and myoclonic jerks, and were
accompanied by severe hypoxemia, tachycardia, diaphoresis, hypotension, and respiratory acidosis. The
man remained in intensive care and subsequently died after 50 days in the hospital. The autopsy
attributed the cause of death to hypoxic ischemic encephalopathy from γ-HCH poisoning. The dose of
γ-HCH was not estimated, and neither blood nor tissue levels of γ-HCH were measured during
hospitalization or at autopsy (Sudakin 2007).
In an acute animal study of rats exposed nose-only to γ-HCH aerosol for 4 hours, the LC
50
was
determined to be 1,560 mg/m
3
(Ullmann 1986b); the lowest concentration associated with lethality was
378 mg/m
3
. No rats exposed whole body to γ-HCH for 4 hours up to a concentration of 603 mg/m
3
died
HEXACHLOROCYCLOHEXANE (HCH) 101
2. HEALTH EFFECTS
throughout the 14-day observation period (Oldiges et al. 1980). In the beginning of a 14-week study,
12/45 female and 2/45 male mice that were exposed to 10 mg/m
3
of γ-HCH dust aerosol via whole body
(6 hours/day) died during the first week (Klonne and Kintigh 1988). In this study, the concentration was
decreased from 10 to 5 mg/m
3
after the first week; additional deaths occurred at 5 mg/m
3
(two males and
three females) and 1 mg/m
3
(one male and one female), but there were no deaths at 0.3 mg/m
3
. One
unexplained death in a control mouse was also reported (Klonne and Kintigh 1988).
Studies in laboratory animals have reported deaths after acute-duration oral administration of γ-HCH
doses ≥20 mg/kg/day (Chadwick et al. 1988; Fitzhugh et al. 1950; Gaines 1960; Hassoun and Stohs
1996a; Liu and Morgan 1986; Martinez et al. 1991; Matsuura et al. 2005; Thorpe and Walker 1973;
Tusell et al. 1988; Yuksel et al. 2009). In studies of rats administered a single dose of γ-HCH via gavage,
LD
50
values of 88 mg/kg in males and 91 mg/kg in females were obtained (Gaines 1960). One of seven
male rats died following a single administration of γ-HCH at a dose of 60 mg/kg via gavage (Martinez et
al. 1991), while 2/18 rats died within the third day of exposure to doses of 20 mg/kg/day (Tusell et al.
1988). Acute exposure-duration studies in mice showed similar lethal doses in some strains. Pregnant
DBA/2J and C57BL/6J mice both exhibited mortality (14–25%) upon single gavage doses of 30 and
45 mg/kg, respectively, administered on gestation day (GD) 12 (Hassoun and Stohs 1996a). In another
study, no mortality was reported in nonpregnant adult female C57BL/6 mice; however, six of six DBA/2
mice died after 20 mg γ-HCH/kg was administered by daily gavage for up to 10 days (Liu and Morgan
1986).
In intermediate- and chronic-duration studies, the doses inducing lethality were similar to those seen after
acute-duration exposure. Two F0 female rats exposed to doses of 26.1 mg/kg/day in a 2-generation
reproductive toxicity study died, but the times of death were not reported (Matsuura et al. 2005). In F344
rats administered γ-HCH for up to 15 weeks, 2/12 animals died at 20 mg/kg/day and 7/12 died at
40 mg/kg/day (Chadwick et al. 1988). When groups of 10 Sprague-Dawley rats were administered 20 or
40 mg/kg/day γ-HCH by gavage for 30 days, one animal in each group died during week 3 (Yuksel et al.
2009). The age at death in Wistar rats was significantly decreased to 39.7 weeks in animals administered
60–70 mg/kg/day, compared to 58.3 weeks in control animals (Fitzhugh et al. 1950). In a 2-year study
with interim sacrifices, dietary administration of technical-grade γ-HCH at a dose of 32 mg/kg/day
resulted in significantly decreased survival when compared with controls (Amyes 1990).
Acute dermal exposure to γ-HCH resulted in death. Dermal LD
50
values in rats exposed to γ-HCH once
and observed for 10 days were 1,000 mg/kg in males and 900 mg/kg in females (Gaines 1960). In an
HEXACHLOROCYCLOHEXANE (HCH) 102
2. HEALTH EFFECTS
acute dermal study in which male and female rats were exposed to γ-HCH for 24 hours, mortality rates
across both sexes were 0, 20, 40, and 30% at 250, 600, 1,000, and 2,000 mg/kg, respectively (Ullmann
1986a). Weanling rabbits were more sensitive to γ-HCH treatment than young adults, as seen by
increased mortality rates accompanied by excitement and convulsions after a single whole-body treatment
with a 1% solution at a dose of 60 mg/kg γ-HCH (Hanig et al. 1976). Significant mortality (47%) was
seen in female rats, but not male rats, exposed dermally to γ-HCH at 400 mg/kg/day for 6 hours/day,
5 days/week, for 13 weeks (EPA 1988a). No studies regarding chronic dermal exposure to γ-HCH were
located.
Technical HCH or Unspecified Isomers of HCH. Joseph et al. (1992a) reported an LD
50
of 2,428 mg/kg
in male rats administered a single gavage dose of technical-grade HCH (72.8% α-HCH, 12.6% γ-HCH,
7.95% δ-HCH, 5% β-HCH). Technical-grade HCH administered to rats for 90 days resulted in increased
mortality: 6/12 males and 4/12 females exposed to 5 mg/kg/day died, and a 58% increase in mortality
(incidence not reported) was observed at 25 mg/kg/day (Dikshith et al. 1991b). When Wistar rats were
administered technical-HCH in the diet as part of a chronic study, age at death was significantly
decreased to 32.9 weeks in animals administered 64 mg/kg/day compared to 58.3 weeks in control
animals (Fitzhugh et al. 1950). Exposure to low levels (0.4 mg/kg/day) of technical-grade HCH in the
diet for 360 days resulted in deaths of 4/20 rats (Dikshith et al. 1991a).
Dikshith et al. (1978) reported that guinea pigs dermally exposed to 200 mg technical-grade HCH/kg died
within 5–12 days. Four of 20 rats died from dermal exposure to technical-grade HCH at 100 mg/kg/day
for 15–30 days (Dikshith et al. 1991c). Rabbits treated with 25 mg/kg/day technical-grade HCH for
30 days by skin painting on shaved dorsal, ventral, or thigh areas exhibited no deaths in the group
exposed by dorsal application, but two of eight rabbits died in the group exposed by ventral application,
and four of eight died in the group exposed by thigh application (Dikshith et al. 1989b).
2.3 BODY WEIGHT
Epidemiological Studies. Studies of body weight effects in humans include three studies of β-HCH;
these are summarized in Table 2-8. In a cohort of women residing in an agricultural area of California,
serum levels of β-HCH were associated with increased body mass index (BMI), waist circumference,
body fat percent, and obesity when measured over the 3 years after serum collection (Warner et al. 2018).
A cross-sectional study of surgical patients in Spain provided support for an association between β-HCH
(measured in serum or adipose tissue) and increased BMI (Arrebola et al. 2014). In another cross-

HEXACHLOROCYCLOHEXANE (HCH) 103
2. HEALTH EFFECTS
sectional study in adults from Seoul, South Korea, serum levels of β-HCH were associated with increased
BMI (Seo et al. 2022).
Table 2-8. Summary of Epidemiological Studies of β-Hexachlorocyclohexane
(β-HCH) Exposure and Body Weight Effects
Reference, study type, and
population
Biomarker
Concentration
Outcome evaluated
Result
Arrebola et al. 2014
Cross-sectional, 298 noncancer
surgical patients >16 years old,
Spain
Serum or
adipose
19.60±28.74 ng/g
lipid (mean)
BMI
↑
a
Warner et al. 2018
Cohort, 468 women >18 years old,
residing in agricultural area of
California, United States
Serum
>5.2 ng/g lipid
(median)
BMI
↑
Waist circumference
↑
Body fat %
↑
Obesity
↑
Seo et al. 2022
Cross-sectional, 880 adults, ages
20–80 years, South Korea
Serum
40.4 ng/g lipid
BMI
↑
a
BMI exhibited a quadratic association with β-HCH, increasing at low concentrations and then decreasing at higher
concentrations.
↑ = association with increase; BMI = body mass index
Data on body weight changes in animals exposed by inhalation or dermal contact were limited to γ-HCH.
α-HCH. Sumida et al. (2007) reported no body weight changes in male rats administered α-HCH via
gavage at 20 mg/kg/day for up to 28 days. In rats exposed for 24 weeks to 45 mg/kg/day in feed, terminal
body weight was decreased by 15% (Nagasaki et al. 1975); food intake was not reported. Significantly
decreased body weight gain in the absence of changes in food intake was also seen in rats treated with
60–70 mg/kg/day of α-HCH in the diet for 6 months (Fitzhugh et al. 1950). In studies of several mouse
strains given 90 mg/kg/day α-HCH in feed for 24 weeks, a significant (17%) decrease in terminal body
weight was observed in male C57BL/6 mice, but not in other strains (dd, DDY, ICR, DBA/2, or C3H/He)
or in females of any strain (Ito et al. 1973; Nagasaki et al. 1975). These authors conducted a similar
experiment in male hamsters given 45 mg/kg/day in feed for 24 weeks. Terminal body weight was 14%
lower than controls in the hamsters (Nagasaki et al. 1975).
β-HCH. Intermediate-duration oral studies of body weight effects after exposure to β-HCH exposure
showed effects in rats, but not in mice. Body weight decreases of at least 10% were observed in Wistar
HEXACHLOROCYCLOHEXANE (HCH) 104
2. HEALTH EFFECTS
rats administered dietary β-HCH at a dose of 22.5 mg/kg/day in males or 25 mg/kg/day in females for
13 weeks; however, at this dose, half of the animals were sacrificed moribund prior to study termination
(Van Velsen et al. 1986). No body weight effects were observed at doses up to 5 mg/kg/day in males or
females (Van Velsen et al. 1986). After 6 months of β-HCH administration in the diet, body weight gain
was decreased by 11% in female Wistar rats exposed to 9 mg/kg/day (Fitzhugh et al. 1950); body weight
data for the lower dose group were not reported. No effect on body weight was observed in mice
administered β-HCH at doses of 60 mg/kg/day for 30 days (Cornacoff et al. 1988) or 90 mg/kg/day for
24 weeks (Ito et al. 1973).
γ-HCH (Lindane). Limited information is available on body weight effects of γ-HCH in animals exposed
by inhalation. In Wistar rats exposed for 4 hours to 603 mg/m
3
γ-HCH, females lost weight for the first
post-exposure observation week. Neither mice nor rats exposed to γ-HCH aerosols at concentrations up
to 5 mg/m
3
for 6 hours each day (5 or 7 days/week) for 13–14 weeks exhibited any change in body weight
(Oldiges et al. 1983; Klonne and Kintigh 1988).
No effects on body weight were observed in rats administered acute-duration oral doses ranging from
8.8 to 15 mg/kg/day (Parmar et al. 2003; Sinha and Shukla 2003; Sumida et al. 2007) or in mice at doses
ranging from 5.9 to 40 mg/kg/day (Di Consiglio et al. 2009; Hong and Boorman 1993; Maranghi et al.
2007; Serrano et al. 1990; Sinha and Shukla 2003). One study of Wistar rat dams exposed to
≥8 mg/kg/day exhibited decreased body weight gain (25% less than controls) on GDs 6–20; decreased
food consumption was seen at the same dose (EPA 1999c). Body weights of pregnant mice administered
15–25 mg/kg/day γ-HCH via gavage during gestation were not affected (Maranghi et al. 2007; Traina et
al. 2003). In a 7-week study of beagle dogs given γ-HCH in the diet, Rivett et al. (1978) reported
suppression of body weight gain at 7 mg/kg/day; however, only two dogs per group were used in this
study.
In intermediate-duration studies, there were no effects on body weight in rats administered γ-HCH via
gavage at doses between 2.5 and 30 mg/kg/day for 15–28 days (Ahmed et al. 2008; Andrews and Gray
1990; Parmar et al. 2003; Sumida et al. 2007; Yang et al. 2014; Zhang et al. 2016) or up to
26.1 mg/kg/day for about 10 weeks in a 2-generation reproductive toxicity study (Matsuura et al. 2005).
In another 2-generation reproductive toxicity study (EPA 1991a), body weight gain decreased, without
changes in food intake, in high-dose (13.1 mg/kg/day) F0 parental females during gestation. Mice
exposed to γ-HCH in feed for 24 weeks exhibited no body weight changes at doses up to 90 mg/kg/day
(Ito et al. 1973).
HEXACHLOROCYCLOHEXANE (HCH) 105
2. HEALTH EFFECTS
Significantly decreased body weight gain was seen after 6 months in rats treated with 120–140 mg/kg/day
γ-HCH in feed as part of a chronic-duration study, but this dose was also associated with significantly
reduced survival (Fitzhugh et al. 1950). Beagle dogs given doses up to 2.92 mg/kg/day γ-HCH in feed
displayed no changes in body weight over the 102-week exposure duration (Rivett et al. 1978).
Technical HCH or Unspecified Isomers of HCH. Wistar rats administered technical-grade HCH via
gavage at doses up to 20 mg/kg/day for 7 days exhibited no effect on body weight (Samanta et al. 1999).
Swiss albino mice had significantly decreased body weight after administration of a single dose of
100 mg/kg technical-grade HCH via gavage in oil (Dikshith et al. 1990). Significantly decreased body
weight gain has been observed in rats treated orally with 3 or 20 mg/kg/day technical-grade HCH for up
to 6 months (Gautam et al. 1989; Nagaraja and Desiraju 1994; Roy Chowdhury and Gautam 1990). Rats
administered technical-grade HCH via gavage at a dose of 50 mg/kg/day for 30 days exhibited a 21%
body weight loss (Khanna et al. 1990). In an 80-week study, Swiss mice administered technical-grade
HCH at 17 mg/kg/day exhibited no changes in body weight or body weight gain, despite decreased food
consumption (Kashyap et al. 1979). After 6 months of technical-grade HCH administration, no effect on
body weight was observed at 7–9 mg/kg/day, but body weight was decreased by 16% in female rats
exposed to 70 mg/kg/day and by 26% in males exposed to 60 mg/kg/day (Fitzhugh et al. 1950). No effect
on body weight was observed in mice chronically administered technical-grade HCH at 10 mg/kg/day by
gavage or 17 mg/kg/day in the diet (Kashyap et al. 1979).
2.4 RESPIRATORY
α-HCH. No studies were located regarding respiratory effects in humans after exposure to α-HCH. In
rats exposed via diet to α-HCH doses up to 70 mg/kg/day for an average of 9 months or up to
9 mg/kg/day for 2 years, there were no histopathology findings in the lungs (Fitzhugh et al. 1950).
β-HCH. In a cross-sectional study in southern Ghana, vegetable farmers who reported pesticide use
showed a significant, positive association between serum levels of β-HCH and respiratory symptoms of
cough, phlegm production, and wheezing (Quansah et al. 2016). In another cross-sectional study in
Ghana, children (up to 5 years old) of vegetable farmers who had accompanied their parents to the farm
showed an association between urine concentrations of β-HCH and lower (but not upper) respiratory tract
infections (Akyeampong et al. 2022). Rats given doses up to 70 mg/kg/day for up to 10 weeks or up to
9 mg/kg/day for 2 years exhibited no microscopic pathology in the lungs (Fitzhugh et al. 1950).
HEXACHLOROCYCLOHEXANE (HCH) 106
2. HEALTH EFFECTS
γ-HCH (Lindane). In a cross-sectional study of vegetable farmers in southern Ghana who used
pesticides, no association was observed between serum levels of γ-HCH and respiratory symptoms
(cough, phlegm production, and wheezing) (Quansah et al. 2016). In humans, mucous membrane
irritation of the nose and throat was observed after acute exposure to the HCH products dispensed by an
overheated γ-HCH vaporizer (Conley 1952). Exposure levels were not reported, and dermal exposure
may also have occurred, although the observed irritation was probably due to direct action upon the
mucous membranes. An acute dermal poisoning of a 2-month-old infant exposed to a whole-body
application of 1% γ-HCH lotion resulted in death. The autopsy revealed pulmonary petechiae (Davies et
al. 1983).
No respiratory effects and no histopathology changes in the nasal cavities or lungs were observed in rats
exposed to γ-HCH aerosol (up to 5 mg/m
3
) 6 hours/day for 90 days (Oldiges et al. 1983) or in mice
similarly exposed for 14 weeks (Klonne and Kintigh 1988). Mononuclear cell infiltrates in peribronchial
and perivascular regions of the lung were observed in male mice administered 0.25 mg/kg/day γ-HCH by
gavage in groundnut oil for 61 days (Tewari et al. 2017). Bronchoalveolar lavage (BAL) from the treated
mice contained increased total leukocyte counts and neutrophil percentages.
In rats, dietary exposure to γ-HCH at doses up to 140 mg/kg/day for ~10 months or 30 mg/kg/day for
2 years resulted in histopathology changes in the lungs (Fitzhugh et al. 1950). Slight dyspnea was
reported in rats exposed dermally for 24 hours to 1,000 or 2,000 mg/kg γ-HCH on a shaved patch of
dorsal skin (Ullmann 1986a). The dyspnea was severe in one female administered the high dose. Rapid
respiration or wheezing was noted in rats exposed dermally to ≥10 mg /kg/day γ-HCH for 13 weeks (EPA
1988a).
δ-HCH. No association between serum concentrations of δ-HCH and respiratory symptoms was
observed in a cross-sectional study of vegetable farmers using pesticides in southern Ghana (Quansah et
al. 2016). In another cross-sectional study in Ghana, children of vegetable farmers who had accompanied
their parents to the farm showed a positive association between urine concentrations of δ-HCH and acute
lower respiratory tract infections in children below the age of 5 years (Akyeampong et al. 2022). No
association was observed with upper respiratory tract infections.
HEXACHLOROCYCLOHEXANE (HCH) 107
2. HEALTH EFFECTS
Technical HCH or Unspecified Isomers of HCH. Neither intermediate- nor chronic-duration dietary
exposure to technical HCH resulted in lung histopathology changes in rats given doses up to
70 mg/kg/day for 6 months or 9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
2.5 CARDIOVASCULAR
Epidemiological Studies. Epidemiological studies of associations between cardiovascular effects in
humans and biomarkers of exposure to β-HCH have been conducted. Table 2-9 provides a summary of
the epidemiological data pertaining to cardiovascular effects and exposure to β-HCH in humans.
Arrebola et al. (2015a) observed an association between serum β-HCH concentrations and incident
hypertension in a cohort of 297 surgical patients followed for 10 years. A positive association between
serum β-HCH concentrations and hypertension was seen among surgical patients with a BMI >26.3,
whereas no association was observed in those with a BMI <26.3 (Arrebola et al. 2015a). In cross-
sectional studies, no association was reported between blood β-HCH concentrations and hypertension in a
study of 1,615 Inuit adults in Greenland (Valera et al. 2013b), while an inverse association with
hypertension was reported in a smaller study of Inuit adults in Quebec (Valera et al. 2013a). No
association with peripheral artery disease was observed in a cross-sectional study of U.S. adult
participants in the National Health and Nutrition Examination Survey (NHANES) (1999–2004) (Min et
al. 2011). Epidemiological studies of cardiovascular effects and exposure to other HCH isomers were not
located, but there are case reports of these effects in humans accidentally or intentionally exposed to
γ-HCH.
Table 2-9. Summary of Epidemiological Studies of β-Hexachlorocyclohexane
Exposure and Cardiovascular Effects
Reference, study type, and
population
Outcome
evaluated
Biomarker
Mean concentration
(unless otherwise noted)
Result
Arrebola et al. 2015a
Cohort, 297 noncancer surgical
patients >16 years old, Spain,
follow-up 10 years
Hypertension
Serum
11.2 ng/g lipid (median) (BMI
>26.3 kg/m
2
)
↑
6.6 (BMI <26.3kg/m
2
)
↔
Valera et al. 2013a
Cross-sectional, 315 Inuit
≥18 years, Quebec, Canada
Hypertension
Plasma
0.13 μg/L lipid (GM)
↓
Valera et al. 2013b
Cross-sectional, 1,614 Inuit aged
≥18 years, Greenland
Hypertension
Plasma
27.0 μg/kg lipid (GM)
↔

HEXACHLOROCYCLOHEXANE (HCH) 108
2. HEALTH EFFECTS
Table 2-9. Summary of Epidemiological Studies of β-Hexachlorocyclohexane
Exposure and Cardiovascular Effects
Reference, study type, and
population
Outcome
evaluated
Biomarker
Mean concentration
(unless otherwise noted)
Result
Min et al. 2011
Cross-sectional, 2,032 adults
>40 years old, NHANES (1999–
2004)
PAD
Serum
15.37 ng/g lipid (obese with
PAD)
10.05 (non-obese with PAD)
10.90 (obese without PAD)
7.92 (non-obese without PAD)
↔
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association;
GM = geometric mean; NHANES = National Health and Nutrition Examination Survey; PAD = peripheral artery
disease
Data regarding cardiovascular effects in animals are limited to the γ-HCH isoform.
α-HCH. No histopathology changes were noted in the hearts of rats given α-HCH via the diet at doses up
to 70 mg/kg/day for ~9 months or up to 9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
β-HCH. There were no microscopic lesions in the heart when rats were exposed by dietary
administration to β-HCH doses up to 70 mg/kg/day for up to 10 weeks or up to 9 mg/kg/day for 2 years
(Fitzhugh et al. 1950).
γ-HCH (Lindane). Autopsy findings in a 2-month-old infant who expired after whole-body application
of 1% γ-HCH lotion were minimal but revealed epicardial petechiae (Davies et al. 1983). In a suicide
attempt, a 56-year-old man intentionally ingested approximately 12 ounces of an insecticide containing
20% γ-HCH (Wiles et al. 2015). Cardiac symptoms of premature atrial and ventricular contractions, and
atrial fibrillation after 3–5 days were observed. The man died on day 12 by committing suicide by means
unrelated to γ-HCH, and no cardiac abnormalities were noted at autopsy.
There were no treatment-related changes to cardiac histopathology findings in rats or mice exposed by
inhalation to γ-HCH concentrations up to 5 mg/m
3
for 13–14 weeks (Klonne and Kintigh 1988; Oldiges et
al. 1983). Evidence for cardiac effects comes from studies of animals exposed orally. Increased serum
levels of lactate dehydrogenase (LDH) and creatine phosphokinase and cardiac histopathological changes
including separated muscle fibers and inflammatory cells were observed in male rats administered 100
mg/kg/day γ-HCH by gavage in olive oil for 30 days (Vijaya Padma et al. 2013). Rats receiving gavage
doses of 3 mg/kg/day γ-HCH for 6 weeks exhibited tachycardia, increased blood pressure and plasma
calcium levels, an increase in myocardial calcium influx, and decreased calcium-potassium-ATPase
HEXACHLOROCYCLOHEXANE (HCH) 109
2. HEALTH EFFECTS
activity. Electrocardiographic changes included increased ST segment and T-wave amplitude and
reduced R-R interval and P-wave (Anand et al. 1995). Rats exposed to γ-HCH in utero exhibited
alterations in cardiac electrophysiology and histopathology; see Section 2.17 for details. Fitzhugh et al.
(1950) reported no histopathology findings in the hearts of rats given γ-HCH in feed at doses up to
140 mg/kg/day for 10 months or 30 mg/kg/day for 2 years.
Mechanisms. Oxidative stress may contribute to the cardiovascular effects of γ-HCH. Lipid peroxidation
was increased and antioxidant enzyme activities (superoxide dismutase, catalase, glutathione peroxidase,
and glutathione) were reduced in cardiac tissue from male rats treated with 8.8 mg/kg/day γ-HCH via
gavage for 3 weeks (Kamal El-Dein et al. 2016). Serum lipid and creatine phosphokinase levels were
also increased at this dose. Pre-treatment with the antioxidant α-lipoic acid attenuated the γ-HCH-
induced effects on serum lipids, CPK, lipid peroxidation and antioxidant enzyme activities in the heart.
Technical HCH or Unspecified Isomers of HCH. Kashyap (1986) reported electrocardiogram (ECG)
abnormalities in 15% of 45 factory workers involved in the production of technical-grade HCH; exposure
concentrations were not reported, and dermal exposure may have occurred. Technical HCH administered
to rats in feed did not induce cardiac histopathology changes at doses up to 70 mg/kg/day for 6 months or
9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
2.6 GASTROINTESTINAL
Data on gastrointestinal effects in humans were limited to γ-HCH. No studies were located regarding
gastrointestinal effects in animals following dermal exposure to any of the HCH isomers.
α-HCH. There were no microscopic gastrointestinal lesions in rats given α-HCH via the diet at doses up
to 70 mg/kg/day for ~9 months or up to 9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
β-HCH. Dietary administration of β-HCH doses up to 70 mg/kg/day for up to 10 weeks or up to
9 mg/kg/day for 2 years did not result in histopathology changes in the gastrointestinal tracts of rats
(Fitzhugh et al. 1950).
γ-HCH (Lindane) Lindane exposures reported to the Texas poison control network between 1998 and
2002 were reviewed by Forrester et al. (2004). Ingestion was the primary exposure route (79%), and
reported symptoms included vomiting, nausea, and abdominal pain (doses were not specified). Vomiting
HEXACHLOROCYCLOHEXANE (HCH) 110
2. HEALTH EFFECTS
and diarrhea occurred in a child (Ramchander et al. 1991) and a woman (Hall and Hall 1999) who were
exposed to 1% γ-HCH applied to the skin to treat rash or scabies.
Rats exposed to 5 mg/m
3
γ-HCH aerosol for 6 hours/day, 7 days/week exhibited persistent diarrhea
beginning after 2 weeks of exposure and continuing for nearly 3 weeks; exposure to 1 mg/m
3
did not
induce diarrhea (Oldiges et al. 1983). No gross necropsy or histopathology changes were observed in the
gastrointestinal tracts of these rats (Oldiges et al. 1983). In mice exposed to concentrations up to
5 mg/mg
3
for 14 weeks, no gastrointestinal effects were noted, and there were no gross or microscopic
changes to the gastrointestinal tract at necropsy (Klonne and Kintigh 1988). Microscopic examination of
the stomach, small intestine, and colon showed no treatment-related changes in rats given γ-HCH via feed
for 10 months at up to 140 mg/kg/day or 2 years at up to 30 mg/kg/day (Fitzhugh et al. 1950).
Technical HCH or Unspecified Isomers of HCH. Fitzhugh et al. (1950) did not observe any effects of
treatment on the gastrointestinal tract of rats given technical HCH via diet for 6 months (up to
70 mg/kg/day) or 2 years (up to 9 mg/kg/day).
2.7 HEMATOLOGICAL
Epidemiological Studies. In a cross-sectional study of adolescents and adults living near a former HCH
production facility in Brazil, an increase in eosinophilia was associated with serum β-HCH levels (Freire
et al. 2015). The results of a case-control study showed a positive association between serum levels of
α-HCH and childhood aplastic anemia, but no association with serum β- or γ-HCH (Ahamed et al. 2006).
α-HCH. Dietary administration of α-HCH doses up to 70 mg/kg/day for up to 9 months or up to
9 mg/kg/day for 2 years did not result in histopathology changes in the spleen or bone marrow of rats
(Fitzhugh et al. 1950).
β-HCH. Exposure to β-HCH at doses of 22.5–25 mg/kg/day in the diet for 13 weeks in rats resulted in
statistically significant decreases in numbers of red blood cells and white blood cells, as well as reduced
hemoglobin and packed cell volume values (Van Velsen et al. 1986). At this dose, 50% of the exposed
animals were sacrificed moribund. Extramedullary hematopoiesis was observed in males and females
surviving for 13 weeks at 23-26 mg/kg/day, but not in the early decedents. There were no histopathology
findings in the spleen or bone marrow of rats given β-HCH in the diet for up to 10 weeks at 70 mg/kg/day
HEXACHLOROCYCLOHEXANE (HCH) 111
2. HEALTH EFFECTS
or 2 years at 9 mg/kg/day (Fitzhugh et al. 1950). No other data on hematological effects in animals
exposed to β-HCH were located.
γ-HCH (Lindane). Hematological effects have been reported in case reports of humans following
exposure to γ-HCH. Hypochromic anemia was reported in a 2.5-year-old boy who was exposed to
γ-HCH in a home in which a pesticide vaporizer was operated. Air γ-HCH concentrations measured in
the basement and living room of the house were 2.4–5.5 μg/m
3
; however, the actual concentration the
child was exposed to, and the duration of exposure were not determined (Morgan et al. 1980). Aplastic
anemia was reported in a boy exposed to γ-HCH used as an insecticide in his home and in a man exposed
at work (Rugman and Cosstick 1990). In both cases, the anemia was reversible and was not present in
other family members. The levels and routes of exposure are not known, although they are presumed to
be inhalation and dermal. Aplastic anemia was also documented in a man who applied γ-HCH to his skin
for 3 weeks for treatment of scabies (Rauch et al. 1990).
A woman who committed suicide by drinking γ-HCH was found to have disseminated intravascular
coagulation (a condition where abnormal blood clotting occurs in blood vessels throughout the body)
during the period when serum γ-HCH levels were elevated (Sunder Ram Rao et al. 1988). Reduced
hemoglobin and hematocrit values and a nearly complete absence of red blood cell precursors in bone
marrow were reported in a 2-year-old boy exposed to a family dog that was dipped regularly in mange
treatment containing 12% γ-HCH (Vodopick 1975).
In an inhalation study of rats exposed to 5 mg/m
3
γ-HCH aerosol for 90 days, bone marrow myelogram
changes were observed, including increased reticulocytes in males and females, increased stem cells and
myeloblasts in males, and decreased lymphocytes in females (Oldiges et al. 1983). No changes to blood
parameters were noted in this study (Oldiges et al. 1983). Mice exposed to concentrations of γ-HCH up
to 5 mg/m
3
for 14 weeks exhibited no hematological changes or effects on bone marrow smears (Klonne
and Kintigh 1988).
Hong and Boorman (1993) reported significant suppression in bone marrow cellularity, erythrocyte
precursors, and granulocyte-macrophage progenitor cells, and residual progenitor cell damage in male
B6C3F
1
mice given 20 or 40 mg γ-HCH/kg/day by gavage in corn oil for 3 days. In a similar experiment,
dose-dependent decreases in bone marrow cellularity, granulocyte-macrophage progenitor cells, and
pluripotent bone marrow stem cells were noted following 10 days of exposure to 10 or 20 mg
γ-HCH/kg/day (Hong and Boorman 1993). No effects on blood leukocytes were reported in male mice
HEXACHLOROCYCLOHEXANE (HCH) 112
2. HEALTH EFFECTS
administered 0.25 mg/kg/day γ-HCH by gavage in oil for 61 days (Tewari et al. 2017). No hematological
effects were noted in rats exposed to 10 mg γ-HCH/kg/day in the diet for 12 weeks (Suter 1983) or in
beagle dogs exposed to 2.92 mg/kg/day γ-HCH in the diet for 104 weeks (Rivett et al. 1978). No
histopathology changes were observed in the spleen or bone marrow of rats exposed to γ-HCH in feed for
up to 10 months at 140 mg/kg/day or up to 2 years at 30 mg/kg/day (Fitzhugh et al. 1950).
Technical HCH or Unspecified Isomers of HCH. The results of a case-control study of childhood
aplastic anemia indicated no association between this disease and serum levels of total-HCH (including
α-, β-, γ-, and δ-HCH) (Ahamed et al. 2006). Hematological abnormalities, including alterations in
polymorphonuclear leukocyte count, lymphocyte count, reticulocyte count, and prothrombin time have
been reported following chronic human occupational exposure to γ-HCH (Brassow et al. 1981). Exposure
concentrations were not specified in these studies and concomitant dermal exposure probably occurred.
Granulocytopenia, aplastic anemia, and pancytopenia have been reported in several case reports of
individuals following exposure to γ-HCH and other pesticides such as DDT in the home, during the
handling of the pesticide, or from a nearby formulating plant (Danopoulos et al. 1953; Friberg and
Martensson 1953; Gewin 1939; Loge 1965; Mendeloff and Smith 1955). Exposure concentrations were
not reported, dermal exposure was likely, and in many cases, there was concomitant exposure to other
pesticides. Excessive dermal exposure to HCH was reported to result in aplastic anemia and bone
marrow hyperplasia in a woman who bathed her dog once a week for 2 years with a preparation that
reportedly contained 2% HCH (Woodliff et al. 1966).
No hematological effects were seen in rats following oral exposure to 60 mg/kg/day technical-grade HCH
for 30 days (Dikshith et al. 1989a). In a study evaluating the influence of vitamin A on the toxicity of
technical-grade HCH, significant decreases in total white blood cell counts and clotting time were
reported in rats fed vitamin A-deficient diets containing technical-grade HCH at a dose level of
90 mg/kg/day for 7 weeks (Joseph et al. 1992c). In a similar study, rats fed a vitamin A-supplemented
diet containing the same dose level of technical-grade HCH for 7 weeks exhibited a significant reduction
in total white blood cell count, but not red blood cell count (Joseph et al. 1992c). No treatment-related
histopathology changes were seen in the spleen or bone marrow of rats after 6 months of dietary exposure
to technical HCH at 70 mg/kg/day or 2 years of dietary exposure at 9 mg/kg/day (Fitzhugh et al. 1950).
HEXACHLOROCYCLOHEXANE (HCH) 113
2. HEALTH EFFECTS
2.8 MUSCULOSKELETAL
Data regarding musculoskeletal effects in humans were limited to oral exposure to γ-HCH (see γ-HCH
subsection below).
α-HCH. There were no microscopic lesions in the leg muscles or bones of rats given α-HCH via the diet
at doses up to 70 mg/kg/day for ~9 months or up to 9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
β-HCH. Dietary administration of β-HCH at doses up to 70 mg/kg/day for up to 10 weeks or up to
9 mg/kg/day for 2 years did not induce histopathology changes in the leg muscles or bones of rats
(Fitzhugh et al. 1950).
γ-HCH (Lindane) Ingestion of a single dose of approximately 15–30 mL γ-HCH powder (was associated
with seizures and limb muscle weakness and necrosis in an adult man (Munk and Nantel 1977); a muscle
biopsy conducted 15 days after ingestion showed no evidence of denervation or neuropathy. Widespread
striatal muscle necrosis was seen in a woman who died 11 days after intentionally ingesting 8 ounces of a
20% γ-HCH solution (Sunder Ram Rao et al. 1988). A suicidal 21-year-old male developed
rhabdomyolysis as indicated by muscle pain, muscle tenderness, proteinuria, myoglobinuria, elevated
serum levels of aspartate aminotransferase (AST), potassium, creatinine, and creatinine protein kinase
(CPK) 1–3 days following ingestion of a single unknown dose of γ-HCH (Shah et al. 2013). The man
recovered after 3 weeks of clinical care.
Microscopic examination of skeletal muscle in mice and rats (as well as femur in mice) exposed by
inhalation to concentrations up to 5 mg/m
3
for 13–14 weeks did not show any treatment-related effects
(Klonne and Kintigh 1988; Oldiges et al. 1983). Decreased medullary area in the femur bone was found
in young rats treated with 20 mg/kg/day of γ-HCH by gavage for 10 weeks (Andrews and Gray 1990).
Fitzhugh et al. (1950) reported no treatment-related histopathology changes in the leg muscles or bones of
rats exposed via diet for 10 months at doses up to 140 mg/kg/day or for 2 years at 30 mg/kg/day.
Technical HCH or Unspecified Isomers of HCH. No microscopic lesions were observed in the leg
muscles or bones of rats given dietary technical HCH doses up to 70 mg/kg/day for 6 months or
9 mg/kg/day for 2 years (Fitzhugh et al. 1950).

HEXACHLOROCYCLOHEXANE (HCH) 114
2. HEALTH EFFECTS
2.9 HEPATIC
Epidemiological Studies. Human epidemiological data pertaining to HCH exposure and hepatic effects
(see Table 2-10) are limited to two cross-sectional studies of β-HCH (Arrebola et al. 2014; Freire et al.
2015). No association between serum lipids and serum or adipose concentrations of β-HCH was
observed in a study of 298 non-surgical cancer patients in Spain (Arrebola et al. 2014). In a study of
adolescents and adults residing near a former HCH production facility in Brazil, serum β-HCH was
associated with increased risk of elevated total and indirect serum bilirubin in females, but not in males
(Freire et al. 2015). There were no associations between serum β-HCH and risk of elevated direct serum
bilirubin or serum enzymes (Freire et al. 2015).
Table 2-10. Summary of Epidemiological Studies of β-Hexachlorocyclohexane
Exposure and Hepatic Effects
Reference, study type, and
population
Outcome evaluated
Biomarker
Mean
concentration
Result
Arrebola et al. 2014
Cross-sectional, 298 noncancer
surgical patients >16 years old,
Spain
Measures of serum lipids
(total triglycerides, HDL,
LDL, and total cholesterol)
Serum or
adipose
19.60±28.74 ng/g
lipid (biomarker
not reported)
↔
Freire et al. 2015
Cross-sectional, 339 males and
375 females, age >14 years
residing near former HCH
production facility, Brazil
Elevated total and
indirect serum bilirubin
Serum
Males: 3.72 µg/g
lipid
Females:
3.09 µg/g lipid
M: ↔
F: ↑
Elevated direct serum
bilirubin
M: ↔
F: ↔
Elevated serum AST, ALT,
GGT
M: ↔
F: ↔
↑ = association with increase; ↔ = no association; ALT = alanine aminotransferase; AST = aspartate
aminotransferase; GGT = γ-glutamyl transferase; HCH = hexachlorocyclohexane; HDL = high-density lipoprotein;
LDL = low density lipoprotein
α-HCH. Hepatic effects have been observed in rats, mice, and hamsters after intermediate- and chronic-
duration oral exposures to α-HCH. Increases in absolute and relative liver weight, coupled with
hepatocellular hypertrophy and/or hyperplasia, have been observed in F344 rats at doses of at least
20 mg/kg/day for 28 days (Sumida et al. 2007) and in Wistar rats at doses of 45 mg/kg/day for 24 weeks
(Nagasaki et al. 1975) or 35 mg/kg/day for 48 weeks (Ito et al. 1975). Rats receiving 60–70 mg/kg/day
α-HCH in the diet as part of a chronic study died early (mean survival 35.9 weeks compared with
58.3 weeks in controls); at necropsy, these animals exhibited 2-fold increases in liver weight and
histopathology changes of moderate severity, including focal necrosis and fatty degeneration (Fitzhugh et
HEXACHLOROCYCLOHEXANE (HCH) 115
2. HEALTH EFFECTS
al. 1950). Hypertrophied liver cells were reported in mice fed 18 mg/kg/day α-HCH for 24 weeks (Ito et
al. 1973). More severe effects, including hepatomegaly, bile duct proliferation, oval cells, nodular
hyperplasia, megalocytosis, and a doubling of liver weight, were observed in several strains of mice
(DDY, ICR, DBA/2, C57BL/6, C3H/He, and HPBC57BL) given feed containing 90 mg/kg/day for at
least 21 weeks (Nagasaki et al. 1975; Tryphonas and Iverson 1983). This dose (90 mg/kg/day) yielded a
significant increase in hepatocellular carcinomas in all but the C57BL/6 strain of mouse (Nagasaki et al.
1975; Tryphonas and Iverson 1983). Nagasaki et al. (1975) also conducted an experiment using male
Syrian hamsters exposed to α-HCH in the diet for 24 weeks. In hamsters, a 38% increase in relative liver
weight, with increased liver cell hypertrophy, was observed at 45 mg/kg/day.
Chronic (107 weeks) exposure to α-HCH in feed resulted in dose-related increases in the severity of liver
histopathology changes in rats (Fitzhugh et al. 1950). Very slight to slight microscopic damage in the
liver, along with ≥32% increase in relative liver weight, was seen at ≥4 mg/kg/day (Fitzhugh et al. 1950).
The microscopic changes were described as “characteristic of certain chlorinated cyclic compounds”
without further detail. Rats at the highest dose in this study (60–70 mg/kg/day) exhibited reduced
survival (mean <1 year); these animals exhibited more severe liver effects, as described above with the
intermediate-duration studies.
Both rats and mice exposed to α-HCH have developed liver cancers. Hepatocellular carcinomas were
reported in rats administered 70 mg/kg/day in the diet for 48 or 72 weeks (Ito et al. 1975), and liver
cancers were observed in mice given 18–90 mg α-HCH/kg/day for 16–36 weeks (Hanada et al. 1973; Ito
et al. 1973, 1976; Nagasaki et al. 1975; Tryphonas and Iverson 1983; Tsukada et al. 1979) (see
Section 2.19). Hamsters exposed to 45 mg/kg/day for 24 weeks did not develop liver tumors (Nagasaki et
al. 1975).
Mechanisms: Little information is available on potential mechanisms of α-HCH-induced hepatotoxicity
but is possible that oxidative stress and/or mitotic disturbances may be involved. Administration of
1.8 mg/kg/day α-HCH in the diet to rats for 15 or 30 days resulted in increases in lipid peroxidation and
microsomal superoxide production in the liver (Barros et al. 1991). In male Donryu rats, a 3-week dietary
exposure to α-HCH resulted in mitotic disturbances including an increased mitotic rate and an increased
frequency of polyploid hepatic cells (Hitachi et al. 1975).
HEXACHLOROCYCLOHEXANE (HCH) 116
2. HEALTH EFFECTS
β-HCH. Hepatic effects have been observed in intermediate-and chronic-duration studies of β-HCH in
rats and mice exposed via the diet. Moderate to marked liver damage, including fatty degeneration and
focal necrosis, was reported in rats that died prematurely (within 10 weeks of study initiation) after
dietary exposure to doses of 60–70 mg/kg/day (Fitzhugh et al. 1950). In a comprehensive 13-week study
of rats (Van Velsen et al. 1986), dose-related increases in hepatic effects were seen at all doses
(≥0.18 mg/kg/day) in males. At 0.18 mg/kg/day, the effects consisted of hyalinization of centrilobular
cells; at ≥4.5 mg/kg/day, increased mitoses in females, periportal fat accumulation in both sexes, and
isolated instances of focal necrosis in males were observed. Relative liver weights were increased by
10% or more at 1.0 mg/kg/day in females and 4.5 mg/kg/day in males (Van Velsen et al. 1986). At the
highest dose in this study (22.5–25 mg/kg/day), liver weights were doubled; at this dose, 50% of the
animals were sacrificed moribund before the end of the study (Van Velsen et al. 1986). Liver cell
hypertrophy was reported in rats fed 35 mg/kg/day in the diet for 48 weeks or 70 mg/kg/day for 24 weeks
(Ito et al. 1975). In mice, exposure for 24 weeks to 18 mg/kg/day in the diet resulted in an 18% increase
in liver weight, and liver cell hypertrophy at higher doses (≥45 mg/kg/day) (Ito et al. 1973). Mice
exposed to 50–60 mg/kg/day in the diet for 32 weeks exhibited hepatic foci of degeneration (Hanada et al.
1973).
Chronic dietary exposure of rats to lower doses of β-HCH resulted in increased liver weight and dose-
related histopathology changes in the liver (Fitzhugh et al. 1950). At 0.7–0.9 mg/kg/day, a 34% increase
in liver weight and slight microscopic changes described as “characteristic of certain chlorinated cyclic
compounds” were observed.
Liver tumors were not reported in mice exposed to β-HCH for 24–32 weeks (Hanada et al. 1973; Ito et al.
1973) or in rats exposed for 24–48 weeks (Ito et al. 1975); however, Thorpe and Walker (1973) reported
liver cancer in mice fed 34 mg/kg/day for 26 months.
γ-HCH (Lindane). Two case reports of intentional ingestion of γ-HCH have documented increases in
serum liver enzymes; neither report provided an estimate of the associated dose of γ-HCH. A 30-year-old
male farmer from rural India ingested a single dose of approximately 50 mL of 2% γ-HCH solution in a
suicide attempt (Paul et al. 2013). Six hours after the ingestion, the man went to the emergency
department where initial examination and laboratory tests were normal. Nausea was noted on the second
day, and abdominal tenderness and increased serum levels of bilirubin, AST, and alanine
aminotransferase (ALT) were observed on day 5. Treatment with hemodialysis for acute kidney injury
spontaneously reduced the hepatic enzymes and the man recovered after 3 weeks. A 56-year-old man
HEXACHLOROCYCLOHEXANE (HCH) 117
2. HEALTH EFFECTS
ingested approximately 12 ounces of an insecticide containing 20% γ-HCH intentionally in a suicide
attempt (Wiles et al. 2015). Hepatic enzymes (AST, ALT, alkaline phosphatase [ALP], and γ-glutamyl
transferase [GGT]) were increased after 3 days. The man died on day 12 after committing suicide by
other means; at autopsy, no gross or microscopic abnormalities were noted.
Rats exposed to γ-HCH aerosol (5 mg/m
3
for 6 hours/day) exhibited increased hepatic cytochrome P450
concentration after 90 consecutive days, but this level returned to control values after a 4-week recovery
period (Oldiges et al. 1983). Statistically significant, but modest, increases in absolute and relative liver
weights (up to 12%) were observed at this exposure concentration, but there were no concomitant effects
of treatment on serum chemistry or liver histopathology in the rats (Oldiges et al. 1983). Mice exposed to
the same concentration 5 days/week for 14 weeks exhibited no changes in clinical chemistry, liver
weights, or liver histology (Klonne and Kintigh 1988).
Hepatic effects have been documented in rats and mice exposed to γ-HCH via oral administration for
acute, intermediate, and chronic durations. At lower doses, effects include increased serum enzymes,
increased liver weight, and hepatocellular hypertrophy. Higher doses and/or longer exposure durations
result in liver effects of increasing severity, including vacuolar degeneration, necrosis, and congestion.
Acute-duration oral studies in rats show increases in liver weight as well as histopathology changes in
animals exposed to γ-HCH. In male Wistar rats, a single gavage dose of 60 mg/kg/day resulted in marked
centrilobular hepatic necrosis (Singh and Sharma 2011), and fatty degeneration, vacuolation, and necrosis
were observed after gavage doses of 5 mg/kg/day for 3 days (Hfaiedh et al. 2012). Ultrastructural
changes observed in the liver of Sprague-Dawley rats (sex not specified) after 2 days of exposure to
30 mg/kg/day in feed were reduced number of cells per field; increased cell, nucleus, and nucleolus size;
and slight cellular disorganization (Ali and Shakoori 1998). Although no histopathological examinations
were performed, no significant increase in liver weight was noted in Sprague-Dawley rats exposed to
10 mg γ-HCH/kg/day for a minimum of 4 days (Joy et al. 1982). A significant increase (15% relative to
controls) in absolute, but not relative, liver weight was observed in rats exposed to 15 mg γ-HCH/kg/day
for 5 days (Parmar et al. 2003). A significant, but modest, increase (6% relative to controls) in relative
liver weight was observed in a small group of male F344 rats given 10 mg/kg/day γ-HCH by gavage for
7 days, but not in a group similarly exposed for 14 days (Sumida et al. 2007). Histopathology evaluations
of these animal were not reported.
HEXACHLOROCYCLOHEXANE (HCH) 118
2. HEALTH EFFECTS
Increases in serum enzymes indicative of hepatotoxicity have been reported in rats exposed once to
12 mg/kg/day (Attia et al. 2011) or for 3 days to 5 mg/kg/day (Hfaiedh et al. 2012). Male Wistar rats fed
13.5 mg γ-HCH/kg/day in their diet for 12 days exhibited decreased activities of liver enzymes (malic
enzyme, glucose-6-phosphate dehydrogenase, phosphogluconate dehydrogenase, citrate cleavage enzyme,
and fatty acid synthase) and increased levels of serum triglycerides (Boll et al. 1995). Significantly
increased liver microsomal 7-ethoxycoumarin-O-dealkylase activity was found in Osborne-Mendel rats
exposed to 11.2 mg/kg/day γ-HCH and in CF1 and B6C3F1 strain mice exposed to 23.6 and
50.5 mg/kg/day in the diet for 3 days (Oesch et al. 1982).
The most common histopathology finding in the livers of rats and mice exposed to oral doses of γ-HCH
for intermediate durations is hepatocellular hypertrophy. A dose-dependent increased incidence of liver
centrilobular hypertrophy was reported in Wistar rats dosed with ≥0.4 mg γ-HCH/kg/day in the diet for
12 weeks (Suter 1983). In multigeneration reproductive toxicity studies in rats, hepatocellular
hypertrophy reportedly occurred at increased incidence in F0 and F1 male and female Sprague-Dawley rat
parents exposed to 3–6 mg/kg/day (Matsuura et al. 2005) and in F1 male CD rat parents exposed to
1.7 mg/kg/day (EPA 1991a). In both studies, no hepatic effects were seen at a dose of about 1 mg/kg/day
γ-HCH (EPA 1991a; Matsuura et al. 2005). Rats exposed to 35 mg/kg/day γ-HCH for 48 weeks in the
diet exhibited hepatocellular hypertrophy (Ito et al. 1975). Similar findings were reported in a study of
Wistar rats given ≥7–8 mg γ-HCH/kg/day in the diet for up to 52 weeks, in which a dose-related increase
in periacinar hepatocytic hypertrophy was seen (Amyes 1990). In mice, administration of 90 mg
γ-HCH/kg/day in the diet for 24 weeks was reported to result in centrilobular hypertrophy and a
significant increase (33%) in relative liver weight (Ito et al. 1973).
In other intermediate-duration studies, more severe lesions have been noted in the liver. An early study
(Ortega et al. 1957) reported the development of liver cell “lipospheres” in rats fed 2.5 mg γ-HCH/kg/day
in the diet for 32 weeks; in older literature, these changes were described as spherical cytoplasmic
inclusions of a fatty nature. Ali and Shakoori (1998) reported ultrastructural changes in the livers of
Sprague-Dawley rats exposed for 15 days to a dose of 18 mg/kg/day in food. Findings observed in the
treated animals included reduced number of cells per field; increased cell, nucleus, and nucleolus size;
vacuoles in the cytoplasm; and apparent fatty degeneration. At a dose of 20 mg/kg/day γ-HCH
administered by daily gavage for 30 days, liver histopathology changes in Sprague-Dawley rats included
megalocytosis, vacuolar degeneration, venous and sinusoidal congestion, and lymphocytic infiltration
(Fatih Fidan et al. 2008). At 100 mg/kg/day for the same duration, the livers of Wistar rats showed
vacuolar degeneration of hepatocytes and marked degradation of the central vein (Vijaya Padma et al.
HEXACHLOROCYCLOHEXANE (HCH) 119
2. HEALTH EFFECTS
2011). Focal degeneration of hepatocytes was noted in rabbits given γ-HCH at a dose of 7 mg/kg/day by
gavage for 4 weeks (Grabarczyk et al. 1990; Kopec-Szlezak et al. 1989).
Two intermediate-duration studies reported no liver effects in rats exposed to γ-HCH. Sumida et al.
(2007) observed no treatment-related hepatic effects (clinical chemistry, liver weight, or histopathology)
in F344 rats exposed for 28 days at 10 mg/kg/day via gavage. The lack of effect in this study may
indicate a lower sensitivity of F344 rats to hepatic effects of this isomer, but also could be a reflection of
the very small numbers of animals tested (four males per group). In an older study focused on
neurotoxicity testing, groups of eight Wistar rats given 50 mg/kg/day for 40 days in feed exhibited
increased liver weight, but normal liver function test results and histology (Desi 1974).
In addition to histopathology changes, intermediate-duration oral exposure to γ-HCH has resulted in
increased liver weights. Rats exposed to 2.5 mg γ-HCH/kg/day for 21 days showed a significant increase
(13% higher than controls) in absolute, but not relative, liver weight (Parmar et al. 2003). Treatment of
female rats with ≥10.6 mg γ-HCH/kg/day or of male and female mice with ≥21.1 mg/kg/day in the diet
for 3 months resulted in significant increases in absolute and relative liver weights; histopathological
examinations were not performed (Oesch et al. 1982). Increased absolute and relative liver weights
occurred at doses (≥3–5 mg/kg/day) associated with increased incidences of hepatocellular hypertrophy in
parental animals exposed to γ-HCH in the diet in a 2-generation reproduction toxicity study (Matsuura et
al. 2005). Exposure of dd strain mice to dietary doses of 90 mg/kg/day for 24 weeks resulted in a 33%
increase in relative liver weight (Ito et al. 1973).
Increased liver weights have also been reported in offspring of rats exposed to γ-HCH during gestation
and/or lactation (Srinivasan et al. 1991). Additional details are provided in Section 2.17 (Developmental).
Increases in serum enzymes and lipids indicating hepatic effects have been observed in rats and rabbits
exposed for intermediate durations to γ-HCH. Significant increases in serum AST, ALT, GGT, ALP,
and/or LDH were observed in Wistar rats exposed to 100 mg/kg/day for 4 weeks (Etim et al. 2006; Vijaya
Padma et al. 2011). Increased serum levels of AST, LDH, cholesterol, total triglycerides, free fatty acids,
and total phospholipids were observed in male Sprague-Dawley rats administered 8.8 mg/kg/day γ-HCH
by gavage in water for 3 weeks (Kamal El-Dein et al. 2016). Rabbits treated with 4.21 mg γ-HCH/kg/day
by gavage for 28 days exhibited significant increases in plasma ALP and ALT activities immediately
following initiation of dosing; these activities returned to control levels by day 14 (Cerón et al. 1995).
HEXACHLOROCYCLOHEXANE (HCH) 120
2. HEALTH EFFECTS
The plasma level of AST activity also increased immediately following dosing and remained elevated up
to 7 days postexposure (day 35) (Cerón et al. 1995).
Oral exposure to γ-HCH for intermediate durations has resulted in induction of hepatic cytochrome P450
levels in rats and mice. Significant increases in hepatic microsomal cytochrome P450 levels were found
in Wistar rats fed diets containing 1.8 mg/kg/day γ-HCH for 15 or 30 days (Barros et al. 1991). A dose-
and time-dependent increase of P450 and P450-dependent enzyme levels was observed in the liver of rats
exposed to γ-HCH (Parmar et al. 2003). P450 content was significantly increased in rats exposed to
10 mg γ-HCH/kg/day for 5 days, and in rats exposed to 2.5 mg γ-HCH/kg/day for 15 and 21 days. There
was no significant increase in P450 content in rats exposed to <10 mg γ-HCH/kg/day for 5 days. Several
P450-dependent enzymes, 7-ethoxyresorufin-O-deethylase (EROD), 7-pentoxyresorufin-O-dealkylase
(PROD), and N-nitrosodimethylamine demethylase (NDMA-d), were significantly increased in rats
exposed to 5 mg γ-HCH/kg/day for 5 days or 2.5 mg γ-HCH/kg/day for 15 and 21 days (Parmar et al.
2003). Increases in liver microsomal mixed-function oxidase activity were observed in rats exposed to
≥10.6 mg γ-HCH/kg/day and mice exposed to ≥21.1 mg/kg/day in the diet for 3 months (Oesch et al.
1982).
Hepatotoxicity has been documented in animals exposed by oral administration to γ-HCH for chronic
durations. After Sprague-Dawley rats were exposed for 18 months to γ-HCH at a dose of 9 mg/kg/day in
feed, the following microscopic findings were observed in the liver: increased cell, nucleus, and nucleolus
size; extensive cytoplasmolysis; slight cytoplasmic degeneration; and increasing nuclear distortion (Ali
and Shakoori 1998). Chronic exposure of rats to 7–9 mg/kg/day γ-HCH in the diet for 107 weeks resulted
in increased liver weight (35% higher than controls) and very slight microscopic liver damage described
as “characteristic of certain chlorinated cyclic compounds” (Fitzhugh et al. 1950). At higher doses, liver
necrosis and fatty degeneration were observed (Fitzhugh et al. 1950). Male CD-1 mice exposed to
20.5 mg/kg/day γ-HCH via feed for 78 weeks exhibited centrilobular hepatocyte hypertrophy (EPA
2000a). No liver lesions were observed by light or electron microscopy in NMRI mice given
~8 mg/kg/day for 80 weeks (Herbst et al. 1975; Weisse and Herbst 1977). At gross necropsy, the livers of
dogs exposed to 2.9 mg/kg/day for 104 weeks were noted to be dark, but no histopathology changes were
reported (Rivett et al. 1978).
Increased incidences of liver tumors (hepatomas, hepatocellular carcinomas) have been observed in mice
exposed via diet to γ-HCH for intermediate and chronic durations at γ-HCH doses as low as
HEXACHLOROCYCLOHEXANE (HCH) 121
2. HEALTH EFFECTS
13.6 mg/kg/day (Hanada et al. 1973; NCI 1977; Thorpe and Walker 1973; Wolff et al. 1987). These
studies are discussed further in Section 2.19 (Cancer).
One study of dermal exposure to γ-HCH reported also reported liver effects. Centrilobular hepatocellular
hypertrophy was reported in male and female rats exposed to γ-HCH ≥60 mg /kg/day by dermal
application for 6 hours/day, 5 days/week, for 13 weeks (EPA 1988a).
Mechanisms. There is some evidence that oxidative stress may contribute to the hepatic effects of
γ-HCH. Significant increases in hepatic microsomal superoxide anion production and cytoplasmic
superoxide dismutase activity and lipid peroxidation were found in the livers of Wistar rats fed diets
containing 1.8 mg/kg/day γ-HCH for 15 or 30 days (Barros et al. 1991). Groups of 10 male rats (strain
not reported) were administered a single dose of γ-HCH (98% purity) in corn oil at a dose of 0 or
12 mg/kg and then sacrificed 24 hours later in a study aimed at evaluating the ameliorating effects of co-
treatment with the antioxidants, nigella sativa oil and omega 3 fatty acids. Co-treatment with nigella
sativa oil and omega 3 fatty acids attenuated the effects of γ-HCH on lipid parameters, clinical chemistry
parameters, lipid peroxidation, and antioxidant enzyme activities (Attia et al. 2011). The mitigating
effects of nigella sativa oil and omega 3 fatty acids could have resulted from their antioxidant activity or
from effects on the absorption or metabolism of γ-HCH.
δ-HCH. Liver cell hypertrophy was observed in rats fed with 70 mg/kg/day of δ-HCH in the diet for
48 weeks (Ito et al. 1975). Similarly, mice exposed for 24 weeks to 90 mg/kg/day δ-HCH in feed
exhibited a 23% increase in relative liver weight and centrilobular hypertrophy (Ito et al. 1973).
Unspecified HCH Isomers or Mixtures of HCH Isomers. In humans, statistically significant increases in
the blood levels of the enzymes LDH (33%), leucine aminopeptidase (45%), and γ-glutamyl
transpeptidase (174%) were reported in 19 individuals occupationally exposed to technical-grade HCH
for over 10 years in an HCH-formulating plant (Kashyap 1986) compared to a control group of workers.
The HCH isomer concentrations in serum were 10-fold higher in the exposed group than in the control
group of workers. Both inhalation and dermal exposure probably occurred.
Increases in liver weight, serum enzymes and lipids, and liver histopathology changes have been observed
in animals exposed to technical-grade HCH by oral administration for acute durations. Technical-grade
HCH was reported to cause increases in liver weight and serum enzyme activities (e.g., ALP,
aminotransferases) in male Swiss mice given 72 mg/kg in the diet for 2 weeks (Ravinder et al. 1989).
HEXACHLOROCYCLOHEXANE (HCH) 122
2. HEALTH EFFECTS
Other effects seen in the mice included significantly increased serum triglycerides, phospholipids, and
cholesterol, as well as hypertrophy of hepatocytes with enlargement of nuclei, centrilobular degeneration,
and focal necrosis (Ravinder et al. 1990). Statistically significant decreases in the liver activities of AST
and LDH were observed in pregnant mice administered a single dose of technical-grade HCH (5 mg/kg)
on GD 9 (Dikshith et al. 1990). Pregnant animals dosed with 25 mg/kg also experienced decreases in
liver ALT and ALP activity (Dikshith et al. 1990). Virgin mice administered a single dose of 5–
200 mg/kg technical-grade HCH had statistically significant decreases in liver activity of ALT and AST,
and increases in liver ALP activity were observed in the virgin mice at doses ≥25 mg/kg. However, with
the exception of decreased AST activity in pregnant mice, the dose-response relationships were
questionable (Dikshith et al. 1990). There were also no corresponding pathological changes in the livers
of the treated mice. However, at a higher dose (50 mg/kg/day) of technical-grade HCH administered to
mice for 1, 5, or 15 days by oil gavage, congestion of hepatic portal vessels and central vein, swollen
hepatic cells with vacuolar or parenchymatous degeneration, and fatty changes in periportal and
centrilobular cells were observed (Philip et al. 1989).
Liver enzyme level changes were seen in male, but not female, rats given 5 or 25 mg/kg/day by gavage
for 90 days; at these doses, there were significant mortalities (Dikshith et al. 1991b). A 65% decrease in
liver weight, decreased liver AST and LDH activities, and increased ALP activity were noted in male rats
given 60 mg/kg by gavage for 30 days, but animals had normal liver histology (Dikshith et al. 1989a).
Technical-grade HCH was reported to deplete the hepatic vitamin A content in male rats fed a diet
containing 90 mg/kg/day HCH for 7 weeks (Joseph et al. 1992b). No adverse hepatic effects were seen in
rats treated with 50 mg/kg/day technical-grade HCH for 30 days (Khanna et al. 1990) or in pigs exposed
to 0.8 mg/kg/day for 90 days (Wang et al. 2006). Mice fed diets containing 90 mg/kg/day of HCH for
8 months exhibited increased liver weight, glycogen accumulation, and decreased glucose-6-phosphatase
and fructose-1,6-di
-
phosphatase activities (Karnik et al. 1981). Enlargement of hepatocytes, nuclear
pyknosis, margination, and vacuolation were observed in rats fed 20 mg/kg/day technical-grade HCH in
the diet for 360 days (Dikshith et al. 1991a). Chronic dietary administration of technical-grade HCH to
rats at a dose of 4 mg/kg/day resulted in slight microscopic liver damage, which the study authors
described as “characteristic of certain chlorinated cyclic compounds” (Fitzhugh et al. 1950).
T
echnical-grade HCH was also reported to cause liver cancer in mice following exposure to
90 mg/kg/day in the diet for time periods ranging from 2 to 8 months (Bhatt and Bano 2009; Bhatt and
Nagda 2012; Karnik et al. 1981; Thakore et al. 1981; Trivedi et al. 2007, 2009) or exposure to 10–
50 mg/kg/day for 80–88 weeks (Kashyap et al. 1979; Munir et al. 1983) (see Section 2.19).
HEXACHLOROCYCLOHEXANE (HCH) 123
2. HEALTH EFFECTS
Hepatic effects have been seen after dermal exposure to technical-grade HCH. Alterations in liver
histopathology, including dilation of sinusoids, focal fatty inclusions, hypertrophy of hepatocytes,
thickened blood vessels, swelling, and proliferation of epithelial cells of bile ducts, was observed in
guinea pigs treated by dermal application of 100 mg/kg/day technical-grade HCH for 30 days (Dikshith et
al. 1978). The area of the abdomen on which the HCH was applied was not covered to prevent licking, so
oral exposure may also have occurred. In rabbits exposed to 25 mg technical-grade HCH/kg/day for
30 days, there were degenerative changes in hepatocytes along with increased liver and serum ALT and
ALP (Dikshith et al. 1989b). Liver cell hypertrophy, fatty degeneration, nuclear pyknosis, and focal and
diffuse necrosis were found in female rats treated with 100 mg/kg/day technical-grade HCH for 7–
30 days, but the time that it took for these lesions to occur, the severity of changes, and the numbers of
animals affected were not reported (Dikshith et al. 1991c).
2.10 RENAL
Epidemiological Studies. Very limited human epidemiological data on renal effects of HCH isomers are
available, as shown in Table 2-11. In a cohort study of 1,545 male pesticide applicators in Iowa and
North Carolina, no association was observed between self-reported pesticide exposure and chronic kidney
disease (Shearer et al. 2021). A cohort study of 31,142 wives of pesticide applicators in Iowa and North
Carolina followed for 15 years did not observe an association between self-reported pesticide exposure
and end-stage renal disease (Lebov et al. 2015). Case-control studies of chronic kidney disease (Ghosh et
al. 2017; Siddarth et al. 2014) did not observe associations with β-HCH in blood or serum, and results
were mixed for α- and γ-HCH. Ghosh et al. (2017) reported an association between blood levels of
γ-HCH and chronic kidney disease and no association for α-HCH, while Siddarth et al. (2014) reported
the converse (association for serum α-HCH and no association for γ-HCH). No association between
serum α-, β,- γ-, or δ-HCH and hyperuricemia (or increased serum uric acid) was noted in cross-sectional
studies of 453 adults in Spain (Arrebola et al. 2019) or 880 adults in South Korea (Seo et al. 2022).
α-HCH. Fitzhugh et al. (1950) reported kidney damage (nephritis tubular dilatation, hyaline tubular
casts, glomerular fibrosis or atrophy, pigment deposition) in rats fed 60–70 mg/kg/day α-HCH for an
average of 35.9 weeks; no such effects were observed in rats fed up to 9 mg/kg/day for 107 weeks.
β-HCH. Renal effects have also been noted in rats exposed to β-HCH in the diet, often at doses
associated with profound toxicity and/or death. Srinivasan et al. (1984) reported significantly increased

HEXACHLOROCYCLOHEXANE (HCH) 124
2. HEALTH EFFECTS
Table 2-11. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Renal Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Arrebola et al. 2019
Cross-sectional, 453 working adults,
median age 35 years, Spain
Hyperuricemia
α-HCH
Serum
<LOQ ng/g lipid (all)
NA
a
β-HCH
<LOQ (median)
0.12 (95
th
percentile)
↔
b
γ-HCH
<LOQ (median)
0.05 (95
th
percentile)
↔
b
Seo et al. 2022
Cross-sectional, 880 adults, ages 20–
80 years, South Korea
Serum uric acid
β-HCH
Serum
40.4 ng/g lipid
↔
α-HCH
1.24
↔
γ-HCH
1.82
↔
δ-HCH
0.17
↔
Shearer et al. 2021
Cohort, 1545 male pesticide applicators,
age ≥50 years, Iowa and North Carolina,
United States
CKD
γ-HCH
NA (self-
reported
pesticide
exposure)
Ever used versus never used
↔
Ghosh et al. 2017
Case-control, 200 cases and 100 controls,
ages 30–50 years, India
CKD of known or
unknown etiology
α-HCH
Blood
1.26 ng/g (median) (CKD, known)
1.68 (CKD, unknown)
0.7 (controls)
↔
β-HCH
2.49 (CKD, known)
2.15 (CKD, unknown)
1.7 (controls)
↔
γ-HCH
2.15 (CKD, known)
2.03 (CKD, unknown)
2.6 (controls)
↑
c
Siddarth et al. 2014
Case-control, 270 cases and 270 controls,
mean ages 46 and 48 years (respectively),
India
CKD
α-HCH
Serum
5.23 ng/mL (median, 3rd tertile)
0.87 (median, 1
st
tertile)
↑
β-HCH
5.50 (3
rd
tertile)
0.20 (1
st
tertile)
↔
γ-HCH
3.89 (3
rd
tertile)
1.86 (1
st
tertile)
↔
Total HCH
12.51(3
rd
tertile)
3.63 (1
st
tertile)
↑

HEXACHLOROCYCLOHEXANE (HCH) 125
2. HEALTH EFFECTS
Table 2-11. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Renal Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Lebov et al. 2015
Cohort, 31,142 wives of pesticide
applicators, ≥18 years at enrollment, Iowa
and North Carolina, United States, mean
follow-up 15.4 years
End-stage renal
disease
γ-HCH
NA (self-
reported
pesticide
exposure)
Ever used versus never used
↔
a
Analysis was not performed because all samples were below the LOQ.
b
Odds ratios comparing serum levels ≥ LOQ to samples < LOQ.
c
Positive association associated with CKD of unknown etiology; no association with CKD of known etiology.
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; CKD = chronic kidney disease; LOQ = limit of
quantification; NA = not applicable
HEXACHLOROCYCLOHEXANE (HCH) 126
2. HEALTH EFFECTS
excretion of glucose in urine and increased excretion of creatinine and urea, as well as hypertrophy and
degeneration of the renal tubular epithelia in rats exposed to 72 mg/kg/day β-HCH for up to 2 weeks.
Van Velsen et al. (1986) reported significantly increased kidney weights in female rats exposed to 0.2 mg
β-HCH/kg/day for 13 weeks, but the change did not exhibit dose-dependence. In males, a significant
increase in kidney weight was observed at 4.5 mg/kg/day. At the highest dose (22.5–25 mg/kg/day), a
dose that was profoundly toxic and led to the humane sacrifice of half of the animals, males exhibited
renal calcinosis in the outer medulla. The study authors noted that renal calcinosis is common in female
rats but that this finding was unusual and therefore of significance in males (Van Velsen et al. 1986).
Fitzhugh et al. (1950) examined the renal effects of exposure to β-HCH (60–70 mg/kg/day) in rats that
died after an average of 4.4 weeks and found nephritis and basal vacuolation. At lower doses in this
study, exposure to doses up to 9 mg/kg/day for up to 2 years did not induce renal histopathology changes
(Fitzhugh et al. 1950).
γ-HCH (Lindane). Renal effects have been documented in case reports of accidental or intentional oral
exposures to γ-HCH. In a suicide attempt, a 30-year-old male farmer from rural India ingested a single
dose of approximately 50 mL of 2% γ-HCH solution (Paul et al. 2013). Six hours after the ingestion, the
man went to the emergency department. On the second day symptoms of lethargy and reduced urinary
output were noted, increasing in severity by day 5 to include additional symptoms of elevated pulse,
increased serum levels of kidney enzymes (blood urea nitrogen [BUN] and creatinine), urinary white
blood cells, red blood cells, and protein. An ultrasound of the kidneys indicated cortical echogenicity and
mild ascites. The man was treated for acute kidney injury by hemodialysis. Urine output started to
increase on day 10 and serum kidney enzymes recovered in 3 weeks, after which he was discharged in
stable condition that persisted though a 3-month follow up (Paul et al. 2013).
A suicidal 21-year-old male ingested γ-HCH (dose unknown) (Shah et al. 2013). One day after ingestion,
the man experienced reduced urine output, dark urine, pedal edema, and muscular pain lasting 2 days
before he went to a clinic. Clinical evaluation at 3 days post-ingestion showed proteinuria,
myoglobinuria, muscle tenderness, elevated serum levels of AST, potassium, creatinine, and CPK, and
metabolic acidosis indicating acute kidney injury from rhabdomyolysis. Treatment included hemodialysis
and supportive care and the man recovered after 3 weeks (Shah et al. 2013). Progressive renal failure was
seen in a woman who died 11 days after intentionally ingesting 8 ounces of a 20% γ-HCH solution
(Sunder Ram Rao et al. 1988). Myoglobin release resulting from muscle lysis in this case led to kidney
shutdown, which was the ultimate cause of death.
HEXACHLOROCYCLOHEXANE (HCH) 127
2. HEALTH EFFECTS
Small (<10%) increases in kidney weights, in the absence of clinical chemistry, urinalysis, and
histopathology changes, were observed in female rats exposed to 5 mg/m
3
γ-HCH aerosol 6 hours/day for
90 consecutive days (Oldiges et al. 1983). Male rats at this concentration exhibited significantly
increased kidney weights. Dose-related increases in the incidences of kidney lesions (dilated tubules with
protein-containing contents; proliferated tubules) were observed in males, but not females, at
concentrations of 0.5 and 5 mg/m
3
(Oldiges et al. 1983). No renal effects (including clinical chemistry,
organ weight, and histopathology) were seen in mice exposed up to 5 mg/m
3
γ-HCH aerosol 6 hours/day,
5 days/week for 14 weeks (Klonne and Kintigh 1988).
In studies of animals exposed orally to γ-HCH, renal effects have largely been limited to male rats,
although many studies did not test females, and two studies reported histopathology changes in females
exposed to higher doses (Suter 1983; Vijaya Padma et al. 2011). In an acute study, male Fischer-344 rats
receiving gavage doses of 10 mg/kg/day of γ-HCH for 4 days showed histopathological changes in the
proximal tubule epithelial cells including accumulation of protein droplets, hypertrophy, necrosis,
pyknotic nuclei, cellular exfoliation, and regenerative epithelium (Dietrich and Swenberg 1990, 1991).
Significantly increased excretion of glucose in urine, and histological changes consisting of hypertrophy
and degeneration of the renal tubular epithelia, were observed in male Wistar rats exposed to
72 mg/kg/day of γ-HCH for up to 2 weeks (Srinivasan and Radhakrishnamurty 1988; Srinivasan et al.
1984).
Male Sprague-Dawley rats exposed for 30 days to γ-HCH via gavage at doses ≥20 mg/kg/day exhibited
medullary and cortical hemorrhage, and degeneration and vacuolation of proximal convoluted tubules
(Fatih Fidan et al. 2008). Similarly, after 30 days of exposure to 20 mg/kg/day, male Wistar rats showed
intertubular hemorrhage, tubular degeneration and desquamation of tubular epithelium, cystic dilatation,
mononuclear cell infiltrate, and necrosis (Prasad et al. 2016). No renal effects other than significantly
increased kidney weight were observed in rats exposed to γ-HCH doses up to 5–50 mg /kg/day in the diet
for up to 40 days (Desi 1974); histological examination of the kidney did not reveal any changes. In
female Wistar rats exposed for 30 days to daily gavage doses of 100 mg/kg/day, glomerular degeneration
and shrinkage and degeneration of the proximal and distal tubules were observed (Vijaya Padma et al.
2011).
Increased kidney weight, hyaline droplet accumulation, and tubular regeneration were observed in male
Long-Evans rats exposed for 10 weeks to 10 mg/kg/day γ-HCH via gavage (Andrews and Gray 1990). In
male rats treated with 0.4–10 mg γ-HCH/kg/day in their diets for 12 weeks, dose-dependent renal effects
HEXACHLOROCYCLOHEXANE (HCH) 128
2. HEALTH EFFECTS
of increasing severity were seen, including basophilic proximal tubules and proximal tubular distention
with cell debris, as well as hyaline droplet formation, and minimal to moderate interstitial nephritis (Suter
1983). Both male and female rats exposed to doses ≥2 mg/kg/day exhibited minimal to slight epithelial
cell necrosis in the proximal tubules (Suter 1983). In a 2-generation reproductive toxicity study, no renal
effects were observed in female adult Crj:CD(SD)IGS rats exposed to doses up to 26.1 mg/kg/day
(Matsuura et al. 2005). In contrast, doses ≥0.56 mg/kg/day resulted in increased incidences and severity
of basophilic tubules and hyaline droplets in the proximal tubules of F0 and F1 parental males (Matsuura
et al. 2005). EPA (1991a) also observed renal histopathological changes characteristic of alpha-
2µ-globulin accumulation in F0 and F1 male CD rats at ≥1.7 mg/kg/day in a 2-generation reproduction
study with γ-HCH. No gross or histopathological changes were observed in kidneys of females in either
generation.
Male Wistar rats exposed for up to 52 weeks to γ-HCH in their diet exhibited hyaline droplets in the renal
proximal tubules, interstitial chronic nephritis, and regeneration in proximal tubules at doses
≥0.07 mg/kg/day; and pale kidneys, increased kidney weights and urine volumes, and higher urinary
protein excretions and tubular necrosis at 7 mg/kg/day (Amyes 1990). In contrast, no renal effects were
seen in females at doses up to 32 mg/kg/day in this study (Amyes 1990). Very slight microscopic kidney
damage (not further specified) was reported in Wistar rats exposed to 7–9 mg γ-HCH/kg/day for up to
104 weeks (Fitzhugh et al. 1950). The histopathology findings were not reported by sex, so it is not clear
whether the effects were limited to males.
Male rats treated dermally with 10 mg/kg/day γ-HCH for 13 weeks exhibited hyaline droplet formation,
and urinalysis showed increased cast formation and turbidity, proteinuria, and hematuria (EPA 1988a).
Females in the same study exhibited a slight increase in the incidence of tubular basophilia at 60
mg/kg/day.
Mechanisms. Available data suggest that the renal effects of γ-HCH may result from at least two possible
mechanisms: (1) alpha-2μ-globulin accumulation in male rats; and (2) increased oxidative stress in both
male and female rats. Dietrich and Swenberg (1990, 1991) demonstrated α-2μ-globulin staining in the
kidney cortex of male F-344 rats exposed for 4 days to 10 mg/kg/day of γ-HCH. No α-2μ-globulin
staining was detected in the kidneys of F-344 male controls, F-344 control or exposed female rats, or
exposed male NBR rats (a strain that does not synthesize α-2μ-globulin). Matsuura et al. (2005) used
immunohistochemistry staining to examine kidneys of male parental rats in a 2-generation reproductive
HEXACHLOROCYCLOHEXANE (HCH) 129
2. HEALTH EFFECTS
toxicity study, and observed that smaller hyaline droplets stained positive for α-2μ-globulin, while larger
ones did not.
Renal effects seen only in male rats that are attributable to alpha-2μ-globulin accumulation are not
relevant to human health (EPA 1991b). However, kidney effects have also been seen in female rats
(Suter 1983; Vijaya Padma et al. 2011), and other mechanisms, such as induction of oxidative stress, may
play a role in these effects. Increases in lipid peroxidation and nitric oxide, as well as depletion of
antioxidant enzyme activities (superoxide dismutase, catalase, and glutathione peroxidase) and reduced
glutathione, have been observed in the kidneys of male rats exposed by gavage to doses of ≥20 mg/kg/day
γ-HCH in intermediate-duration studies (Fatih Fidan et al. 2008; Prasad et al. 2016; Vijaya Padma et al.
2011).
Technical HCH or Unspecified Isomers of HCH. Oral exposure to technical HCH has been shown to
induce kidney effects in mice, rats, and pigs. Mice treated daily with 50 mg/kg/day technical-grade HCH
for 1, 5, or 15 days by oil gavage exhibited renal changes including congestion of blood vessels and
glomerular tufts, swollen tubules with hyaline casts, cystic dilation, fatty changes, some interstitial
hemorrhaging in the medulla, and epithelial cell vacuolation (Philip et al. 1989). No adverse effects were
seen in the kidneys of male rats treated with 50 or 60 mg/kg/day technical-grade HCH for 30 days
(Dikshith et al. 1989a; Khanna et al. 1990). Wang et al. (2006) observed a 24% increase in kidney
weights in pigs given technical-grade HCH at a dose of 0.8 mg/kg/day for 90 days; renal histopathology
was not evaluated. Nephritis, pigmentation, and basal vacuolation were observed in kidneys of rats (sex
not specified) fed 60–70 mg/kg/day technical-grade HCH in the diet for an average of 32.9–64.6 weeks
(Fitzhugh et al. 1950); poor survival (for which there was no explanation) was noted in both control and
treated animals. Tubular necrosis and glomerular degeneration were seen in male rats exposed for
360 days to 20 mg/kg/day of technical-grade HCH (Dikshith et al. 1991a).
Renal changes have been reported in animals exposed to technical-grade HCH by dermal application.
Female rats treated with 100 mg/kg/day of technical-grade HCH for 7, 15, or 30 days had necrosis and
atrophy of the renal tubules and glomeruli, although the number of animals affected and the severity of
the lesions were not reported (Dikshith et al. 1991c). Similar effects were noted in male rabbits treated
with 25 mg/kg/day technical-grade HCH for 30 days (Dikshith et al. 1989b). In both of these studies,
mortalities were seen at the doses associated with renal effects.
HEXACHLOROCYCLOHEXANE (HCH) 130
2. HEALTH EFFECTS
2.11 DERMAL
γ-HCH (Lindane). A 10-year-old boy who was being treated for scabies was exposed to a 1% γ-HCH
solution by dermal application from the neck down for 8 hours/day on 3 consecutive days (Juan et al.
2004). Following the initial application, erythema developed on the neck, extending to the trunk and
axillae after 2 days. On the third day, pustules were present on his neck, trunk, arms, and thighs. He was
examined in a clinic, where symptoms of leukocytosis were noted. A biopsy of a skin lesion showed sub-
corneal and intraepidermal pustule formation with neutrophilic infiltrations and scattered eosinophils in
the dermis, suggesting acute generalized exanthematous pustulosis. After discontinuation of the γ-HCH
solution, the boy recovered within a week (Juan et al. 2004).
A 57-year-old man was admitted to the hospital and diagnosed with scabies for which he was treated with
γ-HCH lotion (concentration not reported) by dermal application to the whole body for 8 hours/day for
3 consecutive days (Yu et al. 2015). Instructions included washing/removal of the lotion after 8 hours,
but the man was only partially washed for unknown reasons. One week after exposure, multiple scattered
and coalescent polymorphic ulcerations with hemorrhagic spots and black burn-like crusted edges
developed. A skin biopsy revealed papillary edema, and acute and chronic inflammation indicating
ulcerative irritant contact dermatitis. Patch testing was performed but the results were unavailable, as the
patient died from sepsis. The time elapsed between exposure and death was not reported and it was not
clear whether the ulceration caused the sepsis (Yu et al. 2015).
In a summary of γ-HCH poisoning cases reported to the Texas poison control network (Forrester et al.
2004), commonly reported symptoms of exposure included erythema and dermal irritation or pain; the
authors did not describe symptoms by routes of exposure, which included oral and dermal. An itchy red
rash was observed in a 10-month-old boy after 7 days of twice-daily application of 1% γ-HCH for scabies
treatment (Bhalla and Thami 2004). Rashes were observed in a boy following treatment with shampoo
containing γ-HCH (Fagan 1981). No exposure level was reported, but the shampoo was rinsed over the
boy's entire body.
Mild dermatitis was observed in rats after 15 skin paintings with 180 mg/kg/day γ-HCH/kg over a period
up to 25 days (Dikshith et al. 1973). Rabbits exposed to 200 mg/kg moistened γ-HCH for 4 hours showed
no primary skin irritation or other toxic symptoms (Ullmann 1986d).
HEXACHLOROCYCLOHEXANE (HCH) 131
2. HEALTH EFFECTS
Technical HCH or Unspecified Isomers of HCH. Rabbits exposed to technical-grade HCH
(25 mg/kg/day for 30 days) had hyperkeratinization of the epidermal layer and swollen collagen fibers in
the dermis, but no scoring level was provided (Dikshith et al. 1989b). Dermal treatment of rats with
100 mg/kg/day technical-grade HCH for 7–30 days resulted in hyperkeratosis, epidermal cell
vacuolization, and thickening of collagen fibers (Dikshith et al. 1991c).
2.12 OCULAR
No studies were located regarding ocular effects in humans following exposure to HCH isomers.
γ-HCH (Lindane). In a survey of γ-HCH exposures reported to the Texas poison control network,
Forrester et al. (2004) reported that ocular irritation and pain were common symptoms. The authors did
not distinguish between effects seen after oral and dermal exposures, both of which were considered in
the survey.
Mice exposed to γ-HCH aerosol (up to 5 mg/m
3
) 6 hours/day for 14 weeks exhibited no ophthalmic
effects (Klonne and Kintigh 1988), and histopathology of the eyes showed no changes in these mice or in
rats exposed similarly (Oldiges et al. 1983). Mild eye irritation was seen in rabbits exposed to 40 mg/kg
γ-HCH in the conjunctival sac for up to 72 hours. The irritation level was given a primary irritation score
of 0.6 out of a maximum possible cumulative score of 16 (Ullmann 1986c).
2.13 ENDOCRINE
Epidemiological Studies. Several epidemiological studies have suggested that exposure to HCH may be
associated with changes in thyroid function (see Table 2-12). In a cohort study of 21,788 male pesticide
applicators, occupational exposure to γ-HCH was associated with increased odds of hypothyroid disease
(Goldner et al. 2013). In other studies, there was suggestive evidence for associations between β-HCH in
blood and alterations in serum thyroid hormone levels, but the direction of change and affected hormones
were not consistent. A case-control study in Chinese subjects with thyroid disease found no association
between serum levels of β-HCH and thyroid disease; however, serum β-HCH levels were associated with
decreased total and free thyroxine (T4) in males and increased levels of free T4 in females (Han et al.
2019). Levels of total T4 in females was not related to serum β-HCH, nor were levels of total
triiodothyronine (T3), free T3, and thyroid-stimulating hormone (TSH) in either sex (Han et al. 2019).

HEXACHLOROCYCLOHEXANE (HCH) 132
2. HEALTH EFFECTS
Table 2-12. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Endocrine
Effects
Reference, study type, and
population
Isomer
Concentration in serum (unless
otherwise noted)
Outcome evaluated
Result
Han et al. 2019
Case-control, 186 cases of
thyroid disease and 186 controls,
mean ages 46 and 44 years,
China
β-HCH
78.96 ng/g lipid (median) (cases)
65.58 (controls)
Thyroid disease
↔
Serum total T4
M: ↓
F: ↔
Serum free T4
M: ↓
F: ↑
Serum total T3, free T3, and TSH
↔
Freire et al. 2013
Cross-sectional, 303 males and
305 females >14 years old, Brazil
β-HCH
6.00 ng/mL (median) (males)
6.98 (females)
Serum total T3
↔
Serum Free T4
M: ↓
F: ↔
Serum TSH
M: ↑
F: ↔
Serum anti-thyroperoxidase
↔
α-HCH
2.52 (males)
2.60 (females)
Serum total T3, free T4, TSH, and anti-
thyroperoxidase
↔
γ-HCH
0.95 (males)
0.97 (females)
Serum total T3, free T4, TSH, and anti-
thyroperoxidase
↔
Alvarez-Pedrerol et al. 2009
Cross-sectional, 1,090 pregnant
women, Spain
β-HCH
32.3 ng/mL (median) (Sabadell [S])
<LOD (median); 22.1 (75
th
percentile)
(Gipuzkoa [G])
Serum total T3
S: ↓
G: ↔
Serum free T4
S: ↔
G: ↑
Dallaire et al. 2009
Cross-sectional, 623 Inuit adults
≥18 years old, Canada
β-HCH
8.33 μg/kg lipid (plasma) (mean)
Serum total T3
↓
Serum thyroxine binding globulin
↓
Serum free T4, TSH
↔

HEXACHLOROCYCLOHEXANE (HCH) 133
2. HEALTH EFFECTS
Table 2-12. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Endocrine
Effects
Reference, study type, and
population
Isomer
Concentration in serum (unless
otherwise noted)
Outcome evaluated
Result
Freire et al. 2012
Cross-sectional, 193 children
<15 years old, Brazil
β-HCH
479 ng/mL (mean)
Serum total T3
↑
Serum free T4, TSH
↔
α-HCH
300
Serum total T3
↑
Serum free T4, TSH
↔
γ-HCH
77.5
Serum total T3
↑
Serum free T4, TSH
↔
Seo et al. 2022
Cross-sectional, 880 adults, ages
20–80 years, South Korea
β-HCH
40.4 ng/g lipid (mean)
Serum free T4
↓
Serum TSH
↔
γ-HCH
1.82
Serum TSH and free T4
↔
α-HCH
1.24
↔
δ-HCH
0.17
↔
Kim et al. 2013
Cross-sectional, 105 pregnant
women 22–46 years old, South
Korea
β-HCH
7.58 ng/g lipid (median)
Serum total T3, free T3, total T4, free T4,
TSH
↔
Piccoli et al. 2016
Cross-sectional, 275 farmers and
farm residents, 18–69 years old,
Brazil
β-HCH
<LOD ng/g (median)
77.87 (95
th
percentile)
Serum total T3
↑
Serum free T4, TSH
↔
γ-HCH
3.71 (median)
24.35 (95
th
percentile)
Serum total T3, free T4, TSH
↔
α-HCH
<LOD (median)
21.8 (95
th
percentile)
Serum total T3, free T4, TSH
↔
Yamazaki et al. 2020
Cross-sectional, 333 pregnant
women 17–48 years old, Japan
β-HCH
235.6 pg/g (late gestation or post-
partum) (75
th
percentile)
Serum TSH (early gestation)
↔
Serum free T4 (early gestation)
↔

HEXACHLOROCYCLOHEXANE (HCH) 134
2. HEALTH EFFECTS
Table 2-12. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Endocrine
Effects
Reference, study type, and
population
Isomer
Concentration in serum (unless
otherwise noted)
Outcome evaluated
Result
Goldner et al. 2013
Cohort, 21,788 male private
pesticide applicators, Iowa and
North Carolina, United States
Lindane
NA (occupational, ever use)
Hypothyroid disease
↑
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; F= female; LOD = limit of detection; M = male; NA = not
applicable; T3 = triiodothyronine; T4 = thyroxine; TSH = thyroid stimulating hormone
HEXACHLOROCYCLOHEXANE (HCH) 135
2. HEALTH EFFECTS
Several cross-sectional studies evaluated effects of β-HCH on thyroid hormone levels. Decreased total
T3 and thyroxine binding globulin in serum were associated with higher plasma β-HCH levels in
Canadian Inuit adults, while there were no associations with free T4 or TSH (Dallaire et al. 2009). In a
study of pregnant women in Spain, there was an association between serum β-HCH and decreased total
T3 levels in women in the city of Sabadell, but no association was seen in women from the city of
Gipuzkoa (Alvarez-Pedrerol et al. 2009). In contrast, free T4 levels were not associated with serum
β-HCH in Sabadell women, while there was an increased association in Gipuzkoa women (Alvarez-
Pedrerol et al. 2009). No relationship between serum β-HCH levels and total T3, free T3, total T4, free
T4, or TSH was observed in pregnant women or adults of both sexes in South Korea (Kim et al. 2013) or
serum TSH and free T4 levels in pregnant women in Japan (Yamazaki et al. 2020). A cross-sectional
study in adults from South Korea observed an inverse association between serum levels of β-HCH and
serum free T4 (Seo et al. 2022). No association was observed between serum levels of β-HCH and TSH,
or between serum α-, γ-, or δ-HCH concentrations and serum TSH or free T4.
A population-based survey of residents living near an HCH production factory (operating from late 1940s
to 1955) in Brazil examined associations of serum levels of α-, β-, and γ-HCH and thyroid function in
193 children <15 years old (Freire et al. 2012) and in adolescent and adult (>14 years old) males (n=303)
and females (n=305) (Freire et al. 2013). In children, serum levels of α-, β-, and γ-HCH were associated
with increased serum total T3 levels, while no association was observed for serum free T4 or TSH levels
(Freire et al. 2012). In adults, serum levels of β-HCH were associated with decreased free T4 and
increased TSH levels in men, while no association was determined in women for either parameter.
Further, there were no associations between serum β-HCH and total T3 or anti-thyroperoxidase levels and
there were no associations between α-HCH or γ-HCH and total T3, free T4, TSH, and anti-
thyroperoxidase (Freire et al. 2013). Increased total T3 levels corresponded to serum β-HCH levels in
275 adult Brazilian farmers and farm residents, while no relationship was found with α- or γ-HCH
(Piccoli et al. 2016). In this study, there was also no association between serum α-, β-, or γ-HCH and free
T4 or TSH levels (Piccoli et al. 2016).
α-HCH. No histopathology changes were noted in the adrenal glands or thyroids of rats given α-HCH via
the diet at doses up to 70 mg/kg/day for ~9 months or up to 9 mg/kg/day for 2 years (Fitzhugh et al.
1950).
HEXACHLOROCYCLOHEXANE (HCH) 136
2. HEALTH EFFECTS
β-HCH. There were no microscopic lesions in the thyroid or adrenal glands when rats were exposed by
dietary administration to β-HCH doses up to 70 mg/kg/day for up to 10 weeks or up to 9 mg/kg/day for
2 years (Fitzhugh et al. 1950).
γ-HCH (Lindane). In 13- and 14-week inhalation studies of rats and mice (respectively) exposed to
concentrations up to 5 mg/m
3
for 6 hours/day, there were no effects of treatment on the histology of the
pancreas, thyroid, or adrenal glands (Klonne and Kintigh 1988; Oldiges et al. 1983) or on the histology of
the pituitary in the mice (Klonne and Kintigh 1988). Wistar rats administered 50 mg/kg/day γ-HCH in
drinking water for 30 days had 84% higher serum levels of free T4 and 74% lower serum TSH compared
to controls (Hfaiedh et al. 2011). In a 2-generation reproductive toxicity study, F0 and F1 male and/or
female parental rats exposed via feed to doses of 17.2–26.1 mg/kg/day exhibited endocrine effects
including decreased absolute and relative pituitary weights (F0 and F1 females), altered serum thyroid
hormone levels, and increased incidences of thyroid follicular cell hypertrophy (F0 females and F1 males)
(Matsuura et al. 2005). Fitzhugh et al. (1950) reported no histopathology findings in the thyroid or
adrenal glands of rats given γ-HCH in feed at doses up to 140 mg/kg/day for 10 months or 30 mg/kg/day
for 2 years. No effect on adrenal gland weights or adrenal, thyroid, or parathyroid histopathology
findings in CD-1 mice given γ-HCH in the diet at doses up to 26.8 mg/kg/day for 78 weeks (EPA 2000a).
Technical HCH or Unspecified Isomers of HCH. Technical HCH administered to rats in feed did not
induce thyroid or adrenal gland histopathology changes at doses up to 70 mg/kg/day for 6 months or
9 mg/kg/day for 2 years (Fitzhugh et al. 1950).
2.14 IMMUNOLOGICAL
Epidemiological Studies. Few studies of immune system effects in humans exposed to HCH isomers
were located, and the available studies examined limited endpoints. Table 2-13 provides an overview of
the epidemiological studies. Landgren et al. (2009) followed a cohort of 678 male pesticide applicators in
the United States for 9 years and evaluated the risk of monoclonal gammopathy of undetermined
significance (MGUS). MGUS is a condition in which an abnormal protein (monoclonal or M protein)
accumulates in the blood; this condition sometimes progresses to lymphoma or multiple myeloma. There
was no increase in risk for MGUS among applicators who reported use of γ-HCH compared with those
who had never used γ-HCH (Landgren et al. 2009). Ryu et al. (2018) reported a positive association
between serum levels of β-HCH and specific T-lymphocyte frequencies (CD8+ CD57+ and CD8+

HEXACHLOROCYCLOHEXANE (HCH) 137
2. HEALTH EFFECTS
Table 2-13. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Immune
Effects
Reference, study type, and
population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Meng et al. 2016
Case-control, 124 cases of asthma,
109 controls, children ages 3–6 years,
China
Asthma
α-HCH
Plasma
Cases: 40.73±36.01 ng/g lipid
Controls: 12.52±16.03
↑
β-HCH
Cases: 111.11±70.1
Controls: 31.49±74.02
↑
γ-HCH
Cases: 27.5±12.13
Controls: 9.34±20.21
↑
Severe asthma
a
α-HCH
See above
↔
β-HCH
See above
↔
γ-HCH
See above
↔
Landgren et al. 2009
Cohort, 678 male pesticide applicators
followed for at least 9 years, Iowa and
North Carolina, United States
Monoclonal gammopathy of
undetermined significance
γ-HCH
None
(occupational)
Ever versus never used
↔
Ryu et al. 2018
Cross-sectional, 95 healthy adults, age
30–69 years, Korea
T-lymphocyte frequencies:
CD8+ CD57+
β-HCH
Serum
10.7 ng/g lipid
(median of 4
th
quartile)
↑
CD8+ CD28-
↑
CD4+ CD57+
↔
CD4+ CD28-
↔

HEXACHLOROCYCLOHEXANE (HCH) 138
2. HEALTH EFFECTS
Table 2-13. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Immune
Effects
Reference, study type, and
population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Seth et al. 2005
Case-control, 20 patients hospitalized
with lindane poisoning and
20 unexposed age- and sex-matched
healthy subjects, India
Serum immunoglobulins and
cytokines:
γ-HCH
None
(exposure
assessed
based on
clinical
symptoms,
history, and
AChE activity)
Exposed versus unexposed
IgG, IgM, IgA, and IgE
↔
IL-2,IL-4, and TNF-α
↑
IFN-γ
↓
Wang et al. 2021a
Cross sectional, 10 women (5 from
rural and 5 from urban areas), mean
age 37 years, China
IL-8, MCP-1
α-HCH
Estimated
dietary intake
0.471 ng/kg/day
↔
β-HCH
0.185
γ-HCH
0.043
δ-HCH
0.104
a
Severe asthma was defined as “asthma attacks more than 10 times over the year with repeated episodes (more than 3 times) during the last month, or if severe
oxygen deficiency occurred in one attack.”
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; AChE = acetylcholinesterase; Ig = immunoglobulin;
IL = interleukin; TNF = tumor necrosis factor; IFN = interferon; MCP = monocyte chemotactic protein.
HEXACHLOROCYCLOHEXANE (HCH) 139
2. HEALTH EFFECTS
CD28-) in a cross-sectional study of healthy adults over 30 years of age. In a case-control study of
children 3–6 years old, associations between asthma and increased plasma α-, β-, and γ-HCH levels were
reported (Meng et al. 2016); however, in this study, exposure (blood level) was measured after the
outcome (asthma) occurred. A small cross-sectional study of 10 women in China found no association
between any of the HCHs and serum interleukin-8 (IL-8) or monocyte chemotactic protein-1 (MCP-1)
(Wang et al. 2021a).
β-
HCH. Decreased lympho
-p
roliferative responses to mitogens were seen in mice exposed to
60 mg/kg/day β-HCH in the diet for 30 days (Cornacoff et al. 1988). There were no associated changes in
immunoglobulins, red blood cell counts, or histology of the thymus, spleen, or lymph nodes (Cornacoff et
al. 1988). Cortical atrophy of the thymus and depletion of splenic lymphoid tissue were observed in rats
fed 22.5–25 mg/kg/day β-HCH in the diet (Van Velsen et al. 1986). In this 13-week study (Van Velsen et
al. 1986), 50% of the rats exposed at this dose were sacrificed humanely before study termination (as
early as the first 3 weeks) due to moribund condition.
γ-
HCH (Lindane) Immune system parameters in blood were evaluated in a group of 20 patients seen in a
hospital in India with γ-HCH poisoning and compared with results in a group of age- and sex-matched
controls without pesticide exposure (Seth et al. 2005). The dose, route, nature, and timing of γ-HCH
exposures were not reported, and there was no effort to adjust for potential confounders. No differences
in serum immunoglobulin levels (IgG, IgM, IgA, or IgE) were seen; however, several serum cytokine
levels, including interleukin-2 (IL-2), interleukin-4 (IL-4), and tumor necrosis factor-alpha (TNF-α) were
higher in the poisoning victims, and serum IFN-γ levels were lower (Seth et al. 2005).
A 14-day exposure to 10 mg/kg/day γ-HCH in male rats previously sensitized to Keyhole Limpet
Hemocyanin (KLH) resulted in a reduction in delayed-type hypersensitivity response (measured as a 43%
decrease in foot pad thickness in response to KLH challenge) (Mediratta et al. 2008). Decreased relative
thymus weight (28% less than controls) was observed in mice gavaged with 20 mg/kg/day γ-HCH for
3 days; at 40 mg/kg/day, atrophy of the thymic cortex was seen (Hong and Boorman 1993). Another
experiment by these authors showed significant decreases in relative weights of thymus (≥7%
decrements) and spleen (≥17% decrements) in mice exposed to 10–20 mg/kg/day γ-HCH for 10 days
(Hong and Boorman 1993).
Immunosuppression, as measured by decreased antibody titers against typhoid vaccine and Salmonella
vaccine, was reported in rats exposed by gavage to doses of ≥6.25 mg/kg/day γ-HCH for 5 weeks (Dewan
HEXACHLOROCYCLOHEXANE (HCH) 140
2. HEALTH EFFECTS
et al. 1980) and in rabbits exposed by capsules 5 times each week to 1.5, 6, and 12 mg/kg/day for 5–
6 weeks (Desi et al. 1978). Humoral immune response, as indicated by serum antibody response to sheep
red blood cells (SRBC), was suppressed in rats that were exposed to γ-HCH in estimated dietary doses of
3.6 or 7 mg/kg/day for 8 weeks (Koner et al. 1998). The primary antibody response to SRBC was also
suppressed in albino mice after exposure to 9 mg/kg/day γ-HCH in the diet for 12 weeks (Banerjee et al.
1996). Suppression of secondary antibody response (response after repeat exposure) was also observed
after 3 weeks of exposure to 9 mg/kg/day γ-HCH and after 12 weeks of 5.4 mg/kg/day γ-HCH exposure
(Banerjee et al. 1996). A biphasic, dose-dependent immunological effect of γ-HCH on components of
cell- and humoral-mediated immunity, characterized by initial stimulation followed by immuno-
suppression, was reported in mice fed 0.012, 0.12, or 1.2 mg γ-HCH/kg/day for 24 weeks (Meera et al.
1992). Histological examinations in these animals revealed decreased lymphocyte populations in the
thymus and lymph nodes, a reduction in overall cellularity in the spleen, and necrosis of the thymus at
1.2 mg/kg/day. Cell-mediated immune response, as measured by delayed-type hypersensitivity reaction
to dinitrofluorobenzene antigen, was suppressed in sheep that were exposed to 1.25 ppm γ-HCH in the
diet for 6 months (Khurana et al. 1999).
Technical HCH or Unspecified Isomers of HCH. A statistically significant increase (approximately
18%) in the level of immunoglobulin M (IgM) was noted in 19 workers occupationally exposed to
technical-grade HCH during pesticide formulation, as compared to 14 nonexposed workers (Kashyap
1986). The HCH isomer concentrations in serum showed a 10-fold increase when compared to the
control group. Both inhalation and dermal exposure probably occurred, and the measurement of IgM
alone is not a reliable measure of immune function in adults.
2.15 NEUROLOGICAL
Epidemiological Studies. Epidemiological studies of neurological effects in humans exposed to HCH
isomers are summarized in Table 2-14. Most of the studies used serum or blood levels of β-HCH to
assess exposure; only Singh et al. (2012, 2013, 2014) and Xu et al. (2022) measured α- γ-, and/or δ-HCH
levels as well. A cohort study of 669 Canadian adults at least 65 years old who were followed for 10
years showed no association between blood levels of β-HCH and dementia, Alzheimer’s disease, or
cognitive deficits (Medehouenou et al. 2019). In contrast, case-control studies reported increased risks of
Parkinson’s disease, Alzheimer’s disease, and cognitive deficits with higher β-HCH levels in blood or
serum (Kim et al. 2015; Petersen et al. 2008; Richardson et al. 2009, 2011; Singh et al. 2012, 2013, 2014;
Xu et al. 2022). The study by Xu et al. (2022) also reported increased risk of Parkinson’s disease
HEXACHLOROCYCLOHEXANE (HCH) 141
2. HEALTH EFFECTS
associated with increased serum levels of α- and δ-HCH. However, in the case-control studies, exposures
were measured after the outcome occurred, so there is no clear temporal relationship between exposure
and effect. A small cross-sectional study that suffered from the same limitation reported no association
between β-HCH in serum and tremors at rest or cognitive deficits (Steenland et al. 2014).
Some studies have reported impairments in sensory function associated with HCH exposure. Serum
levels of α-HCH were associated with hearing loss in a small case-control study (87 pairs) in China
(Zhang et al. 2021), but the exposure was measured after the outcome in this study. Shrestha et al. (2021)
observed an association between γ-HCH lifetime days of use and self-reported olfactory impairment
20 years after enrollment in a very large cohort of pesticide applicators in the United States. The odds of
olfactory impairment increased with intensity-weighted days of γ-HCH use in this cohort. The absence of
an objective measure of olfactory impairment limits the conclusions that can be drawn from this study.
α-HCH. Muller et al. (1981) reported no delay in tail nerve conduction velocity in rats fed 5.1, 54.2, or
106.2 mg α-HCH/kg/day for 30 days. No other data on neurological effects of α-HCH were located.
β-HCH. Clinical signs of neurotoxicity have often preceded death in rats and mice exposed to β-HCH via
oral administration. Mice treated with 60 or 200 mg/kg/day β-HCH in the diet in a 30-day study
developed ataxia within the first week of treatment (Cornacoff et al. 1988). The animals receiving
60 mg/kg/day recovered within a few days, while those receiving 200 mg/kg/day became markedly
worse, leading to humane sacrifice of 80% of the animals in this group (Cornacoff et al. 1988). In the
first 2 weeks of a 13-week study, male and female rats exposed to 38 mg/kg/day in the diet exhibited
ataxia and hypoactivity, progressing to coma within 3 days (Van Velsen et al. 1986). The animals were
humanely sacrificed, as were five additional males and six additional females that showed similar signs
later in the study. A single study of electrophysiology was located; in this study, Muller et al. (1981)
reported a significant delay in tail nerve conduction velocity in rats fed 66.3 mg β-HCH/kg/day for
30 days. No comprehensive tests of sensitive neurotoxicity endpoints other than electrophysiology in
animals exposed to β-HCH were located.
γ-HCH (Lindane). Neurological effects have been seen in humans and animals exposed to γ-HCH by
inhalation, oral, and dermal routes. The effects range in severity from subtle neurobehavioral changes
and altered neurotransmitter levels to tremors, convulsions, and ultrastructural changes in the brain.

HEXACHLOROCYCLOHEXANE (HCH) 142
2. HEALTH EFFECTS
Table 2-14. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Neurological
Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Exposure
Biomarker
Mean concentration
(unless otherwise noted)
Result
Richardson et al. 2009, 2011
Case-control, 149 cases of Parkinson’s
disease, 134 controls, four sites in Texas
and Georgia, United States
Parkinson’s disease
β-HCH
Serum
10.77–79.43 ng/mg
cholesterol
(range of medians among
cases across four sites)
↑
Petersen et al. 2008
Case-control, 79 cases of Parkinson’s
disease, 154 controls, mean ages 74 and
75 years, respectively, Faroe Islands
Parkinson’s disease
β-HCH
Serum
0.06 µg/g lipid (GM) (cases)
0.04 (controls
↑
Xu et al. 2022
Case-control, 90 cases of Parkinson’s
disease, 90 controls who were spouses of
the cases, mean ages 65.76 and 64.23,
respectively, China
Parkinson’s disease
α-HCH
Serum
80.19 ng/g lipid (cases)
1.79 ng/g lipid (controls)
↑
β-HCH
1126.4 ng/g lipid (cases)
349.88 ng/g lipid (controls)
↑
γ-HCH
583.30 ng/g lipid (cases)
39.62 ng/g lipid (controls)
↔
δ-HCH
12.09 ng/g lipid (cases)
5.82 ng/g lipid (controls)
↑
Singh et al. 2012, 2013, 2014
Case-control, 100 patients with Alzheimer’s
disease, 100 age-matched controls, Delhi,
India
Alzheimer’s disease
β-HCH
Serum
4.42±0.54 ng/mL (in cases)
↑
α-HCH
Serum
0.37±0.11 ng/mL
↔
γ-HCH
Serum
0.78±0.23 ng/mL
↔
Kim et al. 2015
Cross-sectional, 633 adults aged 60–85
years, NHANES 1999–2002, United States
Cognitive deficit
β-HCH
Blood
89.6 ng/g lipid (median of 4
th
quartile)
↑
Medehouenou et al. 2019
Cohort, 669 adults ≥65 years old, Canadian
Study of Health and Aging (CSHA), Canada
Dementia, Alzheimer’s disease,
cognitive deficit
β-HCH
Blood
0.13 µg/L (median) (cases)
0.12 (controls)
↔
Steenland et al. 2014
Cross-sectional, 89 adults >65 years of age,
Costa Rica
Movement disorder (tremor at
rest)
β-HCH
Serum
≥0.88 ng/mL (4
th
quartile
cutoff)
↔
Cognitive deficit
β-HCH
Serum
≥0.88 ng/mL (4
th
quartile
cutoff)
↔

HEXACHLOROCYCLOHEXANE (HCH) 143
2. HEALTH EFFECTS
Table 2-14. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Neurological
Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Exposure
Biomarker
Mean concentration
(unless otherwise noted)
Result
Sullivan et al. 2018
Cohort, 159 Gulf War veterans who had
worked in pest control, mean age 48 years,
United States
Depression, fatigue scores on
mood test
γ-HCH
NA (self-
reported
exposure)
(high exposure versus low
exposure)
↑
Anger, confusion, tension
scores on mood test
↔
Zhang et al. 2021
Case-control, 87 cases and 87 age- and
gender-matched controls
, mean age
51 years, China
Hearing loss
β-HCH
Serum
27.0 ng/g lipid (cases)
(geometric mean)
29.6 ng/g lipid (controls)
↔
α-HCH
14.4 ng/g lipid (cases)
12.2 ng/ml (controls)
↑
δ-HCH
8.98 ng/g lipid (cases)
9.54 ng/g lipid (controls)
↔
Shrestha et al. 2021
Cohort, 20,409 adult, mostly male pesticide
applicators, Iowa and North Carolina, United
States
Olfactory impairment
γ-HCH
NA (self-
reported
exposure)
Quartiles of intensity-weighted
lifetime days of use
↑
↑ = association with increase; ↔ = no association; GM = geometric mean; NA = not applicable; NHANES = National Health and Nutrition Examination Survey
HEXACHLOROCYCLOHEXANE (HCH) 144
2. HEALTH EFFECTS
Studies in animals exposed in utero and/or during lactation show that γ-HCH can also elicit these
neurological effects in offspring of exposed parents; neurotoxic effects in animals exposed during
development are discussed in Section 2.17.
Abnormal electroencephalographic (EEG) patterns (increased variation in the frequency and amplitude of
wave pattern or more serious changes without specific EEG signs) were recorded in 16 of 37 workers
following exposure to γ-HCH for 0.5–2 years in a fertilizer plant (Czeglédi-Jankó and Avar 1970).
Exposure concentrations were not reported; however, these EEG changes were found to correlate with
blood levels of γ-HCH. Effects on mood were examined in a cohort of 159 Gulf War veterans who had
been engaged in pesticide application (Sullivan et al. 2018). In this group, higher self-reported exposure
to γ-HCH was associated with higher scores for depression and fatigue in mood tests, while no
association was seen with anger, confusion, or tension scores on the tests (Sullivan et al. 2018).
Seizures and convulsions have been observed in individuals who accidentally or intentionally ingested
γ-HCH in insecticide pellets, liquid scabicide, or contaminated food (Davies et al. 1983; Forrester et al.
2004; Harris et al. 1969; Munk and Nantel 1977; Nordt and Chew 2000; Powell 1980; Ramabhatta et al.
2014; Starr and Clifford 1972; Storen 1955; Wiles et al. 2015). In most cases, the amount of γ-HCH
ingested could not be determined. A 56-year-old man intentionally ingested approximately 12 ounces of
an insecticide containing 20% γ-HCH in a suicide attempt (Wiles et al. 2015). Thirty minutes later, he
developed a progressive decline in consciousness and multiple seizures ensued between 3 and 14 hours
later. After 18 hours, the man was conscious and responsive, but neurological symptoms were noted
6 days later, including ataxia, slurred speech, paranoia, depression, and defects in higher mental
functioning. The man committed suicide 12 days later by other means. At autopsy, γ-HCH levels in
blood and adipose tissue were 0.248 µg/mL and 132.83 μg/g, respectively.
Several case studies of acute γ-HCH exposure to children ingesting liquid scabicide reported similar
neurological effects, including tremors and tonic/clonic seizures (Aks et al. 1995; CDC 2005; Lifshitz and
Gavrilov 2002; Wheeler 1977). One hour after a 3-year-old boy ingested approximately one teaspoon of
a 1% γ-HCH shampoo, the boy had a tonic-clonic seizure for a duration of 4–5 minutes, despite the
mother’s efforts to induce vomiting (CDC 2005). Three hours later, the boy’s condition was stable, and
he was discharged from the hospital emergency room. Ramabhatta et al. (2014) reported two cases of
neurological effects in children after accidental γ-HCH ingestion. In the first case, a 3-year-old boy was
orally administered a single dose of 10 mL of a γ-HCH lotion (due to a mix-up of prescribed oral and
dermal medications). The child had convulsions 1 hour after ingestion and recovered in 24 hours with
HEXACHLOROCYCLOHEXANE (HCH) 145
2. HEALTH EFFECTS
supportive measures. In the second case, a 6-year-old girl ingested a γ-HCH lotion (amount and
concentration not reported) and had a generalized seizure lasting 10–15 minutes. The child recovered
24 hours after clinic admission (Ramabhatta et al. 2014).
T
here have been many reports of human intoxication involving seizures or convulsions in adults and
children after excessive topical application of γ-HCH (Boffa et al. 1995; Fischer 1994; Hall and Hall
1999; Lee and Groth 1977; Matsuoka 1981; Ramchander et al. 1991; Solomon et al. 1995; Sudakin 2007;
Telch and Jarvis 1982; Tenenbein 1991); exposure levels were generally not quantified. Central nervous
systems symptoms of severe γ-HCH poisoning, including uncontrollable shaking and myoclonic jerking
and tonic-
cl
onic movements of the extremities, developed in a woman following three dermal
applications of a considerable amount (not quantified) of an anti-scabies product over a period of
approximately 2 weeks (Hall and Hall 1999). Fever, tachycardia, grand mal seizure, and hallucinations
were reported in a teenager treated with a 1% γ-HCH lotion for 3 consecutive nights (Boffa et al. 1995).
Weakness of the left and right limbs, dysarthria, and dysphagia were seen in an agricultural worker
exposed by inhalation and dermal contact to unspecified levels of several organochlorine pesticides,
including γ-HCH (Fonseca et al. 1993).
A 1
0-month-old boy exposed to 1% γ-HCH by repeated dermal application to the whole body 2 times/day
for the treatment of scabies developed jerky movements, listlessness, and loss of consciousness after
7 days (Bhalla and Thami 2004). Upon examination by a medical professional after 10 days, the boy
exhibited apathy, semi-consciousness, absence of superficial reflexes, reduced response to touch, pain,
and pressure, and tremor in tongue and limbs. Use of γ-HCH was immediately discontinued and the
infant regained normal consciousness and interaction with environmental stimuli over the subsequent
2 weeks (Bhalla and Thami 2004). The study authors reported that the boy showed evidence of anemia
and malnutrition, which were described as risk factors for γ-HCH-induced neurotoxicity. A 7-year-old
boy exposed to γ-HCH by dermal application 3 times in 4 days (dose not reported) exhibited ataxia,
weakness, and burning paresthesia, and following the third application, the boy had myoclonic jerks and
tonic-clonic seizures, whereupon he was brought to the hospital (Daud et al. 2010). No details of the
extent of exposure were reported. After treatment to control the seizures, induction of diuresis, and
frequent bathing and changing of clothing, the boy was discharged after 2 days (Daud et al. 2010).
Rats exposed to various concentrations of γ-HCH aerosol via nose-only inhalation for 4 hours exhibited
concentration-related neurological effects (Oldiges et al. 1980; Ullmann 1986b). Slight-to-moderate
sedation was observed after exposure to 101 mg/m
3
; slight-to-severe sedation was noted after exposure to
HEXACHLOROCYCLOHEXANE (HCH) 146
2. HEALTH EFFECTS
378 mg/m
3
; restlessness, excitation, and ataxia were seen after exposure to ≥273 mg/m
3
; and spasms were
also noted at the highest concentration (2,104 mg/m
3
). Concentrations ≥378 mg/m
3
were also associated
with mortality in one study (Ullmann 1986b) but not in the other even at concentrations up to 603 mg/m
3
(Oldiges et al. 1980). Rats exposed to 0.02–5 mg/m
3
γ-HCH aerosol for 90 consecutive days exhibited a
"slightly disturbed general condition" (not further characterized) within 2 weeks (Oldiges et al. 1983). At
the end of the 90-day exposure, there were no treatment-related changes in brain weight or histology, or
on histology of the sciatic or optic nerves. Mice were exposed to similar concentrations (0.3–5 mg/m
3
)
for 14 weeks (5 days/week) and exhibited no clinical signs of neurotoxicity (Klonne and Kintigh 1988).
Neurotoxic effects have been reported in several species of animals exposed to γ-HCH. The most serious
effects were seizures and/or convulsions following intragastric administration of approximately 15–
60 mg/kg for ≥1 day in rats (Amyes 1990; EPA 1999a; Fitzhugh et al. 1950; Gilbert and Mack 1995;
Johri et al. 2008; Joy et al. 1982; Martinez and Martinez-Conde 1995; Martinez et al. 1991; Matsuura et
al. 2005; Parmar et al. 2003; Tusell et al. 1988; Vendrell et al. 1992a, 1992b; Woolley and Griffith 1989).
Kindling, the induction of seizures with repeated application of subthreshold electrical or chemical stimuli
to the brain, has been used as a method of investigating neurological response to γ-HCH poisoning. A
single oral dose of 5–20 mg/kg γ-HCH to either naïve or rats previously kindled by electrical stimulus
produced myoclonic jerks and clonic seizures, which increased in a dose-dependent manner and were
increased in kindled animals (Gilbert and Mack 1995). Enhanced susceptibility to kindled seizures
brought on by electrical stimulation was seen in rats exposed for 10 weeks to 10 mg/kg/day γ-HCH,
3 days/week (Gilbert 1995). Increased rates of acquisition of kindled seizures were observed following
dosing of rats with 3–10 mg γ-HCH/kg/day for 4 days (Joy et al. 1982). Single daily doses of 20 mg/kg
γ-HCH in mice significantly reduced the convulsive threshold, as measured by the dose of
pentylenetetrazol required to induce seizures 1–4 hours after treatment, but increased the convulsive
threshold 48 hours following treatment (Hulth et al. 1978). A dose of 50 mg/kg γ-HCH significantly
increased the convulsive threshold 2, 4, and 10 days following dosing (Hulth et al. 1978).
Two studies of rats showed that oral administration of γ-HCH can alter neurotransmitter levels in the
brain. Decreased levels of brain serotonin were reported in rats exposed for 6 days to a dose of
3 mg/kg/day γ-HCH (Attia et al. 1991), while 10 doses totaling 60 mg/kg γ-HCH over a period of 30 days
resulted in decreased brain dopamine levels (Martinez and Martinez-Conde 1995).
HEXACHLOROCYCLOHEXANE (HCH) 147
2. HEALTH EFFECTS
Acute and subchronic neurotoxicity screening bioassays including functional observational battery, motor
activity assessments, and neuropathology were reported in unpublished Confidential Business
Information (CBI) submissions summarized by EPA (1999a, 1999b). In the acute neurotoxicity screening
study, exposure to γ-HCH caused decreased motor activity 3 hours after gavage dosing of female rats
with ≥20 mg/kg and males at 60 mg/kg (EPA 1999a). Females also had increased forelimb grip strength
and decreased grooming behavior at 20 mg/kg, and an absence of grooming behavior at 60 mg/kg. Other
effects at 60 mg/kg included clinical signs (e.g., piloerection, urine-stained fur, tremors, and/or
convulsions) in both sexes and increased hindlimb foot splay in males (EPA 1999a). A 13-week
neurotoxicity screening study in Crl:CDBR rats by the same author (EPA 1999b) showed neurological
effects in both sexes at the highest dose (28.1–30.2 mg/mg/day), including clinical signs (e.g.,
piloerection, abnormal grooming behavior), increased rearing, walking on tiptoes, hypersensitivity to
touch, hunched posture, and several deaths. There were no effects on forelimb or hindlimb grip strength,
hindlimb splay, motor activity, or neuropathology (EPA 1999b).
Neurobehavioral testing in rats exposed for acute and intermediate durations have shown effects on
activity, cognition, and memory. Increased anxiety (Llorens et al. 1990) and decreased motor activity
(EPA 1999a) were reported in rats following a single gavage dose of 20 mg/kg, and increased
spontaneous motor behavior was observed at 10 mg/kg (Llorens et al. 1989). Avoidance response latency
was significantly increased in rats administered a single dose of 15 mg/kg by gavage (Tilson et al. 1987).
Impaired neurocognition, measured as decreased step-down latency in passive avoidance test and
prolonged transfer latency in the elevated plus maze test, occurred in rats exposed for 6 weeks to a γ-HCH
dose of 15 mg/kg/day (Sahaya et al. 2007). Srivastava et al. (2019) observed behavioral changes (reduced
locomotor activity and impaired spatial memory) in rats exposed to 2.5 mg/kg/day for 21 days. A longer
exposure (40 days) at this dose (2.5 mg/kg/day) resulted in significantly altered Skinner box behavior
(operant conditioning) in a small number of rats (Desi 1974).
At a γ-HCH dose (2.5 mg/kg/day for 21 days) that induced changes in locomotor activity and spatial
memory, Srivastava et al. (2019) detected ultrastructural changes in the hippocampus and substantia nigra
of rats. Changes in the brain included swollen mitochondria with disintegrated cristae, shortened fuzzy
synapse, disintegrated myelin layer, and autophagosomes (Srivastava et al. 2019). Peripheral nerve
effects were seen in one oral study of γ-HCH. Significantly decreased nerve conduction velocity was
measured in rats exposed to 25.4 mg/kg/day for 30 days (Muller et al. 1981).
HEXACHLOROCYCLOHEXANE (HCH) 148
2. HEALTH EFFECTS
While data are more limited, neurotoxicity has been documented in rats and rabbits exposed to γ-HCH via
dermal application. Clinical signs such as excitability, seizures, and convulsions were observed in rabbits
following a single topical application of 60 mg/kg γ-HCH in a 1% solution (Hanig et al. 1976); young
rabbits were more susceptible than older rabbits. Slight sedation was observed in rats exposed once for
24 hours to 1,000 mg/kg γ-HCH through shaved dorsal skin (Ullmann 1986a). One female exposed to
2,000 mg/kg in this study exhibited severe sedation and spasms (Ullmann 1986a). Aggressiveness or
hyperactivity were noted in rats exposed dermally for 13 weeks to ≥10 mg γ-HCH/kg/day, while ataxia,
tremors, and convulsions were seen in females at 60 mg/kg/day (EPA 1988a).
Mechanisms. Gavage administration of 2.5, 5, 10, or 15 mg γ-HCH/kg/day for 5 days produced a dose-
dependent increase in the activities of EROD, PROD, and NDMA-d in the brain of Wistar rats (Parmar et
al. 2003). In the same study, Parmar et al. (2003) examined the effect of metabolism on the convulsive
effect of γ-HCH in rats. A single dose of 35 mg/kg of γ-HCH induced convulsions in 4 out of 10 animals.
Pretreatment of the rats with 3-methylcholanthrene (MC), an inducer of CYP1A1/1A2, had no significant
effect in the incidence of convulsions induced by γ-HCH. However, induction of CYP 2B1/2B2 (by
pretreatment with phenobarbital) or CYP2E1 (by pretreatment with ethanol) significantly increased the
incidence of convulsions caused by γ-HCH, as did blocking of cytochrome P450-mediated metabolism
with cobalt chloride (Parmar et al. 2003). Taken together, the results suggest that the convulsive activity
is due to γ-HCH per se and/or to metabolites formed by phenobarbital- or ethanol-inducible P450
isoenzymes.
Decreased myelin was observed in rats exposed to 5 mg/kg/day by gavage for 3 days (Serrano et al.
1990). These authors also detected a significant decrease in 2',3'-cyclic nucleotide 3'-phosphodiesterase
(CNP) activity in treated animals, although no dose-response was seen. This enzyme is myelin-specific,
but its exact function in normal myelin in unknown (Serrano et al. 1990).
Increased lipid peroxidation (thiobarbituric acid reactive substances [TBARS]) and decreases in
antioxidant enzyme activities (superoxide dismutase, catalase, and glutathione peroxidase) were measured
in the brains of Wistar rats after three daily doses of 5 mg/kg/day γ-HCH; neurological endpoints were
not evaluated in these animals (Hfaiedh et al. 2012). Fatih Fidan et al. (2008) reported increased
malondialdehyde and decreased levels of reduced glutathione in the brains of rats exposed to γ-HCH
(≥10 mg/kg/day) by daily gavage for 30 days. Similarly, a 30-day exposure to doses of 50 mg/kg/day
induced lipid peroxidation (measured as TBARS) and depletion of antioxidant enzymes (glutathione
peroxidase and catalase) in the brains of rats exposed by drinking water (Hfaiedh et al. 2011). After
HEXACHLOROCYCLOHEXANE (HCH) 149
2. HEALTH EFFECTS
6 weeks of daily exposures to 15 mg/kg/day, rats exhibited increased malondialdehyde and non-protein
thiols in the brain (Sahaya et al. 2007). In contrast, no significant changes were seen in lipid peroxidation
in brain tissue from rats treated for 90 days with 90 mg γ-HCH/kg/day in food (Arisi et al. 1994).
Technical HCH or Unspecified Isomers of HCH. Paresthesia of the face and extremities, headache, and
vertigo were reported in a group of 45 workers occupationally exposed during manufacture and
formulation of technical-grade HCH for several years (Kashyap 1986); exposure concentrations were not
reported. Both inhalation and dermal exposures were possible. Heiberg and Wright (1955) reported
convulsions in a woman who had treated cattle with an insecticide containing 11% γ-HCH and 16% other
HCH isomers.
In animals exposed to technical-grade HCH by oral administration, effects like those seen with β- and
γ-HCH were seen. Behavioral and neurochemical changes were evaluated in rats that were administered
technical-grade HCH in doses of 10 or 20 mg/kg/day in oil by gavage for 7–30 days (Sahoo et al. 1999).
Assessment of open-field behavior (horizontal motor activity, vertical exploratory rearing, and grooming
activities) and brain biochemistry (ATPases and acetylcholinesterase) showed effects that included
reduced brain total ATPase and Na
+
-, K
+
-, and/or Mg
2+
-ATPase activities after 7–30 days at
≥10 mg/kg/day, reduced brain acetylcholinesterase activity after 15 and 30 days at 20 mg/kg/day,
increased motor activity after 7 days at 20 mg/kg/day, and reduced grooming behavior after 30 days at
20 mg/kg/day (Sahoo et al. 1999). Mudawal et al. (2018) observed impairments in learning (conditioned
avoidance response and Y-maze continuous alternation test) and increased spontaneous locomotor
activity in rats given technical-grade HCH for 21 days beginning at 3, 18, or 48 weeks of age. The most
pronounced effects were observed in the aged rats. After exposure that began at 48 weeks of age, the
animals also exhibited ultrastructural changes in the hippocampus and substantia nigra when examined by
transmission electron microscopy; in contrast, similar exposure beginning at 3 or 18 weeks of age did not
result in ultrastructural changes (Mudawal et al. 2018). Increased motor activity was also observed in rats
exposed to technical-grade HCH at a level of 50 mg/kg/day for 120 days (Gopal et al. 1992).
Alterations in neurotransmitter levels, increased brain wave frequency, and behavioral changes were
reported in male rats administered 50 mg/kg/day technical-grade HCH by gavage for 1 or 3 months
(Anand et al. 1991). Exposure to 0.4 mg/kg/day technical-grade HCH for 360 days resulted in
convulsions, tremors, and paralysis in male rats after 270 days, although the number of animals affected
and the severity of the symptoms were not reported (Dikshith et al. 1991a). This study also found
degeneration of the cerebellum and cerebellar cortex in animals sacrificed after a 1-year exposure to
HEXACHLOROCYCLOHEXANE (HCH) 150
2. HEALTH EFFECTS
20 mg/kg/day. Seizures were noted in mice exposed to technical-grade HCH through feed or gavage at
levels of 10–17 mg/kg/day for 80 weeks (Kashyap et al. 1979). Damage to Purkinje cells in the
cerebellum and tremors were found in female Wistar rats treated with 100 mg/kg/day technical-grade
HCH for 7–30 days (Dikshith et al. 1991c).
Increased levels of brain catecholamines, particularly norepinephrine and dopamine, and associated signs
of toxicity such as mild tremor, lacrimation, salivation, and dyspnea were observed in female rats given
oral doses of 100 mg/kg/day of technical-grade HCH for 7 days (Raizada et al. 1993). The activity of
monoamine oxidase (MAO, an enzyme that oxidizes monoamine neurotransmitters) in the cerebrum
showed a marginal decrease, while significant increases and decreases were observed in the cerebellum
and spinal cord, respectively (Raizada et al. 1993). Rats treated with 20 mg technical-grade HCH/kg/day
in food for 90 days exhibited increased γ-aminobutyric acid (GABA) levels, increased glutamate
decarboxylase (GAD) activity, and decreased glutamate levels in the brain (Nagaraja and Desiraju 1994).
Mechanisms. As with γ-HCH, there is some evidence that oxidative stress may contribute to the
neurotoxic effects of technical-HCH. Mudawal et al. (2018) observed increased lipid peroxidation and
decreases in both antioxidant enzyme activities (superoxide dismutase and catalase) and reduced
glutathione in the hippocampus and substantia nigra of rats given 2.5 mg/kg/day technical HCH for
21 days. These changes correlated with neurobehavioral effects, as discussed above.
2.16 REPRODUCTIVE
Epidemiological Studies. Few epidemiological studies on the reproductive effects of HCH isomers were
located; the available studies are summarized in Table 2-15. With one exception (Freire et al. 2014), the
studies were conducted in populations without known sources of exposure to HCH; in these populations,
consumption of contaminated food is expected to be the primary exposure route. All these studies
measured HCH isomers in serum, fat, or follicular fluid as biomarkers of exposure, and exposure was
measured simultaneously with outcome assessment or after the outcome occurred.
Freire et al. (2014) conducted a cross-sectional study of reproductive hormone and HCH levels in the
serum of 604 people residing near a former HCH manufacturing facility in Brazil. In this population, an
inverse association between serum testosterone concentrations and serum α- and β-HCH concentrations
was observed in men. In women, increases in serum β-HCH were associated with increased serum

HEXACHLOROCYCLOHEXANE (HCH) 151
2. HEALTH EFFECTS
Table 2-15. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Reproductive
Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Akkina et al. 2004
Cross-sectional, 219 menopausal women,
Hispanic Health and Nutrition Examination
survey (HHANES), United States
Age at menopause
β-HCH
Serum
>2.09 ng/g (> median)
↓
Buck Louis et al. 2012
Matched cohort, operative cohort:
473 women undergoing laparoscopy or
laparotomy; population-based cohort:
127
women matched on age and residence,
18–44 years old, United States
Endometriosis
β-HCH
Serum
Operative cohort, medians:
0.0063 ng/g (cases)
0.0063 (non-cases)
↔
Population cohort, medians:
0.0066 (cases)
0.0063 (non-cases)
↑
γ-HCH
Omental fat
Operative cohort, medians:
0.1991 ng/g fat (cases)
0.1200 (non-cases)
↑
Ploteau et al. 2017
Case-control, 55 cases of deep infiltrating
endometriosis and 44 controls, 18–
45 years
old, France
Deep infiltrating
endometriosis
β-HCH
Adipose
tissue
13.62 ng/g lipid (median) (cases)
14.33 (controls)
↔
Deep infiltrating
endometriosis with
ovarian
endometrioma
21.61(cases)
14.33 (controls)
↑
Upson et al. 2013
Case-control, 248 cases of endometriosis
and 538 population-based controls,
Washington, United States
Endometriosis
β-HCH
Serum
>43.06 pg/g (3
rd
quartile)
↑
γ-HCH
>13.89 (4
th
quartile)
↔
Sum HCH
>0.29 mol/g (4
th
quartile)
↔
Ovarian
endometriosis
β-HCH
Serum
>43.06 pg/g (3
rd
quartile)
↑
γ-HCH
>13.89 (4
th
quartile)
↔
Sum HCH
>0.29 mol/g (4
th
quartile)
↔
Al-Hussaini et al. 2018
Cross-sectional, 94 women in infertile
couples undergoing intracytoplasmic sperm
injection, 20–38 years old, Egypt
Endometrial
thickness
γ-HCH
Follicular
fluid
418.6±171.4 µg/L (mean±SD)
↑
Implantation rate
↓

HEXACHLOROCYCLOHEXANE (HCH) 152
2. HEALTH EFFECTS
Table 2-15. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Reproductive
Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Pollack et al. 2021
Operative cohort, 339 women undergoing
laparoscopy or laparotomy, age 18-
44 years, United States
Endometriosis
β-HCH
Omental fat
0.14 ng/g (median)
↑
Serum
0.013
↔
Adipose to
serum ratio
Not reported
↑
γ-HCH
Omental fat
0.137
↑
Serum
0.019
↔
Adipose to
serum ratio
Not reported
↑
Génard-Walton et al. 2023
Case-control, 138 cases of diminished
ovarian reserve and 151 controls,
mean age
33.8 years for cases, 32.4 years for
controls, France
Diminished ovarian
reserve
β-HCH
Serum
4.1 ng/g lipid (cases) (median)
4.5 (controls)
↓
Abou Ghayda et al. 2020
Prospective cohort, 152 males enrolled in
the Russian children’s study at 8–9 years of
age and followed up at 18–23 years of age,
Russia
Semen volume
β-HCH
Serum
172 ng/g lipid (median)
↓
Sperm concentration
↔
Total sperm count
↔
Progressive motility
↔
Madrigal et al. 2021
Cross-sectional, 748 men aged ≥20 years,
NHANES 1999–2004, United States
Serum testosterone
β-HCH
Serum
22.56-1200.0 ng/g lipid (4
th
quartile)
↔
Serum estradiol
↔
Serum sex hormone
binding globulin
↔
Serum androstanediol
glucuronide
↔

HEXACHLOROCYCLOHEXANE (HCH) 153
2. HEALTH EFFECTS
Table 2-15. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Reproductive
Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Miao et al. 2022
Cross-sectional, 387 men seeking semen
analysis, mean age 30.5 years, China
Sperm motility
α-HCH
Serum
0.08 ug/L
↔
β-HCH
0.85
↔
γ-HCH
0.12
↓
δ-HCH
0.70
↔
Sperm concentration,
sperm count
α-HCH
See above
↔
β-HCH
See above
↔
γ-HCH
See above
↓
δ-HCH
See above
↔
Zeng et al. 2022
Cross-sectional, 421 men seeking semen
analysis, mean age 30.6 years, China
Serum testosterone
α-HCH
Serum
0.10 ug/L (75
th
percentile)
↔
β-HCH
1.03
↔
γ-HCH
0.12
↔
δ-HCH
0.89
↔
Freire et al. 2014
Cross-sectional, 604 persons 15–94 years
old, residing near former HCH
manufacturing facility, Brazil
In men:
Serum testosterone
α-HCH
Serum
2.52 ng/mL (median)
↓
a
β-HCH
6.00
↓
γ-HCH
0.95
↔
In premenopausal women:
Serum estradiol,
progesterone,
prolactin, LH, and
FSH
α-HCH
Serum
2.77 ng/mL (median)
↔
β-HCH
6.32
↔
γ-HCH
0.89
↔

HEXACHLOROCYCLOHEXANE (HCH) 154
2. HEALTH EFFECTS
Table 2-15. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Reproductive
Effects
Reference, study type, and population
Outcome evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
In peri- or post-menopausal women:
Serum estradiol
α-HCH
Serum
2.43 ng/mL (median)
↔
β-HCH
11.72
↑
a
γ-HCH
1.07
↔
Serum LH
α-HCH
Serum
See above
↔
β-HCH
See above
↓
γ-HCH
See above
↔
Serum progesterone,
prolactin, and FSH
α-HCH
Serum
See above
↔
β-HCH
See above
↔
γ-HCH
See above
↔
a
Borderline significant.
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; FSH = follicle-stimulating hormone; LH = luteinizing
hormone; NHANES = National Health and Nutrition Examination Survey; SD = standard deviation
HEXACHLOROCYCLOHEXANE (HCH) 155
2. HEALTH EFFECTS
estradiol and decreased serum luteinizing hormone (LH) levels among peri- and post-menopausal women,
but not premenopausal women. No associations were seen between any HCH isomer and serum
progesterone, prolactin, or follicle-stimulating hormone (FSH) in women. Serum α- and γ-HCH levels
showed no association with reproductive hormone levels in women (Freire et al. 2014).
There were no observed associations between serum levels of HCHs and serum testosterone in two cross-
sectional studies (Madrigal et al. 2021; Zeng et al. 2022), and no association between serum β-HCH and
serum estradiol, sex hormone binding globulin, or androstanediol glucuronide in one of the studies
(Madrigal et al. 2021). A decrease in semen volume at adulthood was associated with pre-pubescent
serum levels of β-HCH in a prospective cohort study of 152 Russian men (Abou-Ghayad et al. 2020).
Miao et al. (2022) observed an association between γ-HCH levels in serum and decreased sperm motility
in a cross-sectional study in China. Neither γ-HCH nor other HCH isomers in serum were associated with
changes in sperm count or concentration in this study.
In a matched cohort study of 473 women undergoing laparoscopy or laparotomy (operative cohort) and
127 women matched on age and residence (population-based cohort), both β-HCH levels in serum and
γ-HCH levels in omental fat were associated with increased risk of endometriosis (Buck Louis et al.
2012). Three case-control studies also reported associations between endometriosis and biomarkers of
β-HCH exposure. An association between deep infiltrating endometriosis with ovarian endometrioma
and β-HCH in adipose tissue was seen in a study of 99 adult women in France (55 cases and 44 controls)
(Ploteau et al. 2017). Upson et al. (2013) observed associations between serum levels of β-HCH and both
endometriosis and ovarian endometriosis in a larger study in the United States (248 cases and
538 controls). Serum concentrations of γ-HCH were not associated with endometriosis in this study
(Upson et al. 2013). Pollack et al. (2021) observed an association between incident endometriosis and
β- and γ-HCH concentrations in adipose tissue and adipose to serum concentration ratios; however, no
association was seen with β- or γ-HCH concentrations in serum. Measurements of γ-HCH in follicular
fluid from 94 women undergoing intracytoplasmic sperm injection were associated with increased
endometrial thickness and decreased implantation rate (Al-Hussaini et al. 2018).
A case-control study in France observed an inverse association between diminished ovarian reserve and
serum levels of β-HCH (Génard-Walton et al. 2023). There were no other studies of this endpoint.
HEXACHLOROCYCLOHEXANE (HCH) 156
2. HEALTH EFFECTS
A small cross-sectional study (Akkina et al. 2004) of Hispanic women in the United States reported a
decrease in the age at menopause associated with higher serum levels of β-HCH; no other studies of this
endpoint were located.
α-HCH. No treatment-related histopathology findings were noted in the testes or uterus and ovaries of
rats given α-HCH in feed at doses up to 70 mg/kg/day for an average of 6 months or 9 mg/kg/day for
2 years (Fitzhugh et al. 1950).
β-HCH. Oral exposure to 60 mg β-HCH/kg for 30 days resulted in normal uteri and reproductive cycling
in female mice (Cornacoff et al. 1988). Atrophy of the ovaries and testes, hyperplastic and vacuolized
endometrial epithelium, degeneration of the seminiferous tubules, and disruption of spermatogenesis were
seen in rats exposed to 22.5–25 mg/kg/day β-HCH in the diet (Van Velsen et al. 1986). Half of the
animals in this group showed significant clinical signs of neurotoxicity and were humanely sacrificed
before the end of the 13-week study; abnormal reproductive organ pathology was seen in both survivors
and early decedents (Van Velsen et al. 1986). While no effects were seen upon microscopic examination
of the testes, uteri, and ovaries of rats given β-HCH in the diet at doses up to 70 mg/kg/day for up to
10 weeks, slight testicular atrophy was seen after 2 years of exposure to 7 mg/kg/day (Fitzhugh et al.
1950).
γ-HCH (Lindane). Statistically significant increases in the levels of serum LH were reported in a group
of 54 men occupationally exposed to unspecified concentrations of γ-HCH for approximately 8 years in a
γ-HCH-producing factory (Tomczak et al. 1981). Although the mean serum concentration of FSH was
increased and testosterone was decreased, these differences were not statistically significant compared to
mean values determined in a control group.
Studies of reproductive effects in animals exposed by inhalation are limited to two intermediate-duration
studies focused on systemic toxicity endpoints. Histopathology evaluation of the testes, prostate, ovaries,
and uterus of rats and mice exposed by inhalation to 5 mg/m
3
γ-HCH for 13–14 weeks showed no effects
of treatment (Klonne and Kintigh 1988; Oldiges et al. 1983).
In animals exposed orally for acute and intermediate durations, γ-HCH induced effects on the male
reproductive system, female reproductive system, and on mating, fertility, and early gestation endpoints,
as discussed below.
HEXACHLOROCYCLOHEXANE (HCH) 157
2. HEALTH EFFECTS
Effects on male reproductive system. The male reproductive system appears to be sensitive to the toxic
effects of orally administered γ-HCH. Rats and mice exposed to this isomer have exhibited effects on
spermatogenesis, reproductive organ weight changes, and histopathology changes in the testes, while
altered sexual behavior was reported in sheep. The lowest dose associated with effects on the male
reproductive tract in acute-duration studies is 6 mg/kg/day (Dalsenter et al. 1996); for intermediate-
duration studies, it is 1 mg/kg/day in mink (Beard and Rawlings 1998).
In male rats, oral administration of 6 mg/kg for 5 days or a single dose of 30 mg/kg of γ-HCH resulted in
a reduction in the number of testicular spermatids and epididymal sperm of both treated groups 2 weeks
after treatment (Dalsenter et al. 1996). γ-HCH was detected in the testes of both groups 24 hours and
2 weeks after the last treatment. Histological examination by electron microscopy revealed ballooning of
the Sertoli cells with fragmentation or loss of organelles (Dalsenter et al. 1996). Sharma and Singh
(2010) administered γ-HCH (30 mg/kg/day) by gavage to Wistar rats for 14 and 28 days for evaluation of
effects on the male reproductive tract. After 14 days, the rats had markedly decreased epididymis (27%)
and testes (68%) weights. In addition, substantial and persistent reductions (≥85% less than controls) in
sperm head count, motility, and percent live sperm, and marked and persistent increases (4-fold) in
percent abnormal sperm were observed (Sharma and Singh 2010). After 28 days at this dose, the effects
on epididymis and testes weights were more pronounced, as were the changes in sperm parameters:
decreases of ≥89% compared to controls were seen in sperm head count, motility, and percent live sperm,
as well as a 4-fold increase in percent abnormal sperm (Sharma and Singh 2010). Similar results were
seen in Wistar rats given 50 mg/kg/day γ-HCH in water for 30 days (Hfaiedh et al. 2011). The weights of
the testes, epididymides, and prostate gland were decreased by 42–52% relative to controls, and the
seminal vesicle weight was reduced by 5%. Compared to control values, sperm count was diminished by
56% and sperm motility by 37% at this dose (Hfaiedh et al. 2011). In a 45-day exposure study, Saradha
and Mathur (2006) observed decreased sperm count (~7%) and motility (~15%) in male Wistar rats
administered doses of 1 mg/kg/day by gavage. At the higher dose of 5 mg/kg/day in this study, the
effects were more severe, with a 27% decrease in sperm count and ~25% decrease in sperm motility
(Saradha and Mathur 2006). In a 2-generation reproductive toxicity study in Crj:CD(SD)IGS rats given
dietary doses up to 23.3 mg/kg/day, no statistically significant treatment-related effects on sperm count,
motility, or percent abnormal sperm were noted in F0 or F1 males (Matsuura et al. 2005). Histology of
the parental male reproductive organs was also normal (Matsuura et al. 2005). Dietary exposure to up to
120 mg/day γ-HCH for 10 months or 30 mg/kg/day for 2 years or did not result in histopathology changes
in the testes of rats (Fitzhugh et al. 1950).
HEXACHLOROCYCLOHEXANE (HCH) 158
2. HEALTH EFFECTS
Exposure to γ-HCH has also induced altered levels of reproductive hormones in male rats. When male
Wistar rats were exposed to 50 mg/kg/day γ-HCH in water for 30 days, serum FSH levels were decreased
by 74% relative to controls (Hfaiedh et al. 2011). Effects of γ-HCH on the levels of reproductive
hormones in blood of male animals appear to be more significant in younger animals compared with older
animals. Groups of 30 male Wistar rats were administered 5 mg/kg/day γ-HCH for 5 days beginning at 9,
18, or 27 weeks of age (Agrahari et al. 2019). Animals treated with γ-HCH beginning at 9 weeks of age
exhibited significantly decreased serum testosterone (39%) and growth hormone (29%), and increased
serum LH (42%) and FSH (31%), compared to controls. Similar results were observed in the group
treated at 18 weeks of age, but treatment at 27 weeks of age resulted in smaller decreases in serum
testosterone and growth hormone, and no significant effect on serum LH or FSH. No dose-related effects
on serum hormone levels were observed in F0 or F1 male parents in a 2-generation study of
Crl:CD(SD)IGS rats at doses of 17.2–26.1 mg/kg/day in diet for ~10 weeks (Matsuura et al. 2005).
One study reported male reproductive tract effects in mice exposed to γ-HCH. Nagda and Bhatt (2011)
exposed Swiss mice by gavage (40 mg/kg/day) for 60 days. At sacrifice at the end of exposure, the mice
exhibited a 10% decrease in testes weight as well as histopathology changes in the testes, including
shrunken and distorted seminiferous tubules, sparse Leydig cells, and oligospermia (Nagda and Bhatt
2011). The male reproductive effects of γ-HCH were also studied in young rams given 1 mg/kg/day in
treated feed from conception to sexual maturity (Beard et al. 1999a). The subjectively-scored sexual
behavior in the rams was significantly reduced in treated animals presented with estrous ewes (Beard et
al. 1999a).
Effects on female reproductive system. Studies examining female reproductive effects in animals exposed
to γ-HCH are more limited, but suggest effects on estrous cycling and other endpoints in a variety of
species. Oral administration of γ-HCH for acute and intermediate durations has resulted in alterations in
estrous cycling, sexual behavior, and uterine histopathology. In acute-duration studies, the lowest dose
associated with these effects was 25 mg/kg/day (Uphouse and Williams 1989); in intermediate-duration
studies, the lowest dose associated with these effects was 1 mg/kg/day (Beard and Rawlings 1999).
Increased length of estrous cycle and decreased sexual receptivity were found in female rats treated with a
single dose of γ-HCH (≥25 mg/kg) given by gavage (Uphouse and Williams 1989). Female rabbits
exposed to 0.8 mg γ-HCH/kg/day, 3 days/week for 12 weeks had a reduced ovulation rate (Lindenau et al.
1994). Histopathological changes were observed in the uteri of female Sprague-Dawley rats given
γ-HCH by gavage at a dose of 8 mg/kg/day for 4 weeks. The uterine changes were described as low
columnar endometrial glandular epithelial cells (Yang et al. 2014; Zhang et al. 2016). Delayed vaginal
HEXACHLOROCYCLOHEXANE (HCH) 159
2. HEALTH EFFECTS
opening and disrupted ovarian cycling in female F344 rats given ≥10 mg/kg/day by gavage for 15 weeks
beginning at weaning (Chadwick et al. 1988). Finally, in estrus synchronized ewe lambs dosed with
γ-HCH in feed at 1 mg/kg/day from conception to sexual maturity, significantly shorter estrous cycle
length and reduced number and total volume of corpus lutea were observed (Beard and Rawlings 1999).
No other detrimental fertility effects were observed. No effects on maternal female reproductive organ
weights or histology were noted in CD mice exposed to 15 mg/kg/day via gavage on GDs 9–16
(Maranghi et al. 2007). In a 2-generation reproductive toxicity study in Crj:CD(SD)IGS rats, doses of
28.0 mg/kg/day in diet for ~10 weeks resulted in a significantly decreased estrus cycle length (4 days
versus 4.45 days in controls) in F1, but not F0 female adults (Matsuura et al. 2005). No effects were
observed on ovarian follicle counts at any dose in F1 females, and no changes in reproductive organ
weights or histology were observed at sacrifice of F0 or F1 female parental animals (Matsuura et al.
2005). Fitzhugh et al. (1950) observed no effects on the histopathology of the uterus or ovaries of rats
given γ-HCH via the diet for up to 140 mg/kg/day for 10 months or up to 30 mg/kg/day for 2 years.
In female Wistar rats dosed with γ-HCH by daily gavage for 4 weeks, significantly decreased serum
levels of estradiol (20 and 26%) and testosterone (28 and 37%) were observed at 4 and 8 mg/kg/day
γ-HCH, respectively (Zhang et al. 2016; Yang et al. 2014). Significant, dose-related increases in serum
LH were seen at all doses (23–44% relative to controls at doses from 0.95 to 28 mg/kg/day) in F1 females
during proestrus in a 2-generation study of Crj:CD(SD)IGS rats (Matsuura et al. 2005). Serum hormone
levels in F0 female parents were not impacted by exposure in this study. Female mink exposed to
1 mg/kg/day γ-HCH before mating and through mating, gestation, and lactation exhibited no effects on
serum estradiol or progesterone when evaluated at weaning of their kits (Beard et al. 1997). Sheep
exposed on a similar schedule to the same dose did not show changes in serum LH or FSH (Beard et al.
1999b)
Effects on mating, fertility, and early gestation. In a multigeneration reproduction study with γ-HCH,
Charles River CD rats were exposed to estimated dietary doses of 0, 0.09, 1.7, or 13.1 mg/kg/day for
2 generations (EPA 1991a). No treatment-related effects on mating, fertility, gestation survival, liveborn
indices, or mean litter sizes occurred in either generation, although developmental toxicity occurred at
13.1 mg/kg/day, as shown by reduced body weight and decreased viability in pups of both generations
and delayed maturation of F2 pups (see Section 2.17). Similar findings were noted in the 2-generation
study reported by Matsuura et al. (2005). No effects on mating, fertility, gestation length, birth index, or
gestation index were seen in Crj:CD(SD)IGS rats of either generation at doses up to 28 mg/kg/day, but
there were developmental effects on offspring body weight, viability, and sexual maturation (see
HEXACHLOROCYCLOHEXANE (HCH) 160
2. HEALTH EFFECTS
Section 2.17). Female rabbits dosed with 0.8 mg γ-HCH/kg/day, 3 days/week for 12 weeks followed by
artificial insemination exhibited no effects on the fertilization rate or on pre- or post-implantation losses
(Seiler et al. 1994).
Mice and mink appear to be more sensitive than rats to the effects of γ-HCH on fertility and early
gestation. When mouse dams were treated with γ-HCH (6.2 mg/kg) during GDs 6–12, all fetuses were
resorbed (Sircar and Lahiri 1989). In another experiment by these authors, pregnant mice exposed to
10.8 mg/kg/day on GDs 1–4 exhibited no implantation sites. When pregnant mice were exposed to
3.6 mg/kg/day on GDs 14–19, all pups died (Sircar and Lahiri 1989). Acute preovulatory exposure to
γ-HCH caused embryonic effects in mice (Scascitelli and Pacchierotti 2003). Three consecutive daily
doses of γ-HCH in olive oil were administered to female mice either before mating (during the
preovulatory period) or immediately after mating. Oocyte maturation, ovulation, and fertilization were
evaluated by assessing percentage of vaginal plug positive females, number of embryos/female,
percentage of one-cell embryos (corresponding to unfertilized oocytes or zygotes that did not undergo
cleavage), and gross morphologic alterations of two-cell embryos. Preimplantation embryonic
development was evaluated by morphological examinations of morulae for determinations of one-cell
embryos (unfertilized eggs or zygotes that did not undergo cleavage), embryos retarded in their cleavage,
and abnormal embryos, as well as by cytological examinations of morulae for determinations of
interphase nuclei, meta-anaphases, apoptotic nuclei, micronuclei, and mitotic index. Preovulatory
exposure caused a significant increase of degenerating two-cell embryos (lysis or fragmentation of
blastomeres), but there were no exposure-related effects of post-mating treatment.
Reproductive toxicity studies in mink showed effects on sexual receptivity, whelping rate, and embryo
mortality. A 2-generation reproduction study of γ-HCH was conducted in mink that were exposed to
dietary doses of 0 or 1 mg/kg/day (Beard and Rawlings 1998). The parental (P0) generation was exposed
from 3 weeks before breeding until weaning of the offspring. Following weaning, the F1 females were
exposed throughout growth and mating (to untreated males), and subsequently throughout pregnancy and
lactation until 3 months post-lactation. The F2 females were exposed until they reached full adult body
size at 30 weeks of age. The F1 and F2 males were exposed until the time their testis development was
maximal (sexual maturity) at about 42 weeks of age. In addition to standard reproductive indices, serum
hormone levels (estradiol, testosterone) and histology of male and female reproductive tissues were
evaluated in offspring of both generations. There were no overt signs of toxicity or effects on mating
percentage. Fertility was reduced in both generations, as shown by reductions in whelping rate and litter
size, such that exposed mink produced approximately 60% fewer kits than controls. Other effects
HEXACHLOROCYCLOHEXANE (HCH) 161
2. HEALTH EFFECTS
included reduced testis size in F2 males. In a single-generation study, female mink treated with
1 mg/kg/day γ-HCH in their diet from 3–6 weeks before mating until weaning at 8–10 weeks postpartum
showed effects on reproductive efficiency that included reduced receptivity to a second mating and
reduced whelping rate, although litter size was not affected (Beard et al. 1997). This decreased fertility
effect was primarily a result of embryo mortality after implantation.
Mechanisms. Inhibition of the formation of estradiol-receptor complex in the rat uterus cytosol was
reported in female rats administered 30 mg γ-HCH/kg/day by oral intubation for 7 days (Tezak et al.
1992). Statistically significant increases in the glycogen content of the uterus, cervix, and vagina (but no
increase in organ weight) were reported in female rats exposed to 20 mg γ-HCH/kg/day in the diet for
30 days (Raizada et al. 1980). Antiestrogenic properties were found in female rats given gavage doses of
10 mg/kg/day γ-HCH for 15 weeks (Chadwick et al. 1988). These responses were not seen at
5 mg/kg/day. Ovariectomized rats exposed for 5 days and sexually immature female rats exposed for
7 days to 40 mg γ-HCH/kg/day showed no effects on the number of estrogen and estrogen-dependent
progesterone receptors (Laws et al. 1994). Thus, γ-HCH's antiestrogenic effects in reproductive tissue do
not appear to be due to direct action on estrogen receptors or its induction of progesterone receptors.
In vitro studies have not shown binding of γ-HCH to the estrogen receptor, but one study showed that this
isomer could inhibit the activity of aromatase (the enzyme that forms estrogen in mammals) in human
placental and embryonic kidney cells transfected with the associated gene (reviewed by IARC 2018).
Technical or Unspecified HCH. Studies of reproductive toxicity in animals exposed orally to technical-
grade HCH have shown effects on the male reproductive tract of rats and mice. Dermal exposure of rats
and guinea pigs induced similar changes.
Immature (15-day-old) and mature (90-day-old) rats were administered technical-grade HCH in doses of
10 or 20 mg/kg/day in oil by gavage for 7, 15, or 30 days (Samanta et al. 1999). Exposure to
≥10 mg/kg/day for 7 days caused effects that included reduced epididymis weight in immature rats and
reduced seminal vesicle and ventral prostate weights in adult rats. Effects observed following exposure to
≥10 mg/kg/day for 7–30 days included reduced total sperm count and increased frequencies of damaged
sperm and sperm with anomalous heads in adult rats. Shivanandappa and Krishnakumari (1983) reported
testicular atrophy, degeneration of seminiferous tubules, and disruption of spermatogenesis in male rats
fed technical-grade HCH at 75 mg/kg/day for 90 days. After 180 days of exposure to 3 mg/kg/day
technical-grade HCH, male Charles Foster rats exhibited significant decreases in testes and vas deferens
HEXACHLOROCYCLOHEXANE (HCH) 162
2. HEALTH EFFECTS
weights, as well as decreases in seminiferous tubule diameter and degeneration of muscle tissue in the vas
deferens (Gautam et al. 1989; Roy Chowdhury and Gautam 1990). At a higher dose of 6 mg/kg/day,
complete degeneration of testicular tissue, post-meiotic spermatogenic arrest, and degeneration of
spermatogenic cells were seen (Roy Chowdhury and Gautam 1990). Testicular degeneration was
reported in male rats exposed to 20 mg/kg/day technical-grade HCH in the diet for 360 days (Dikshith et
al. 1991a). Fitzhugh et al. (1950) observed no effects on the histopathology of the testes, uterus, or
ovaries of rats given technical HCH via the diet for up to 9 mg/kg/day for 2 years. Moderate testicular
atrophy was observed in rats given technical HCH in the diet for 6 months at a dose of 60 mg/kg/day
(Fitzhugh et al. 1950). In mice, exposure to 90 mg technical-grade HCH/kg/day (isomer composition
unknown) for 3 months led to increased testicular weight and degeneration of seminiferous tubules
(Nigam et al. 1979).
Male and female Druckrey rats were exposed via diet and drinking water to estimated total daily doses of
0, 16, or 32 mg/kg/day technical-HCH throughout 3 generations (Srivastava and Raizada 2000). There
were no exposure-related effects on reproduction in any of the 3 parental generations, and no
morphological or teratological changes in any of the offspring generations (F1b, F2b, or F3b).
In studies of dermal exposure, the backs of male rats were sprayed with 50 or 100 mg/kg/day technical-
grade HCH for 120 days and the rats were housed in separate cages to prevent licking (Prasad et al.
1995). Depletion of germ cells and impaired function of Leydig and Sertoli cells was suggested by
significant dose-related changes in activities of testicular enzymes such as sorbitol dehydrogenase,
glucose-6-P-dehydrogenase, γ-glutamyl transpeptidase, and β-glucuronidase. Significant reductions in
sperm count and motility and increased percentages of abnormal sperm were also observed in both
groups. A significant reduction in testosterone level was observed in the high-dose group. Dikshith et al.
(1978) reported testicular hypertrophy and atrophy and complete inhibition of spermatogenesis in guinea
pigs dermally treated with technical-grade HCH for 7, 15, or 30 days at doses as low as 100 mg/kg/day.
The patch of the abdomen on which the HCH was applied was not covered to prevent licking, so oral
exposure more than likely occurred.
Mechanisms. There is evidence that oxidative stress may contribute to the effects of technical-grade HCH
on the male reproductive system. Testicular oxidative stress was studied in immature (15-day-old) and
mature (90-day-old) rats that were administered technical-grade HCH in doses of 10 or 20 mg/kg/day in
oil by gavage for 7, 15, or 30 days (Samanta et al. 1999). Endpoints that were evaluated included
testicular protein and lipid peroxidation, testicular levels of antioxidant enzymes (superoxide dismutase,
HEXACHLOROCYCLOHEXANE (HCH) 163
2. HEALTH EFFECTS
catalase, glutathione peroxidase, glutathione reductase) and non-enzymatic antioxidants (reduced
glutathione, ascorbic acid, hydrogen peroxide). Testes from immature and adult rats exposed to
≥10 mg/kg/day for 7–30 days also showed increased lipid peroxidation and changes in glutathione
peroxidase, ascorbic acid, and hydrogen peroxide levels.
2.17 DEVELOPMENTAL
Developmental effects of HCH isomers have been evaluated in human populations and in animals. These
studies are discussed below in the individual isomer subsections. However, the epidemiological studies
of all isomers share limitations that render the reported associations uncertain, especially when considered
without any supporting animal data. Epidemiological studies of developmental effects were conducted in
the general population (without occupational exposure), generally using measurements of HCH isomers
in physiological fluids or tissues of mothers and infants. In the general population, the route(s) of
exposure is unknown. In the studies discussed herein, other organochlorine compounds (such as
hexachlorobenzene, aldrin, heptachlor and its epoxide, DDT and its metabolites, polychlorinated
biphenyls, and/or polychlorinated dioxins and furans) were also present in the blood. Few of the studies
controlled for these co-exposures; thus, the role of HCH isomers in the observed effects, if any, cannot be
ascertained. In addition, the case-control studies examined levels of HCH isomers in blood and tissues
after the outcome was established, rendering the temporal association between exposure and outcome
uncertain.
α-HCH. Data on the developmental effects of α-HCH are limited to a small number of human
epidemiological studies; there are no animal studies of developmental toxicity for this isomer. Table 2-16
provides a summary of human epidemiological data on developmental effects of α-HCH. As the table
shows, the epidemiology data suggest possible associations between growth retardation and maternal or
placental levels of α-HCH. Increased risks of fetal growth restriction (defined as <10
th
percentile of birth
weight for gestational age, and also termed ‘intrauterine growth retardation’ in some studies) were
associated with maternal blood levels of α-HCH in two small case-control studies in India (Sharma et al.
2012; Siddiqui et al. 2003). Reduced birth weight was associated with higher levels of α-HCH in placenta
in a small cross-sectional study in India (Anand and Taneja 2020), but not with maternal serum levels of
α-HCH in a cross-sectional study in an Arctic population in Russia (Bravo et al. 2019) or with cord serum
levels in a large cross-sectional analysis of 1,028 mother-infant pairs in China (Fang et al. 2019a, 2019b).
No association between cord serum α-HCH and infant BMI through age 24 months was observed in a

HEXACHLOROCYCLOHEXANE (HCH) 164
2. HEALTH EFFECTS
Table 2-16. Summary of Epidemiological Studies of α-Hexachlorocyclohexane (HCH) Exposure and
Developmental Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Mean concentration (unless
otherwise noted)
Result
Siddiqui et al. 2003
Case-control, 30 mothers of infants with intra-
uterine growth retardation, 24 mothers of
normal weight infants, India
Intra-uterine growth
retardation
Maternal blood
5.82±3.22 ng/g (mean±SD)
(cases)
3.79±3.14 (controls)
↑
Placenta
9.91±3.89 (cases)
8.88±5.17 (controls)
NR
Cord blood
9.84±5.12 (cases)
6.74±7.83 (controls)
NR
Fang et al. 2019a, 2019b
Cross-sectional, 1,028 pregnant mother-
infant pairs, China
Birth weight
Cord serum
≥0.718 ng/g lipid (3
rd
tertile)
↔
Birth length
↔
Ponderal index
↔
Gestational age
↓ (among
term births)
Anand and Taneja 2020
Cross-sectional, 90 mother-infant pairs, India
Birth weight
Placenta tissue
1.09–211.43 μg/L (range)
↓
Birth length
↔
Head circumference
↔
Ponderal index
↔
Bravo et al. 2019
Cross-sectional, 247 mother-child pairs,
Russia
Gestational age
Maternal serum
(last week of
pregnancy)
3.3 ng/g lipid (median)
↔
Birth weight
↔
Birth length
↔
Head circumference
↔
Yang et al. 2021a
Birth cohort, 1,039 mother-infant pairs
followed for 2 years after birth, China
Infant BMI at birth and
ages 6, 12, and
24 months; risk of
overweight status
Cord serum
0.35 ng/g lipid (median)
↔

HEXACHLOROCYCLOHEXANE (HCH) 165
2. HEALTH EFFECTS
Table 2-16. Summary of Epidemiological Studies of α-Hexachlorocyclohexane (HCH) Exposure and
Developmental Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Mean concentration (unless
otherwise noted)
Result
Mustafa et al. 2013
Case-
control, 156 mothers with preterm births
and 150 mothers with term births, India
Preterm birth
Maternal blood
4.04±2.63 ng/g (mean±SD)
(cases)
2.93±2.59 (controls)
↑
Cord blood
1.91±2.03 (cases)
1.69±2.25 (controls)
↔
Sharma et al. 2012
Case-control, 50 cases delivering babies with
fetal growth restriction and 50 women with
healthy term infants, mean ages 23–24
years,
India
Fetal growth
restriction
Maternal blood
4.55±3.2 ng/g (mean±SD) (cases)
2.92±2.7 (controls)
↑
Cord blood
2.01±1.6 (cases)
1.90±2.3 (controls)
↔
Yin et al. 2021
Case-control, 119 mothers delivering infants
or electively terminating pregnancies with
neural tube defects and 119 controls, China
Neural tube defects
Cord tissue
0.23 ng/g (cases) (median)
0.13 (controls)
↔
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; SD = standard deviation
HEXACHLOROCYCLOHEXANE (HCH) 166
2. HEALTH EFFECTS
birth cohort of 1,039 mother-infant pairs in China (Yang et al. 2021a). None of the available studies
suggested associations between biomarkers of α-HCH exposure in maternal and infant tissues and birth
length, head circumference, or ponderal index (Anand and Taneja 2020; Bravo et al. 2019; Fang et al.
2019a, 2019b).
In cross-sectional studies, a negative association between gestational age among term births and α-HCH
in cord serum was observed in a study of 1,028 mother-infant pairs in China (Fang et al. 2019a, 2019b),
but no association was seen in a smaller group of 247 mother-infant pairs in Russia (Bravo et al. 2019).
Increased risk of preterm birth was associated with higher maternal blood levels of α-HCH in a case-
control study in India (Mustafa et al. 2013).
A small case-control study in China did not observe an association between α-HCH in umbilical cord
tissue and neural tube defects (Yin et al. 2021).
β-HCH. Epidemiological studies of developmental endpoints in humans exposed to β-HCH are
summarized in Table 2-17. As the table shows, most of the studies examined metrics pertaining to fetal
growth and gestational age. The studies provide suggestive evidence for an association between β-HCH
concentrations in maternal or umbilical cord blood and reduced birth weight.
In birth cohorts of mother-infant pairs in California, Lebanon, and China, birth weight showed no
association with β-HCH in maternal serum sampled during the second trimester or maternal or cord serum
at delivery (Fenster et al. 2006, Wang et al. 2022a). An association between increased infant BMI at 1
and 2 years of age and cord serum levels of β-HCH was observed in another birth cohort in China; this
study reported no association with infant BMI at birth or 6 years of age (Yang et al. 2021a). Case-control
studies of fetal growth restriction (<10
th
percentile weight for gestational age) reported conflicting
findings. Siddiqui et al. (2003) reported no association of fetal growth restriction with maternal, cord
blood, or placental concentrations of β-HCH in a small (30 cases and 24 controls) study conducted in
India. However, a slightly larger study of 50 cases and 50 controls in India showed a positive association
between increased risk of fetal growth restriction and β-HCH in maternal blood (but not cord blood)
(Sharma et al. 2012).
Associations between reduced birth weight and increased maternal plasma, umbilical cord blood, or
placental tissue concentrations of β-HCH were reported in cross-sectional studies in Australia (Callan et
al. 2016), Spain (Lopez-Espinosa et al. 2011), China (Fang et al. 2019a, 2019b; Guo et al. 2014; Yang et

HEXACHLOROCYCLOHEXANE (HCH) 167
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Birth outcomes
Siddiqui et al. 2003
Case-control, 30 mothers of infants with intra-
uterine growth retardation, 24 mothers of
normal weight infants, India
Fetal growth restriction
(intrauterine growth
retardation)
Maternal blood
7.95±11.43 ng/g (mean±SD)
(cases)
6.55±5.43 (controls)
↔
Placenta
7.30±10.92 (cases)
7.00±7.14 (controls)
↔
Cord blood
3.03±5.22 (cases)
2.96±3.62 (controls)
↔
Callan et al. 2016
Cross-sectional, 161 mother-infant pairs,
Australia
Birth weight, proportion of
optimal birth weight
Maternal plasma
(2 weeks prior to
birth)
0.18 μg/L (mean)
↓ (boys)
↔ (girls)
Ponderal index
↔
Hjermitslev et al. 2020
Cross-sectional, 468 mother-infant pairs,
Greenland
Birth weight
Maternal serum
3.6 µg/kg lipid (median)
↔
Gestational age
↓
Fenster et al. 2006
Cohort, 385 mother-infant pairs, California,
United States
Length of gestation
Maternal serum
(2
nd
trimester or at
delivery)
37.2 ng/g lipid (median)
↔
Birth weight
See above
↔
Crown-heel length
See above
↔
Khanjani and Sim 2006
Cross-sectional, 815 mother-infant pairs,
Australia
Prematurity
Breast milk
0.0098±0.0286 mg/kg milk fat
(mean±SD)
↔
Previous miscarriage or still
birth
↔
Low birth weight
↔
Small for gestation age
↔
Head circumference
↔
Sex ratio
↔
Gladen et al. 2003
Cross-sectional, 197 mother-infant pairs,
Ukraine
Birth weight
Breast milk
860 ng/g milk fat (3
rd
tertile)
↔

HEXACHLOROCYCLOHEXANE (HCH) 168
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Lopez-Espinosa et al. 2011
Cross-sectional, 494 mothers-infant pairs,
Spain
Birth weight
Cord serum
0.085 ng/mL (median)
↓
(marginal)
Birth length
↔
Head circumference
↔
Fang et al. 2019a, 2019b
Cross-sectional, 1,028 pregnant mother-
infant
pairs, China
Birth weight
Cord serum
≥12.64 ng/g lipid (3
rd
tertile)
↓ (boys)
Birth length
↔
Ponderal index
↓ (boys)
Gestational age
↔
Wang et al. 2022a
Cohort, 1,522 mother-child pairs, China
Birth weight
Cord serum
0.65 ug/L (mean)
↔
Birth length
↔
Head circumference
↓
Yang et al. 2021a
Birth cohort, 1,039 mother-infant pairs
followed for 2 years after birth, China
Infant BMI at ages 12 and
24 months
Cord serum
8. 42 ng/g lipid (median)
↑
Infant BMI at birth and age
6 months
↔
Risk of overweight status
↑ (girls)
↔ (boys)
Guo et al. 2014
Cross-sectional, 81 mother-
infant pairs, China
Birth weight
Maternal serum at
birth
73.96 (median)
↓
Cord serum
35.29 (median)
↓
Anand and Taneja 2020
Cross-sectional, 90 mother-infant pairs, India
Birth weight
Placenta tissue
1.10–678.74 μg/L (range)
↓
Birth length
↔
Head circumference
↔
Ponderal index
↔
Mustafa et al. 2013
Case-
control, 156 mothers with preterm births
and 150 mothers with term births, India
Preterm birth
Maternal blood
5.07±3.40 ng/g (mean±SD)
(cases)
4.03±3.40 (controls)
↔
Cord blood
2.10±1.83 (cases)
1.84±2.10 (controls)
↔

HEXACHLOROCYCLOHEXANE (HCH) 169
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Tan et al. 2009
Cross-sectional, 41 mother-infant pairs,
Singapore
Birth weight
Cord blood
85.4±173 ng/g lipid (mean±SD)
↔
Birth length
↑
Head circumference
↑
Gender
↔
Tyagi et al. 2016
Cross-sectional, 30 mothers with preterm
births and 30 mothers with term births, India
Preterm birth
Maternal blood
6.42±2.158 ng/mL (mean±SD)
(cases)
3.06±2.05 (controls)
↑
Bravo et al. 2019
Cross-sectional, 247 mother-child pairs,
Russia
Gestational age
Maternal serum (last
week of pregnancy)
38 ng/g lipid (median)
↔
↔
Birth weight
↔
Birth length
↔
Head circumference
↔
Yang et al. 2020
Cohort, 102 healthy pregnant women, mean
age 28 years, China
Birth weight
Maternal serum
7.44 ng/mL (mean)
↓
Yang et al. 2021b
Case-control, 89 infants with orofacial clefts
and 129 controls, China
Orofacial cleft
Cord tissue
0.74 ng/g dry weight (cases)
0.66 (controls)
↔
Sharma et al. 2012
Case-control, 50 cases delivering babies with
fetal growth restriction and 50 women with
healthy term infants, mean ages 23–
24 years,
India
Fetal growth restriction
Maternal blood
3.97±3.9 ng/g (mean±SD)
(cases)
↑
Cord blood
2.67±2.4 (cases)
↔
Torres-Arreola et al. 2003
Case-
cohort, 100 mothers with preterm births,
133 controls with full-term births, Mexico
Preterm birth
(<37 weeks)
Maternal serum at
birth
>76.53 ng/g (3
rd
tertile)
↔

HEXACHLOROCYCLOHEXANE (HCH) 170
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Pierik et al. 2007
Case-control nested in birth cohort,
219 mothers of children with cryptorchidism,
564 controls, United States
Cryptorchidism within first
year of life
Maternal serum
(3
rd
trimester)
>3.41 µg/L (90
th
percentile)
↔
Desalegn et al. 2021
Case-cohort, 641 mother-male infant pairs,
Norway
Cryptorchidism
Breast milk
4.43 ng/g (median)
↑
Warembourg et al. 2016
Cross-sectional, 282 newborns, France
Umbilical cord blood free
testosterone, sex hormone
binding globulin, anti-
Müllerian
hormone, estradiol,
aromatase index
Cord blood
11.27 ng/g lipid (median)
↔
Debost-Legrand et al. 2016
Cross-sectional, 268 mother-infant pairs,
France
Umbilical cord serum insulin,
adiponectin
Cord serum
>0.061μg/L (4
th
quartile)
↔
Yin et al. 2021
Case-control, 119 mothers delivering infants
or electively terminating pregnancies with
neural tube defects and 119 controls, China
Neural tube defects
Cord tissue
1.81 ng/g dry weight (cases)
(median)
0.93 (controls)
↑
Postnatal development
Namulanda et al. 2016
Case-control, 218 girls with early menarche
(<11.5 years of age), 230 controls, England
Early menarche
Maternal serum
47.4 ng/g lipid (median)
↔
Marks et al. 2021
Nested case-control, 218 cases of early
menarche (<11.5 years of age),
230 controls, United Kingdom
Early menarche
Maternal serum
45.3 ng/g lipid (cases) (median)
47.5 (controls)
↔
Lam et al. 2014, 2015
Cohort, 350 boys 8–9 years old, followed for
8 years, Russia
Age of pubertal onset
Serum at cohort entry
1.3–14 ng/g (4
th
quartile)
↔
Age of sexual maturity
↑

HEXACHLOROCYCLOHEXANE (HCH) 171
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Garcia-Villarino et al. 2022
Birth cohort, 201 mother-child pairs with
children followed at 8 years of age, Spain
Anogenital index at
8 years of age
Maternal serum
27.23 ng/g lipid (Median)
↓ (boys)
↔ (girls)
Cupul-Uicab et al. 2013
Birth cohort, 1,915 children followed
until age
7 years, United States
Childhood obesity or
overweight and obese
Maternal serum
(3
rd
trimester)
≥2.12 μg/L (4
th
quartile)
↔
Lauritzen et al. 2018
Cohort, 412 mother-child pairs followed until
child age of 5 years, Norway and Sweden
Childhood obesity at
5 years old (BMI, triceps
skinfold, subscapular
skinfold, overweight)
Maternal serum
(2
nd
trimester)
Norway:
21.2 ng/g lipid (median)
Sweden:
25 ng/g lipid (median)
↔
Mendez et al. 2011
Birth cohort, 518 mother-infant pairs
followed
for 14 months, Spain
Rapid infant growth during
first 6 months; elevated BMI
at 14 months
Maternal serum (1
st
trimester)
≥47.28 ng/g lipid
(4
th
quartile)
↔
Salo et al. 2019
Case-control, 40 cases with autoantibodies
and 11 control children up to 6 years old,
Finland
Diabetes-associated
autoantibodies
Cord plasma
>LOQ (not specified)
↔
Alvarez-Pedrerol et al. 2008a
Cross-sectional, 21 newborn infants, Spain
Neonatal plasma TSH
(3 days postpartum)
Cord serum
0.48 ng/mL (geometric mean)
(group with TSH ≥10 mU/L)
0.24 (group with TSH
<10 mU/L)
↑
Alvarez-Pedrerol et al. 2008b
Cross-sectional, 259 children 4 years old,
Spain
Serum free T4, TSH at age
4 years
Serum (child)
≥0.305 ng/mL (4
th
quartile)
↔
Serum total T3 at age
4 years
≥0.191 ng/mL (3
rd
quartile)
↓
Ribas-Fito et al. 2003
Cross-sectional, 98 newborn infants, Spain
Neonatal plasma TSH
≥10 mU/l
Cord serum
0.54 ng/mL (median)
↑
Lopez-Espinosa et al. 2010
Cross-sectional, 453 newborn infants, Spain
Postpartum (≥2 days)
neonatal serum TSH
Cord serum
>104 ng/g lipid (90
th
percentile)
↑

HEXACHLOROCYCLOHEXANE (HCH) 172
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Yamazaki et al. 2020
Cross-sectional, 333 mother-child pairs,
Japan
Serum free T4 or TSH in
infants 7–43 days old
Maternal serum
235.6 pg/g (75
th
percentile)
↔
Li et al. 2014
Cross-sectional, 247 pregnant women,
Yanchen City, China
Umbilical cord serum free
T3, free T4, TSH
Cord serum
13.336 ng/g (median)
↔
Wang et al. 2022b
Birth cohort, 228 mother-child pairs, China
Total or free T3, total or free
T4, TSH in cord serum
Cord serum
0.48 ug/L (median)
↔
Sunyer et al. 2008
Cross-sectional, 52 children, 4 years old,
Spain
Urinary porphyrins (child)
Serum (child)
>0.37 ng/mL
↔
Neurodevelopmental endpoints
Lenters et al. 2019
Birth cohort, 1,199 mother-child pairs,
Norway
ADHD by 13 years of age
Breast milk
4.367 ng/g lipid (median)
↑
Kokroko et al. 2020
Cohort, 256 mother child pairs, United States
IQ at 7 years old (Wechsler
Intelligence Scale for
Children)
Maternal serum during
pregnancy
33.3 ng/g lipid (geometric
mean)
↔
a
Braun et al. 2014
Cohort, 175 mother-child pairs, Ohio, United
States
Social responsiveness scale
score, children aged 4 and
5 years
Maternal serum
<LOD (median)
1.9 ng/g lipids (75
th
percentile)
↔
b
Jeddy et al. 2018
Cohort, 400 mother-daughter pairs, England
Communication
development: nonverbal
communication, social
development, verbal
comprehension, vocabulary
comprehension in daughters
at 15 and 38 months
Maternal serum
>56.15 ng/g lipid (3
rd
tertile)
↔

HEXACHLOROCYCLOHEXANE (HCH) 173
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Wang et al. 2021b
Birth cohort, 242 mother-child pairs, China
Language development at
18 months of age
Cord blood
0.51 ug/L (median)
↔
Motor development at
18 months of age
↓
Cognitive development at
18 months of age
↔
Lee et al. 2007
Cross-sectional, 278 children aged 12–
15 years, NHANES, United States
Learning disability
Serum (child)
17.9 ng/g lipid (median)
↔
Attention deficit disorder
↔
Fabisiková et al. 2012
Cross-sectional, 143 mother-infant pairs,
Slovakia
Bayley mental development
index at 10 months of age
Serum (child)
0.01–82.9 ng/g lipid (range)
↔
Bayley psychomotor
development index at
10 months of age
↔
Sisto et al. 2015
Cohort, 351 infants enrolled at birth, Slovakia
Cochlear deficits
measured as altered
distortion product
otoacoustic emissions at
45 months of age
Cord blood
9.84±8.09 ng/g lipid
↑
Serum at 6 months old
12.24±12.9
↓
Serum at 16 months old
13.36±16.06
↓
Serum at 45 months old
7.70±9.21
↓
Kornvig et al. 2021
Birth cohort, 102 mother-child pairs,
Greenland
Problematic child behavior
at 3–5 years of age
Maternal serum
3.50 ug/kg lipid (median)
↔
Abnormal hyperactivity at 3–
5 years of age
↔

HEXACHLOROCYCLOHEXANE (HCH) 174
2. HEALTH EFFECTS
Table 2-17. Summary of Epidemiological Studies of β-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Cheslack-Postava et al. 2022
Nested case-control, 359 cases of ADHD
and 359 sex-, age- and birthplace-matched
controls, Finland
ADHD
Maternal serum
NR
↑
a
A significant increase in working memory IQ score was observed but is not shown here as it is not considered adverse.
b
A significant decrease in SRS score, corresponding to lower autism-related behaviors, was observed but is shown here as it not considered adverse.
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; ADHD = attention deficit hyperactivity disorder;
BMI = body mass index; IQ = intelligence quotient; LOD = limit of detection; NHANES = National Health and Nutrition Examination Survey; SD = standard
deviation; T3 = triiodothyronine, T4 = thyroxine; TSH = thyroid-stimulating hormone
HEXACHLOROCYCLOHEXANE (HCH) 175
2. HEALTH EFFECTS
al. 2020), and India (Anand and Taneja 2020), but not in cross-sectional studies in Greenland
(Hjermitslev et al. 2020) or Russia (Bravo et al. 2019).
Studies evaluating whether β-HCH concentrations in maternal or infant blood were associated with
preterm birth or gestational age have yielded mixed results. Tyagi et al. (2016) reported a positive
association between risk of preterm birth and β-HCH in maternal blood in a small cross-sectional study in
India, but no association was reported in a case-control study in India (Mustafa et al. 2013) or in a case-
cohort study in Mexico (Torres-Arreola et al. 2003). In 468 mother-infant pairs in Greenland, decreased
gestational age was associated with maternal serum levels of β-HCH (Hjermitslev et al. 2020), but no
association was seen in cross-sectional studies in China (Fang et al. 2019a, 2019b) or Russia (Bravo et al.
2019) or between gestation length and β-HCH in maternal blood in a cohort study in the United States
(Fenster et al. 2006).
No association between umbilical cord levels of β-HCH and orofacial clefts were observed in a case-
control study in China (Yang et al. 2021b). A case-control study in China identified a positive association
between umbilical cord tissue levels of β-HCH and neural tube defects (Yin et al. 2021).
A case-control study nested in a birth cohort in the United States observed no association between
maternal serum concentrations of β-HCH and cryptorchidism or hypospadias (Pierik et al. 2007), but a
case-cohort study of 641 mother-male infant pairs in Norway showed a positive association between
breast milk concentrations of β-HCH and cryptorchidism (Desalegn et al. 2021). Cross-sectional studies
in France reported no association between β-HCH in umbilical cord blood and reproductive hormone
levels (Warembourg et al. 2016), or insulin and adiponectin concentrations in umbilical cord (Debost-
Legrand et al. 2016).
As shown in Table 2-17, serum and cord blood levels of β-HCH were associated with increased serum
levels of TSH in cross-sectional studies conducted in Spain (Alvarez-Pedrerol et al. 2008a, 2008b; Lopez-
Espinosa et al. 2010; Ribas-Fito et al. 2003) but not in a similar study in China (Li et al. 2014). No
association between maternal serum levels of β-HCH and concentrations of free T4 or TSH in infants
from 1 to 6 weeks old (Yamazaki et al. 2020), or between cord serum β-HCH and concentrations of T4,
T3, or TSH in cord serum in a birth cohort in China (Wang et al. 2022b).
In British case-control studies nested in birth cohorts, there was no association between maternal serum
levels of β-HCH and early menarche (Namulanda et al. 2016, Marks et al. 2021). A cohort of 332 mother-
HEXACHLOROCYCLOHEXANE (HCH) 176
2. HEALTH EFFECTS
child pairs found an inverse association between β-HCH levels in serum and anogenital index in boys at
8 years of age (Garcia-Villarino et al. 2022). In a cohort study of 350 boys in Russia, serum levels of
β-HCH were associated with a higher age at sexual maturity (Lam et al. 2014, 2015).
Studies examining neurodevelopmental effects in relationship to maternal or cord serum, breast milk, or
infant or child blood levels of β-HCH are shown in Table 2-17. In general, these studies showed no
associations with IQ, problematic behavior, social responsiveness, communication, or mental and
psychomotor development (see Table 2-17). A birth cohort study in China reported diminished motor
development at 18 months of age with higher β-HCH in cord blood, but no effect on language or
cognitive development (Wang et al. 2021b). Lenters et al. (2019) showed an association between breast
milk concentrations of β-HCH and diagnosis of attention deficit hyperactivity disorder (ADHD) at
approximately 13 years of age. Similarly, β-HCH in maternal serum was associated with ADHD in a
nested case-control study in Finland (Cheslack-Postava et al. 2022).
Sisto et al. (2015) reported an association between serum concentrations of β-HCH in children (obtained
at birth [cord serum] and 6, 16, and 45 months of age) and cochlear deficits (measured as altered
distortion product otoacoustic emissions [DPOAEs] measured at 45 months of age). DPOAEs have been
established as an objective diagnostic tool for assessing the function of cochlear outer hair cells. In this
study, the investigators controlled for co-exposure confounding by evaluating the association between
β-HCH and cochlear function both with and without potentially ototoxic co-exposures. Cord serum
β-HCH concentration was positively associated with the amplitude of otoacoustic emissions across a
range of f2 frequencies, while analyses using β-HCH concentrations from serum samples at 6, 16, and
45 months of age yielded inverse associations with the amplitude of otoacoustic emissions and at
inconsistent frequencies. Significant inverse associations with β-HCH concentrations were seen at low
frequencies in analyses of 6-month blood samples, and at higher frequencies in analyses of 16-month
blood samples. In analyses using the 45-month blood samples, a significant inverse association between
β-HCH concentration and amplitude of otoacoustic emissions was seen for only 1 of 11 frequencies
tested. The study authors suggested that increased exposure during lactation might account for the change
from positive to inverse association between birth and 6 months of age, and indeed, the maximum serum
concentration of β-HCH almost doubled between these two measurements. Sisto et al. (2015) also
assessed tonotopicity (specificity of the effect on different regions of the basal membrane in the organ of
Corti, which correspond to effects on different frequencies), observing that the strength of the association
with each compound varied by noise frequency.
HEXACHLOROCYCLOHEXANE (HCH) 177
2. HEALTH EFFECTS
Limited data are available on developmental effects of β-HCH in animals. Dietary exposure of pregnant
rats to 20 or 25 mg/kg/day of β-HCH during gestation caused increased perinatal mortality, with deaths of
48 or 100% of the pups within 5 days of birth; exposure to 5 mg/kg/day did not influence perinatal
survival (Srinivasan et al. 1991). In another experiment by these authors, a dose 5 mg/kg/day of β-HCH
during gestation and lactation or during lactation only resulted in increased liver weights of pups when
measured at 28 days of age (Srinivasan et al. 1991).
γ-HCH (Lindane). Epidemiological studies of developmental endpoints in humans exposed to γ-HCH
are summarized in Table 2-18. Two case-control studies in India reported associations between maternal
and cord blood levels of γ-HCH and fetal growth restriction (Sharma et al. 2012; Siddiqui et al. 2003). In
a birth cohort of 385 mother-infant pairs in California, birth weight and length were not associated with
γ-HCH concentration in maternal serum collected during the second trimester and at delivery (Fenster et
al. 2006). Birth weight, birth length, and ponderal index showed no association with γ-HCH in cord
serum in a cross-sectional study of 1,028 infants in China (Fang et al. 2019a, 2019b). Higher BMI in
6-month-old infants was associated with increased γ-HCH in cord serum in a cohort in China; however,
BMI at 12 and 24 months of age was not associated with γ-HCH levels (Yang et al. 2021a).
Gestation length was not associated with γ-HCH concentration in maternal serum in a birth cohort of
385 mother-infant pairs in an agricultural area of California (Fenster et al. 2006). Gestational age was
inversely associated with γ-HCH in cord serum in a cross-sectional study of 1,028 infants in China (Fang
et al. 2019a, 2019b). A case-control study in India observed an increased risk of preterm birth associated
with maternal, but not cord blood levels of γ-HCH (Mustafa et al. 2013).
The concentration of γ-HCH in the placenta was associated with an increased risk of male reproductive
tract abnormalities (cryptorchidism or hypospadias observed at birth and 1 month of age) in a case-control
study nested within a birth cohort in Spain (Fernandez et al. 2007). No association between γ-HCH in
maternal serum and anogenital index at age 8 years was observed in a birth cohort of 201 mother-child
pairs in Spain (Garcia-Villarino et al. 2022).
No associations between levels of γ-HCH in umbilical cord tissue and orofacial cleft or neural tube
defects were observed in case-control studies in China (Yang et al. 2021b; Yin et al. 2021).
A cross-sectional study of 220 male newborns in Spain showed no association between placental γ-HCH
and TSH levels in umbilical cord serum (Freire et al. 2011).

HEXACHLOROCYCLOHEXANE (HCH) 178
2. HEALTH EFFECTS
Table 2-18. Summary of Epidemiological Studies of γ-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Siddiqui et al. 2003
Case-control, 30 mothers of infants with
intrauterine growth retardation, 24 mothers of
normal weight infants, India
Intrauterine growth
retardation
Maternal blood
6.30±7.51 ng/g (mean±SD)
(cases)
2.65±2.15 (controls)
↑
Placenta
8.71±4.57 (cases)
6.86±4.46 (controls
↔
Cord blood
9.23±10.31 (cases)
4.23±4.59 (controls)
↑
Fenster et al. 2006
Cohort, 385 mother-infant pairs, California,
United States
Length of gestation
Maternal serum
(2
nd
trimester and
at delivery)
1.0 ng/g lipid (median)
↔
Birth weight
↔
Crown-heel length
↔
Fang et al. 2019a, 2019b
Cross-sectional, 1,028 pregnant mother-infant
pairs, China
Birth weight
Cord serum
≥1.125 ng/g lipid (3
rd
tertile)
↔
Birth length
↔
Ponderal index
↔
Gestational age
↓
Yang et al. 2021a
Birth cohort, 1,039 mother-infant pairs followed
for 2 years after birth, China
Infant BMI at age 6 months
Cord serum
0.70 ng/g lipid (median)
↑
Infant BMI at birth and
ages 12 and 24 months;
risk of overweight status
↔
Mustafa et al. 2013
Case-control, 156 mothers with preterm births
and 150 mothers with term births, India
Preterm birth
Maternal blood
2.63±2.46 ng/g (mean±SD)
(cases)
1.52±1.83 (controls)
↑
Cord blood
0.988±1.31 (cases)
0.887±1.24 (controls)
↔
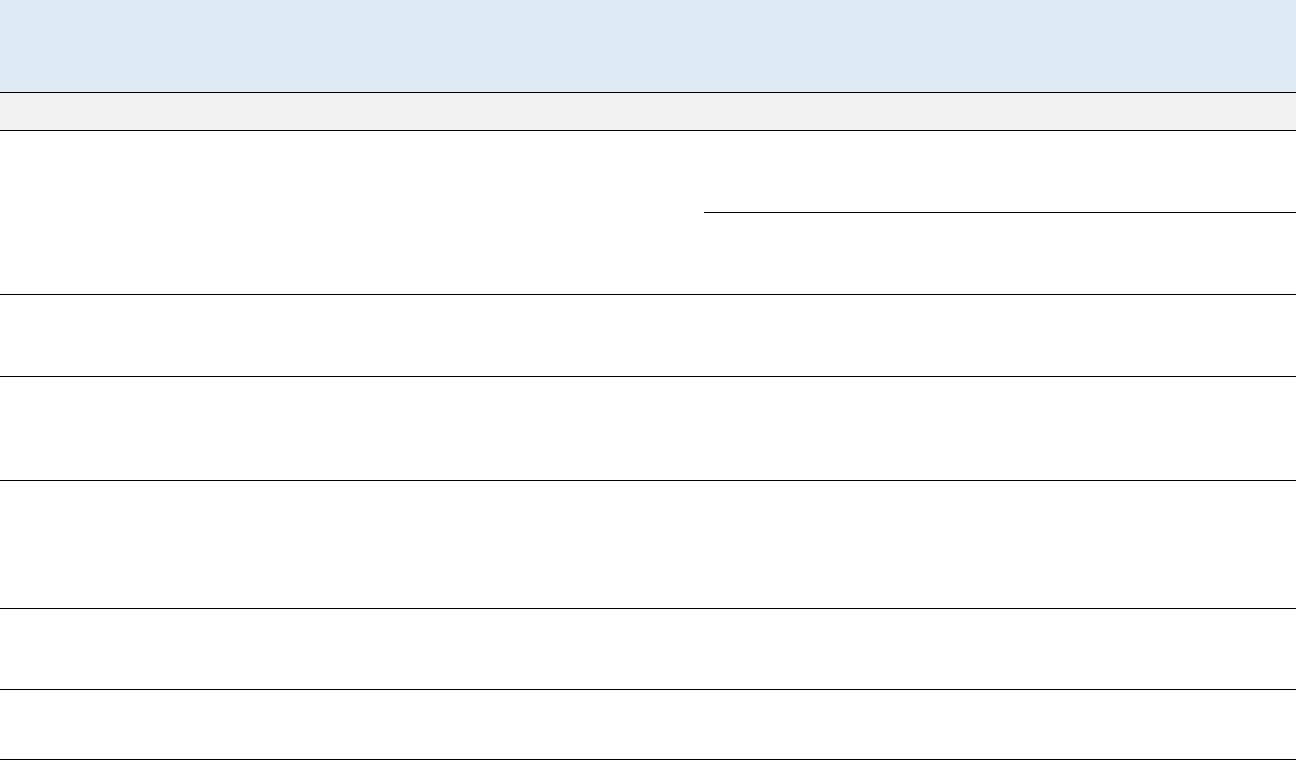
HEXACHLOROCYCLOHEXANE (HCH) 179
2. HEALTH EFFECTS
Table 2-18. Summary of Epidemiological Studies of γ-Hexachlorocyclohexane Exposure and Developmental
Effects
Reference, study type, and population
Outcome evaluated
Biomarker
Concentration
Result
Sharma et al. 2012
Case-control, 50 cases delivering babies with
fetal growth restriction and 50 women with
healthy term infants, mean ages 23–24 years,
India
Fetal growth restriction
Maternal blood
7.06±6.7 ng/g (mean±SD)
(cases)
2.58±3.9 (controls)
↑
Cord blood
3.59±3.8 (cases)
1.44±2.1 (controls)
↑
Yang et al. 2021b
Case-control, 89 infants with orofacial clefts and
129 controls, China
Orofacial cleft
Cord tissue
0.01 ng/g dry weight (cases)
(median)
0.15 (controls)
↔
Yin et al. 2021
Case-control, 119 mothers delivering infants or
electively terminating pregnancies with neural
tube defects and 119 controls, China
Neural tube defects
Cord tissue
9.92 ng/g dry weight (cases)
(median)
7.19 (controls)
↔
Fernandez et al. 2007
Case-control (nested), 50 newborn boys with
cryptorchidism or hypospadias, 114 boys without
malformations, Spain
Cryptorchidism or
hypospadias
Placenta
0.9±0.8 ng/g lipid (mean±SD)
(cases)
0.7±1.0 (controls)
↑
Garcia-Villarino et al. 2022
Birth cohort, 201 mother-child pairs with children
followed to 8 years of age, Spain
Anogenital index at 8 years
of age
Maternal serum
2.49 ng/g lipid (median)
↔
Freire et al. 2011
Cross-sectional, 220 mother-infant son pairs,
Spain
Umbilical cord serum TSH
Placenta
0.25 ng/g placenta (median)
↔
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; SD = standard deviation; TSH = thyroid-stimulating
hormone
HEXACHLOROCYCLOHEXANE (HCH) 180
2. HEALTH EFFECTS
The effects of oral exposure to γ-HCH on development in animals have been extensively studied,
primarily in rats and mice. Developmental effects of γ-HCH in these species include reduced viability
and pup body weight; perturbation of male and female reproductive tract development; alterations in the
developing liver, thymus, spleen, and heart; and developmental neurotoxicity.
Birth outcomes. Effects of γ-HCH administered orally to animals include stillbirths, reduced viability,
and decreased body weight; in addition, some studies have suggested delays in developmental milestones.
There is no evidence for teratogenic effects in animals exposed orally to γ-HCH.
In Wistar rats exposed from GD 6 through lactation to doses of 0, 0.8–0.9, 4.2–4.6, or 8.0–
10.5 mg/kg/day during gestation and 0, 1.2–1.7, 5.6–8.3, or 13.7–19.1 mg/kg/day during lactation, effects
observed at 8.0 to 19.1 mg/kg/day included increased stillbirths (live birth index of 77% compared to
99% in controls), and increased neonatal mortality (postnatal day [PND] 4 viability index of 71%
compared to 89% in controls) (EPA 1999c). In a 2-generation reproductive toxicity study of rats, a dose
of 13.1 mg/kg/day γ-HCH in food resulted in significant reductions in the pup survival on lactation day
(LD) 4; for the F1 and F2 pups, survival was 81 and 85%, respectively, compared with ≥96% for the
controls (EPA 1991a). In another 2-generation rat study, F0 dams exhibited normal lactation and
maternal behavior, but six F1 dams exposed to 26–28 mg/kg/day γ-HCH showed abnormal lactation and
retrieving behavior, leading to death of nearly all their offspring by PND 4, and a significant (49%)
reduction in the F2 PND 0–4 viability index for this group (Matsuura et al. 2005). In rats, dietary
exposure to 25 mg/kg/day of γ-HCH during gestation (GDs 0–21) did not result in changes in numbers of
litter or pup survivals (Srinivasan et al. 1991). When minks were treated with 1 mg/kg/day γ-HCH in
their diet (Beard et al. 1997), the proportion of embryos lost after implantation was increased.
Reduced pup body weights have been reported in rats and mice exposed to γ-HCH during gestation and/or
lactation. Sauviat et al. (2005) observed a 21% reduction in body weight on PND 42 in rats exposed to
0.0003 mg/kg/day γ-HCH in drinking water during gestation, lactation, and growth; body weight was not
affected at 0.00015 mg/kg/day. Exposure of female Wistar rats from GD 6 through lactation to doses
≥4.2 mg/kg/day resulted in decreased pup body weights (up to 18% less than controls at the mid-dose and
up to 20% less than controls at the high dose), and body weight gains (16–24% less than controls at the
mid-dose, and up to 40% less than controls at the high dose) in both sexes during LDs 1–11 (EPA 1999c).
Similarly, body weights of the pups of both generations were significantly lower than controls in
2-generation studies of γ-HCH in rats exposed via feed at doses of 13.1 mg/kg/day (EPA 1991a) and
26.1 mg/kg/day (Matsuura et al. 2005). In the latter study, F2 female offspring also exhibited a 10%
HEXACHLOROCYCLOHEXANE (HCH) 181
2. HEALTH EFFECTS
reduction in body weight at 5.6 mg/kg/day (Matsuura et al. 2005). Administration of 30 mg/kg to
pregnant C57BL/6J mice and 45 mg/kg to pregnant DBA/2J mice on GD 12 resulted in significant
decreases in fetal and placental weights (Hassoun and Stohs 1996a).
No malformations were observed in the fetuses of pregnant C57BL/6J or DBA/2J mice dosed with 30 and
45 mg/kg/day γ-HCH by gastric intubation on GD 12, even though both doses caused maternal deaths
(Hassoun and Stohs 1996a). A dose-related increase in the incidence of fetuses with an extra 14th rib was
reported in CFY rats exposed to 5, 10, or 20 mg/kg γ-HCH by gavage during GDs 6–15; statistical
significance was attained only at 20 mg/kg (Palmer et al. 1978). The incidence of fetuses with an extra
13th rib was statistically increased in rabbits exposed to 20 mg/kg γ-HCH by gavage during GDs 6–18
(Palmer et al. 1978). In both rats and rabbits, the incidences of extra ribs were within or just greater than
the ranges recorded for the control groups, and therefore, may not be sufficient evidence of teratogenicity
of γ-HCH. Maternal toxicity (reduced body weight gain and food consumption) occurred at doses
≥10 mg/kg/day in the rats, but not in rabbits (highest tested dose 20 mg/kg/day) (Palmer et al. 1978). No
effects on embryonic development were seen in rabbits treated by gavage with 0.8 mg/kg/day γ-HCH
3 times/week for 12–15 weeks before artificial insemination (at week 15) and throughout gestation (Seiler
et al. 1994).
In a 2-generation study of γ-HCH in CD rats exposed via feed to a dose of 13.1 mg/kg/day, the onset and
completion of tooth eruption and completion of hair growth were delayed by 10.5, 11.6, and 24% in the
high-dose F2 pups, respectively, compared to controls (EPA 1991a). In contrast, doses up to 26–
28 mg/kg/day in a 2-generation reproductive toxicity study of Crj:CD(SD)IGS rats did not influence
offspring developmental landmarks (pinna unfolding, incisor eruption, or eye opening) (Matsuura et al.
2005).
Male reproductive system development. Studies in rats and mice exposed to γ-HCH via oral
administration during gestation and lactation show effects on the developing male reproductive system,
including effects on serum hormone levels, spermatogenesis, reproductive organ weights, and testicular
histopathology; effects on sexual behavior and fertility have not been seen in these studies. Serum
testosterone was reduced in 7-month-old male rats exposed to 30 mg/kg/day γ-HCH on GD 15 (Dalsenter
et al. 1997a) and in PND 65 male rats exposed to 6 mg/kg/day on LD 9 or 14 or 1 mg/kg/day on LDs 9–
14 (Dalsenter et al. 1997b).
HEXACHLOROCYCLOHEXANE (HCH) 182
2. HEALTH EFFECTS
Lactational exposure of rats to 6 mg/kg/day on LD9 or 14 resulted in significantly reduced spermatid and
sperm counts (~8–10%) at PND 140 (Dalsenter et al. 1997b). In rats exposed to 1 mg/kg/day on LDs 9–
14, reduced spermatid numbers at PNDs 65 and 140 (29 and 12.8% less than controls, respectively) and
reduced sperm number at PND 140 (13.2%) were observed (Dalsenter et al. 1997b). Similarly, CD-1
mice that were administered 15 or 25 mg/kg/day doses of γ-HCH in olive oil by gavage on GDs 9–16
showed reduced sperm count at ≥15 mg/kg/day on PND 60 and reduced sperm concentration at
25 mg/kg/day on PNDs 60–69 and 100 (Traina et al. 2003; Di Consiglio et al. 2009). La Sala et al.
(2009) reported decreased numbers of primordial germ cells in male CD1 mouse embryos exposed to
≥15 mg/kg/day on days 8.5–11.5 post-coitum and collected on day 12.5.
Exposure of rats to 1 mg/kg/day on LDs 9–14 resulted in statistically significant reductions in relative
testicular weight at PND 140 and relative epididymis weight at PND 65 (Dalsenter et al. 1997b). In
another experiment by these authors, a single dose of 6 mg/kg/day on LD 9 or 14 resulted in 10%
reductions in relative testicular weights on PNDs 66 and 140 (Dalsenter et al. 1997b). Traina et al. (2003)
observed reduced absolute (8%) and relative (10%) testicular weights in male mouse offspring of dams
exposed to 15 mg/kg/day during GDs 9–16, but not at 25 mg/kg/day; in addition, no change in testes
weight was evident in 25 mg/kg/day F1 males sacrificed on PND 100. No change in testis weight was
observed in PND 50 or 100 mouse offspring exposed in utero to 25 mg/kg/day γ-HCH on GDs 9–16 (Di
Consiglio et al. 2009).
Gestational or lactational exposure to γ-HCH also resulted in histopathology changes in male
reproductive organs. Dalsenter et al. (1997b) reported that microscopic examination of the testes in male
rat pups exposed to 6 mg/kg/day on LD 9 or 14 showed large areas of normal tissue, but some areas had
distinct changes ranging from small alterations to a pronounced effect, including necrotic changes and
reductions in Leydig cell numbers and spermatogenesis. However, there were no significant effects on
sexual behavior or fertility in the group exposed to 1 mg/kg/day on LDs 9–14 or to 6 mg/kg on LD 9 or
14 (Dalsenter et al. 1997b). In CD-1 mice administered 15 or 25 mg/kg/day doses of γ-HCH by gavage
on GDs 9–16, testicular histological alterations (increased number and size of Leydig cells) were seen
along with increased number of epididymal sperm with chromatin abnormalities at ≥15 mg/kg/day on
PND 60 and altered testicular germ cell distribution at 25 mg/kg/day on PNDs 60 and 100 (Traina et al.
2003). A multigeneration study in mink exposed to 1 mg/kg/day γ-HCH in the diet observed that testis
size was reduced in F3 males, although there were no effects on testicular development in the second
generation (Beard and Rawlings 1998).
HEXACHLOROCYCLOHEXANE (HCH) 183
2. HEALTH EFFECTS
Agrahari et al. (2019) showed that gestational exposure to γ-HCH could influence reproductive hormone
levels in male offspring. Pregnant Wistar rats were administered γ-HCH by gavage in corn oil at doses of
0 or 0.25 mg/kg/day on GDs 5–21. Serum hormone levels were evaluated in male offspring at 9, 18, and
27 weeks of age. In offspring evaluated at 9 weeks of age, decreased serum levels of testosterone (16%
compared to controls) and growth hormone (15%) were observed, while serum LH (22%) and FSH (18%)
levels were significantly increased. Offspring evaluated at 18 and 27 weeks of age exhibited no
statistically significant changes in serum hormone levels (Agrahari et al. 2019). In other groups of male
offspring that were exposed similarly during gestation and then treated by gavage with 5 mg/kg/day for
5 days at 9, 18, or 27 weeks of age, significant hormone level changes were seen in all groups, including
decreased testosterone and growth hormone, and increased LH and FSH (Agrahari et al. 2019).
The results of another study with γ-HCH, reported only as an abstract, indicate that the male reproductive
system may be a particularly sensitive target of developmental toxicity in rats (Pages et al. 2000). Male
Sprague-Dawley rats were exposed to γ-HCH in drinking water for 12 weeks from the beginning of
gestation, lactation, or weaning at concentrations that provided estimated doses of 0.000075, 0.00015, or
0.0003 mg/kg/day. Body weight gain, plasma testosterone, sperm number, and sperm mobility values
were approximately 18, 38, 40, and 52% reduced compared to controls, respectively, in groups exposed to
0.0003 mg/kg/day during gestation or lactation. The pup rate was normal when treated males were mated
with untreated females, but newborn mortality was higher when treated males were exposed to treated
females. Given the lack of a complete report, the results of this study cannot be regarded as conclusive.
A multigeneration reproduction toxicity study in Crj:CD(SD)IGS rats exposed to γ-HCH via diet showed
a delay of 1.5 days in preputial separation in F1 animals exposed to 26.1 mg/kg/day (Matsuura et al.
2005). In contrast, the mean day of preputial separation was not affected by treatment in Wistar rat pups
exposed to doses up to 10.5–19.1 mg/kg/day from GD 6 through lactation (EPA 1999c) or in mouse pups
exposed to doses up to 25 mg/kg/day during GDs 9–16 (Traina et al. 2003).
Female reproductive system development. There are few data on the effects of γ-HCH on development of
the female reproductive tract, but available studies show effects in mice exposed during gestation to oral
doses of 15 mg/kg/day. La Sala et al. (2009) exposed CD-1 mice to 15 or 30 mg/kg/day γ-HCH by
gavage for 3 days during gestation (days 8.5–11.5 post-coitum) and collected embryos 1 day after the last
dose. The female embryos exhibited reduced numbers of primordial germ cells in the ovaries at both
doses (La Sala et al. 2009). In female pups of CD mice exposed to 15 mg/kg/day γ-HCH on GDs 9–16
and sacrificed on PND 22, significant increases in uterine weight (13–17% higher than controls) were
HEXACHLOROCYCLOHEXANE (HCH) 184
2. HEALTH EFFECTS
observed, while those pups sacrificed on PND 60 exhibited decreased oocyte diameter (21%) in primary
follicles (Maranghi et al. 2007). Exposed female pups also developed vaginal patency earlier than
controls; by PND 33, 93% of female pups exposed to 15 mg/kg/day and 36% of control females exhibited
complete vaginal opening (Maranghi et al. 2007). No effect on vaginal opening was observed in Wistar
rat pups exposed to doses as high as 10.5–19.1 mg/kg/day from GD 6 through lactation (EPA 1999c) or in
CD-1 mouse pups exposed on GDs 9–16 to doses up to 25 mg/kg/day (Traina et al. 2003). In contrast,
vaginal opening was delayed by 1.4 days in Crj:CD(SD)IGS rats exposed to 26.1 mg/kg/day γ-HCH via
diet in a multigeneration reproductive toxicity study (Matsuura et al. 2005).
Systemic development. Oral exposure to γ-HCH during gestation and/or lactation has resulted in
significant effects on liver, thymus, and spleen weights in the offspring, and on cardiac development.
Srinivasan et al. (1991) observed significant increases in pup relative liver weight when rat dams were
exposed to 25 mg/kg/day during gestation and lactation or during lactation only (LDs 0–28). When
administered by gavage on GD 12 to pregnant C57BL/6J mice, a dose of 30 mg/kg γ-HCH resulted in
significant decreases in fetal thymic weight; fetal body weight was also reduced (Hassoun et al. 1996). At
doses of 26–28 mg/kg/day administered in feed to rats through 2 generations, treatment-related decreases
in absolute and relative thymus (13–31%) and spleen (13–32%) weights were observed in both
generations (Matsuura et al. 2005).
Development of the heart was examined in a study of female Sprague-Dawley rats exposed to very low
doses (0.000076, 0.00015, or 0.0003 mg/kg/day) of γ-HCH in drinking water (0.5, 1, and 2 μg/L,
respectively) prior to mating; during mating, gestation, and lactation; and for 3 weeks post-weaning
(Sauviat et al. 2005). The pups were sacrificed at 6 weeks of age for evaluation of heart weight,
morphometry, and lipid content and electrophysiology of dissected left ventricular papillary muscles.
Heart weights and lipid content of exposed rats did not differ from control. Morphometry analysis
showed that hearts of pups in the 0.0003 mg/kg/day group had a 9% increase in heart width (relative to
controls), but no significant change in length, with a corresponding 9% decrease in length-to-width ratio.
At this dose, offspring heart morphology was described as rounder and “cherry like.” The study authors
reported that hearts of treated offspring showed hypertrophied areas with thinning of the left ventricular
wall and few developed papillary muscles. Histopathological examination in offspring exposed to
0.0003 mg/kg/day showed that the heart tissue muscle bundles and layers were unorganized and
dissociated, with large hemorrhagic interspaces and dispersion of cell nuclei, destruction of fibroblasts,
and dispersion and disorganization of collagen bundles, compared to control heart muscle.
Histopathology was not assessed in the groups exposed to 0.000076 or 0.00015 mg/kg/day.
HEXACHLOROCYCLOHEXANE (HCH) 185
2. HEALTH EFFECTS
Electrophysiology changes were also evident in the dissected left ventricular muscles of exposed pups.
Action potential durations were unchanged at 0.000076 mg/kg/day, but the plateau was shortened
moderately at 0.00015 mg/kg/day, and significantly shortened at 0.0003 mg/kg/day. At
0.0003 mg/kg/day, the slow repolarizing phase was also significantly shortened. The effects of γ-HCH on
action potential durations were mitigated by addition of quinidine or E-4031 (blockers of the rapid
delayed rectifier potassium current or I
Kr
) to the test solution, indicating that γ-HCH may act directly on
the I
Kr
.
Sauviat et al. (2005) noted that the I
Kr
channel is involved in long QT syndrome (a disorder that
increases the risk of cardiac arrhythmias).
These authors conducted a related study examining whether the cardiac effects could be induced by
paternal exposure to γ-HCH in drinking water (Sauviat et al. 2007). In this study, male rats were exposed
to a concentration of 2 μg/L for an unspecified “chronic” duration prior to mating with untreated females.
The lack of information on exposure duration in the males precluded estimation of doses. In offspring
sacrificed at 6 weeks of age, there were no effects on heart weight or shape or electrophysiology, but there
were histopathology changes in the hearts similar to those reported by Sauviat et al. (2005) at the same
water concentration.
Developmental neurotoxicity. Convulsions and seizures have been observed in offspring of rats exposed
to γ-HCH during gestation and/or lactation. Johri et al. (2008) observed convulsions in 8/10 male rat
pups that had been exposed during gestation (GDs 5–21) to 0.25 mg/kg/day γ-HCH and then received a
single gavage dose of 30 mg/kg on PND 45. In pups exposed only during gestation, no convulsions were
observed (Johri et al. 2008). Epileptiform seizures were reported in male rats fed milk from dams that
were gavaged with 20 mg/kg/day γ-HCH on PNDs 3–15 (Albertson et al. 1985).
In a developmental neurotoxicity study, Han Wistar rats were exposed to 0, 10, 50 or 120 ppm γ-HCH in
the diet from GD 6 through LD 10 (EPA 1999c). Reported daily maternal dose levels were 0, 0.8–0.9,
4.2–4.6, or 8.0–10.5 mg/kg/day during gestation, and 0, 1.2–1.7, 5.6–8.3, or 13.7–19.1 mg/kg/day during
lactation. The F1 offspring were evaluated for functional observational battery, motor activity, auditory
startle response, learning and memory, and brain endpoints (weight, histology, and morphometrics) on
postpartum days 11 and 65. The offspring showed increased motor activity (both sexes) and decreased
habituation of motor activity (females) at the two highest dose levels. Reduced auditory startle response
habituation was observed at the high dose in both sexes on day 28 and day 60 postpartum (EPA 1999c).
No significant changes in brain weight, morphometry, or histology were detected in the pups. This study
was classified as an unacceptable developmental neurotoxicity study by EPA (2000a) because there was
HEXACHLOROCYCLOHEXANE (HCH) 186
2. HEALTH EFFECTS
no laboratory validation of the neurobehavioral tests and the number of animals (six per dose level) was
insufficient.
Increased motor activity was also reported in rat pups exposed during gestation only. Exposure on
GDs 5–21 to doses ≥0.25 mg/kg/day resulted in increased locomotor activity (increases in distance
traveled, ambulatory time, horizontal count, stereotypic time, and stereotypic movement burst) in 3-week-
old rat pups, and some of the changes (increased distance traveled) persisted through 9 weeks of age
(Johri et al. 2007). At a dose of 0.125 mg/kg/day, distance traveled was increased at 3 weeks of age, but
not at subsequent evaluations (Johri et al. 2007). Srivastava et al. (2019) observed no significant changes
in spontaneous locomotor activity or spatial memory (Y-maze activity) in 12-week-old male rat pups
whose mothers were exposed by gavage from GD 5 through GD 21 to 0.25 mg/kg/day γ-HCH. However,
other groups of pups similarly exposed in utero and then rechallenged at 12 weeks of age with 21 daily
gavage doses of 2.5 mg/kg/day γ-HCH exhibited significantly reduced spontaneous locomotor activity
(reductions in distance travelled, moving time, numbers of rearings, and stereotypic counts, along with
significantly increased resting time) and spatial memory effects (significant reduction in percent
alterations in Y-maze testing) (Srivastava et al. 2019). Acquisition of a passive avoidance task was
improved in 15-day-old rat pups that were treated with γ-HCH by gavage as either a single 20 mg/kg dose
or 7-day repeated 10 mg/kg/day doses (Rivera et al. 1998). Exposure to the single 20 mg/kg dose resulted
in a decrease in motor activity, while repeated exposure to the lower dose increased motor activity in this
study (Rivera et al. 1998).
Neurobehavioral testing of F1 offspring exposed to doses up to 26–28 mg/kg/day in a 2-generation
reproductive toxicity study showed no effects in tests of reflex, sensory function, surface righting reflex,
corneal reflex, startle response, pain response, or mid-air righting reflex at 4–6 weeks of age (Matsuura et
al. 2005). In addition, no effects were observed in the open field test, rotarod test, or pole climbing test
(Matsuura et al. 2005).
S
tudies of neurotransmitter levels in rats exposed to γ-HCH showed that the effects depended on the
treatment schedule and brain region (Rivera et al. 1991, 1998). In suckling Wistar rats treated once with
20 mg/kg γ-HCH by gavage at PNDs 8, 15, 22, or 29, regional changes in brain noradrenaline, serotonin,
and the dopamine metabolite, 3,4-dihydroxyphenyl- acetic acid (DOPAC) levels were seen, with
differential effects depending on age at the time of exposure (Rivera et al. 1991). Similarly, Rivera et al.
(1998) observed different patterns (e.g., ratios of 5-HIAA/serotonin and DOPAC/dopamine) in brain
monoaminergic levels in rat pups after exposure on PND 15 to a single 20 mg/kg dose or 7 consecutive
HEXACHLOROCYCLOHEXANE (HCH) 187
2. HEALTH EFFECTS
daily doses of 10 mg/kg/day γ-HCH. The patterns suggested that monoaminergic turnover was increased
by the single dose but decreased by repeated exposure at lower doses (Rivera et al. 1998).
A study of rats exposed to γ-HCH in drinking water at very low doses (0.000055–0.00011 mg/kg/day) for
12 days prior to mating and through gestation and lactation examined effects of treatment on wake-sleep
cycle in male offspring at 14 weeks of age (Breton et al. 2005). Sleep cycle was analyzed by EEG in
three phases: wakefulness, slow wave sleep, and paradoxical sleep, and there were no treatment-related
changes in the sleep cycle. Spectral electrocorticographic analysis showed slight changes in brain activity
relative to controls (increased relative energy in the 11–15 Hz frequency during wakefulness and slow
wave sleep, and increased activity in the 7–15 Hz range in all sleep phases) in both exposure groups, but
the changes did not show dose-dependence. No histopathological effects were reported in the brain at any
dose (Breton et al. 2005).
Srivastava et al. (2019) examined transmission electron ultrastructural microscopy of the brains in
15-week old male rat pups whose mothers were exposed by gavage from GD 5 through GD 21 to
0.25 mg/kg/day γ-HCH. Although neurobehavioral changes were not seen in these pups, ultrastructural
changes were detected in the hippocampus and substantia nigra of exposed pups, including moderately
distorted mitochondria, demyelinated neurons, and autophagic vesicles with damaged cytoplasmic
contents (Srivastava et al. 2019). In another experiment by these authors, electron microscopy of the
hippocampus and substantia nigra showed loss of mitochondrial integrity (loss of cristae and number),
severe loss of synaptic structure, severe demyelination, and highly condensed nuclei with cytoplasmic
content showing necrotic effects after similar prenatal exposure followed by a rechallenge exposure at
12 weeks of age (21 days at 2.5 mg/kg/day) (Srivastava et al. 2019).
Mechanisms. Superoxide production, lipid peroxidation, and deoxyribonucleic acid (DNA) single-strand
breaks were increased in fetal and placental tissues, and lipid peroxidation markers were increased in
maternal sera and amniotic fluid 48 hours after administration of a single dose of 30 mg/kg γ-HCH to
pregnant mice on GD 12 (Hassoun and Stohs 1996b; Hassoun et al. 1996). Significant increases in lipid
peroxidation also occurred in fetal livers collected on GD 18. Thus, it was suggested that fetotoxic effects
of γ-HCH may be due to induced oxidative stress, enhanced lipid peroxidation, and DNA-single strand
breaks in mice.
There is evidence that the developmental neurotoxicity effects of γ-HCH may be mediated by metabolites
and involve alterations in the blood-brain barrier. Johri et al. (2007) detected dose-dependent increases in
brain cytochrome P450 (CYP1A1, 1A2, 2B1, 2B2, and 2E1) protein expression and mRNA, and CYP-
HEXACHLOROCYCLOHEXANE (HCH) 188
2. HEALTH EFFECTS
dependent enzyme (EROD, PROD, and NDMA-d) activities in F1 offspring exposed to 0.0625–
0.25 mg/kg/day γ-HCH. The enzyme changes persisted longer at the high dose, correlating with the
effects on locomotor activity and suggesting that metabolites of γ-HCH may be responsible for this effect
(Johri et al. 2008). γ-HCH exposure causes functional impairment of the developing blood brain barrier
in young rats (Gupta et al. 1999). The integrity (impermeability) of the blood brain barrier was studied by
assessing uptake of sodium fluorescein (a micromolecular tracer dye) into the brain of neonatal rats
following single or repeated acute gavage doses of γ-HCH. The brain uptake index of fluorescein was
significantly increased in 10-day-old pups treated with a single 2 mg/kg dose (72 and 23% higher than
controls after 2 hours and 3 days, respectively), as well as in those treated with 2 mg/kg/day for 8 days
(50% higher than controls 7 days after the first exposure, with recovery 20 days after the first exposure).
The effect appeared to be age-related because the brain uptake index was lower when rats were
administered a single 2 mg/kg dose at 15 days of age (20% higher than controls after 2 hours) or a higher
dose of 4 mg/kg/day for 3 days as adults (no effect on brain permeability).
δ-HCH. Epidemiological studies that have examined relationships between δ-HCH in maternal or fetal
blood or tissues and developmental outcomes are shown in Table 2-19. A small case-control study in
India reported positive associations between fetal growth restriction and higher δ-HCH concentrations in
maternal and umbilical cord blood, but not with concentrations in placenta (Siddiqui et al. 2003). No
association between infant birth size and δ-HCH in placental tissue was observed in a cross-sectional
study in India (Anand and Taneja 2020). No studies of developmental outcomes in animals exposed to
δ-HCH by any exposure route were located.
Technical HCH or Unspecified Isomers of HCH. A dose-related increase in fetal resorptions was seen
in pregnant female mice treated once with 25–200 mg/kg technical-grade HCH by gavage on GD 9, but
fetal development was normal (Dikshith et al. 1990). Srivastava and Raizada (2000) further studied the
prenatal effect of orally administered technical-grade HCH. While mice exposed to HCH during the
preimplantation period (GDs 2–6) did not show fetolethality, exposure during the post-implantation
period (GDs 6–12) to 25 and 50 mg/kg/day HCH produced significant increases in resorption of fetuses,
inhibition of maternal serum progesterone levels, and higher levels of HCH in fetal tissues. Oral exposure
to Benesan (a pesticidal formulation containing 50% γ-HCH) given at doses of 6.25, 12.5, or
25 mg/kg/day by gavage on GDs 6–15 failed to produce teratogenic effects in rats (Khera et al. 1979).
Alterations in levels of brain dopamine, serotonin, GABA, glutamate, glutamate decarboxylase, and
noradrenaline were seen in various areas of the brains of female rat pups treated with 10 mg technical-
grade HCH/kg/day for 60 days beginning at birth (Nagaraja and Desiraju 1994).

HEXACHLOROCYCLOHEXANE (HCH) 189
2. HEALTH EFFECTS
Table 2-19. Summary of Epidemiological Studies of δ-HCH and Total HCH Exposure and Developmental Effects
Reference, study type, and
population
Outcome
evaluated
Isomer
Biomarker
Mean concentration (unless
otherwise noted)
Result
Siddiqui et al. 2003
Case-control, 30 mothers of infants with
fetal growth restriction (intrauterine
growth retardation), 24 mothers of normal
weight infants, India
Fetal growth
restriction
δ-HCH
Maternal blood
2.14±1.94
↑
Placenta
2.92±3.99
↔
Cord blood
4.51±4.63
↑
Anand and Taneja 2020
Cross-sectional, 90 mother-infant pairs,
India
Birth weight
δ-HCH
Placenta tissue
1.18–24.4μg/L (range)
↔
Birth length
↔
Head circumference
↔
Ponderal index
↔
Yin et al. 2021
Case-control, 119 mothers delivering
infants or electively terminating
pregnancies with neural tube defects and
119 controls, China
Neural tube defects
δ-HCH
Cord tissue
0.81 ng/g dry weight (cases)
(median)
0.57 (controls)
↔
↑ = association with increase; ↔ = no association; HCH = hexachlorocyclohexane
HEXACHLOROCYCLOHEXANE (HCH) 190
2. HEALTH EFFECTS
2.18 OTHER NONCANCER
Epidemiology Studies. Several case-control studies (Berg et al. 2021; Charles et al. 2022; Daniels et al.
2018; Han et al. 2020; Li et al. 2016; Rylander et al. 2015; Tawar et al. 2022; Tyagi et al. 2021; Zong et
al. 2018) and cross-sectional studies (Arrebola et al. 2013; Everett and Matheson 2010; Gasull et al. 2012;
Schwarz et al. 2021; Ukropec et al. 2010) evaluated associations between diabetes and exposure to HCH
isomers (see Table 2-20). There were no associations with serum levels of α-, γ-, or δ-HCH isomers and
type 2 diabetes reported in a case-control study of 723 cases of Chinese type 2 diabetes patients compared
to control subjects, while higher mean serum levels of β-HCH and with total HCH levels were associated
with type 2 diabetes (Li et al. 2016); significant interactions with several ADIPOQ (gene encoding
adiponectin) genotypes were reported for the β-HCH isomer. No association was observed with type 2
diabetes and serum levels of β-HCH or γ -HCH in case-control studies in Norway (Berg et al. 2021;
Charles et al. 2022) or in a case-cohort study in France (Magliano et al. 2021). However, nested case-
control studies reported an association between serum and plasma β-HCH levels and increased incidences
of type 2 diabetes in American nurses (Zong et al. 2018), Norwegian women (Rylander et al. 2015), and
in Chinese adults (Han et al. 2020). One nested case-control study comparing South Asians and European
whites living in London determined a significant association between plasma concentrations of β-HCH
and increased prevalence of diabetes in a Tamil-descent population, while an elevated, but not significant,
association was reported in a Telugu-descent population with much higher plasma concentrations of
β-HCH (Daniels et al. 2018). Tawar et al. (2022) conducted a case-control study in India and reported a
positive association between levels of δ- HCH in adipose tissue and type 2 diabetes, but no association
between α-, β-, or γ-HCH and type 2 diabetes was observed.
In cross-sectional studies evaluating β-HCH, there was no association between the prevalence of diabetes
and levels in adipose tissue (Arrebola et al. 2013), and no association between diabetes and pre-diabetes
and serum levels (Everett and Matheson 2010; Gasull et al. 2012; Schwarz et al. 2021). Another cross-
sectional study similarly found no association with diabetes; however, an association was seen between
serum β-HCH and increased incidence of pre-diabetes (Ukropec et al. 2010). Additionally, a cross-
sectional study using NHANES data reported an association with serum β-HCH and increased incidence
of diabetes without nephropathy, but no association in adults with both diabetes and nephropathy (Everett
and Thompson 2015). There was no relationship between serum β-HCH and insulin sensitivity and
secretion indicators (Lee et al. 2017), nor were there associations with leptin and insulin levels and insulin
resistance (Burns et al. 2014).

HEXACHLOROCYCLOHEXANE (HCH) 191
2. HEALTH EFFECTS
Table 2-20. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Other
Noncancer Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Biomarker
Concentration
Result
Arrebola et al. 2013
Cross-sectional, 386 patients undergoing
noncancer-related surgery, Spain
Type 2 diabetes
β-HCH
Adipose
>16.81 ng/g lipid
↔
Berg et al. 2021
Case-control, 44 cases of Type 2 diabetes and
44 age-matched controls, mean age 52 years,
Norway
Type 2 diabetes
β-HCH
Serum
12.8 ng/g lipid (cases) (median,
prediagnostic sample)
13.9 (controls)
↔
γ-HCH
3.36 (cases)
4.39 (controls)
↓
Charles et al. 2022
Nested case-control, 116 cases of Type 2
diabetes, 139 controls, mean ages 48 and
45 years, respectively, Norway
Type 2 diabetes
β-HCH
Serum
14.0-36.7 ng/g lipid (cases) (mean,
prediagnostic samples)
12.9-31.8 (controls)
↔
Han et al. 2020
Case-control, 158 cases of Type 2 diabetes,
158 controls, China
Type 2 diabetes
β-HCH
Serum
1351 pg/mL (cases) (GM)
695 (controls)
↑
Magliano et al. 2021
Case-cohort, 200 cases of type 2 diabetes and
553 controls, mean ages 51 and 47 years,
respectively, France
Type 2 diabetes
β-HCH
Serum
811 ng/L (cases) (median)
513 (controls)
↔
γ-HCH
18.4 ng/L (cases) (median)
14.7 (controls)
↔
Li et al. 2016
Case-control, 723 cases and 723 controls, mean
age 62 years, China
Type 2 diabetes
α-HCH
Serum
0.012 ng/mL (cases) (GM)
0.011 (controls)
↔
β-HCH
0.575 (cases)
0.266 (controls)
↑
a
γ-HCH
0.020 (cases)
0.018 (controls)
↔
δ-HCH
0.068 (cases)
0.060 (controls)
↔
Total HCH
NR
↑

HEXACHLOROCYCLOHEXANE (HCH) 192
2. HEALTH EFFECTS
Table 2-20. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Other
Noncancer Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Biomarker
Concentration
Result
Zong et al. 2018
Nested case-control, 793 cases and 793 controls
participating in Nurse Health Study II, age 32–
52 years, United States
Type 2 diabetes
β-HCH
Serum
14.3 ng/g lipid (median) (cases)
9.84 (controls)
↑ (trend)
Rylander et al. 2015
Nested case-control, 106 cases and 106 control
women, age 30–70 years, Norway
Type 2 diabetes
β-HCH
Plasma
20.3 ng/g lipid (mean) (cases)
10.0 (controls)
↑
Daniels et al. 2018
Nested case-control, 73 adults of Tamil descent
and 47 adults of Telugu descent, >21 years old,
United Kingdom
Diabetes in Tamil
population
β-HCH
Plasma
≥50.58 ng/g lipid
↑
Diabetes in Telugu
population
≥369.30 ng/g lipid
↔
Everett and Matheson 2010
Cross-sectional, 3,414 adults ≥20 years old,
NHANES, United States
Diabetes
β-HCH
Serum
>9.36 ng/g lipid
↔
Pre-diabetes
↔
Gasull et al. 2012
Cross-sectional, 886 adults 18–74 years, Spain
Diabetes
β-HCH
Serum
>1.547 ng/mL (4
th
quartile cutoff)
↔
Pre-diabetes
↔
Ukropec et al. 2010
Cross-sectional, 2,047 adults 21–75 years old,
Slovakia
Diabetes
β-HCH
Serum
83–781 ng/g lipid (5th quintile)
↔
Pre-diabetes
↑
Schwarz et al. 2021
Cross-sectional, 200 adults 75–76 years old,
Germany
Known diabetes
β-HCH
Serum
0.12 ug/L (median)
↔
Newly diagnosed
diabetes
0.10
↔
Pre-diabetes
0.08
↔
Everett and Thompson 2015
Cross-sectional, 2,992 adults ≥20 years old,
NHANES (1999–2004), United States
Diabetes without
nephropathy
β-HCH
Serum
≥0.1018 ng/g
↑
Diabetes with
nephropathy
β-HCH
Serum
≥0.1018 ng/g
↔

HEXACHLOROCYCLOHEXANE (HCH) 193
2. HEALTH EFFECTS
Table 2-20. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Other
Noncancer Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Biomarker
Concentration
Result
Tawar et al. 2022
Case-control, 70 cases of type 2 diabetes and
70 age-, gender-, and BMI- matched controls,
mean ages 43.65 (control) and 44.9 (cases)
years, India
Type 2 diabetes
α-HCH
Adipose
tissue
0.90 ng/g (cases)
0.41 (controls)
↔
β-HCH
1.25 (cases)
0.89 (controls)
↔
γ-HCH
1.17 (cases)
0.86 (controls)
↔
δ-HCH
2.70 (cases)
0.79 (controls)
↑
Lee et al. 2017
Cross-sectional, 200 adults >30 years old, Korea
Insulin sensitivity
and secretion
indicators
β-HCH
Serum
157.97 pg/mL (4
th
quartile cutoff)
↔
Burns et al. 2014
Cohort, 318 boys, age 8–9 years, Russia
Leptin
β-HCH
Serum
165 ng/g lipid (median)
↔
Insulin
↔
Insulin resistance
↔
Gasull et al. 2018
Cross-sectional, 860 adults 18–74 years old,
Spain
Unhealthy
metabolic
phenotype
b
β-HCH
Serum
≥0.671 ng/mL (4th quartile)
↑
Reina-Pérez et al. 2023
Cross-sectional, 117 adult surgical patients,
median age 44 years, Spain
Metabolic
syndrome
γ-HCH
Adipose
tissue
11.44 ng/g (median)
↑
Elevated waist
circumference
↑
Elevated blood
pressure
↑
Elevated fasting
blood glucose,
triglycerides, or
HDL cholesterol
↔

HEXACHLOROCYCLOHEXANE (HCH) 194
2. HEALTH EFFECTS
Table 2-20. Summary of Epidemiological Studies of Hexachlorocyclohexane (HCH) Exposure and Other
Noncancer Effects
Reference, study type, and population
Outcome
evaluated
Isomer
Biomarker
Concentration
Result
Zhang et al. 2023
Cross-sectional, 1,996 adult residents of Wuhan,
mean age 44.8 years, China
Metabolic
syndrome
β-HCH
Serum
34.5 ng/g lipid (median)
↑
Lee et al. 2016
Cohort, 214 children 7–9 years old, Korea
Measures of
metabolic
syndrome
β-HCH
Serum
6.13 ng/g lipid (median)
↔
Mustieles et al. 2017
Cross-sectional and 10-year longitudinal,
387 noncancer surgical patients (at baseline),
median age 52 years; 154 without metabolic
disease at baseline followed for 10 years, median
age 42 years, Spain
Metabolically
compromised
c
at
baseline
β-HCH
Adipose
tissue
10.6 ng/g lipid (median)
↑
Metabolically
compromised
c
at
follow up
6.9
↑
Wang et al. 2021a
Cross sectional, 10 women, mean age 37 years,
China
Serum lipoprotein
a, total cholesterol,
triglycerides, high-
and low-density
lipoprotein
cholesterol
α-HCH
Estimated
dietary
intake
0.471 ng/kg/day
↔
β-HCH
0.185
↔
γ-HCH
0.043
↔
δ-HCH
0.104
↔
a
Significant interactions with several ADIPOQ genotypes.
b
Unhealthy metabolic phenotype was defined as exhibiting two or more of the following: hypertension, hypertriglyceridemia, low HDL cholesterol, hyperglycemia,
insulin resistance, or systemic inflammation.
c
Metabolically compromised was defined as exhibiting one or more of the following: type 2 diabetes, hypertension, hypertriglyceridemia, or low HDL cholesterol.
↑ = association with increase; ↓ = association with decrease (inverse association); ↔ = no association; GM = geometric mean; HDL = high-density lipoprotein;
NHANES = National Health and Nutrition Examination Survey

HEXACHLOROCYCLOHEXANE (HCH) 195
2. HEALTH EFFECTS
Gasull et al. (2018) reported a positive association between unhealthy metabolic phenotypes (defined as
exhibiting at least two of the following: hypertension, hypertriglyceridemia, low high-density lipoprotein
(HDL) cholesterol, hyperglycemia, insulin resistance, or systemic inflammation) and serum β-HCH
levels. A study of adult noncancer surgical patients also showed an association between β-HCH levels in
adipose tissue and increased likelihood of being metabolically compromised (exhibiting type 2 diabetes,
hypertension, hypertriglyceridemia, or low HDL cholesterol) at baseline and at a 10-year follow-up
(Mustieles et al. 2017). A cross-sectional study of 1,996 adults in China reported a positive association
between β-HCH in serum and metabolic syndrome (Zhang et al. 2023). There was no association
between serum β-HCH levels and measures of metabolic syndrome in a cohort of 214 Korean children
between 7 and 9 years of age (Lee et al. 2016).
In a cross-sectional study of surgical patients in Spain, associations between metabolic syndrome,
elevated waist circumference, and elevated blood pressure with γ-HCH levels in adipose tissue were
observed (Reina-Pérez et al. 2023). In a small cross-sectional study of 10 women in China, Wang et al.
(2021) reported no association between any of the HCH isomers and serum levels of lipids.
2.19 CANCER
Epidemiological Studies. Epidemiological studies of HCH isomers and cancer are shown in Table 2-21.
Studies shown in the table include only those that accounted for at least one potential confounding
variable (i.e., studies reporting only univariate analyses were excluded). In addition, only the most recent
analysis of a given cohort or case-control population is shown in the table.
Table 2-21. Summary of Epidemiological Studies Evaluating Possible
Associations between Hexachlorocyclohexane Exposure and
Risk of Selected Cancer Types
Cancer type
Isomer
Association
a
No association
b
Non-Hodgkin’s lymphoma
Beta (β)
Bassig et al. 2020; Viel et
al. 2011
Brauner et al. 2012; Cantor et al.
2003; Cocco et al. 2008
Gamma (γ)
Alavanja et al. 2014
c
;
Kachuri et al. 2020
Cocco et al. 2008; De Roos et al.
2021; Viel et al. 2011
Multiple myeloma
Beta (β)
Weber et al. 2018
Gamma (γ)
Presutti et al. 2016
HEXACHLOROCYCLOHEXANE (HCH) 196
2. HEALTH EFFECTS
Table 2-21. Summary of Epidemiological Studies Evaluating Possible
Associations between Hexachlorocyclohexane Exposure and
Risk of Selected Cancer Types
Cancer type
Isomer
Association
a
No association
b
Leukemia
Alpha (α)
Rafeeinia et al. 2023;
Beta (β)
Rafeeinia et al. 2023;
Cocco et al. 2008
Gamma (γ)
Purdue et al. 2007;
Rafeeinia et al. 2023
Colon/colorectal
Αlpha (α)
Howsam et al. 2004
Beta (β)
Lee et al. 2018a
Howsam et al. 2004; Park et al. 2021
Gamma (γ)
Howsam et al. 2004; Purdue et al.
2007
Female breast cancer
Alpha (α)
Miao et al. 2021
Beta (β)
Arrebola et al. 2015b
Miao et al. 2021;
Waliszewski et al. 2005
Hoyer et al. 1998; Lopez-Carrillo et
al. 2002; McCready et al. 2004;
Raaschou-Nielsen et al. 2005
d
;
Ward
et al. 2000; Wielsoe et al. 2017; Xu
et al. 2010
Gamma (γ)
Ibarluzea et al. 2004
e
Hoyer et al. 1998; Miao et al. 2021
Delta (δ)
Miao et al. 2021
Prostate cancer
Alpha (α)
Pi et al. 2016
d
Beta (β)
Kumar et al. 2010; Xu et
al. 2010
Aronson et al. 2010; Lim et al. 2017;
Pi et al. 2016; Sawada et al. 2010
Gamma (γ)
Band et al. 2011
Barry et al. 2011; Koutros et al.
2011; Pi et al. 2016
Lung cancer
Gamma (γ)
Purdue et al. 2007
Hepatocellular carcinoma
Beta (β)
Zhao et al. 2012
Bladder cancer
Gamma (γ)
Purdue et al. 2007
Endometrial cancer
Beta (β)
Sturgeon et al. 1998
Melanoma
Gamma (γ)
Purdue et al. 2007
Soft tissue sarcoma
Gamma (γ)
Pahwa et al. 2011
Pancreatic cancer
Beta (β)
Porta et al. 2022
Brain cancer
Alpha (α)
Yousefi et al. 2022
Beta (β)
Yousefi et al. 2022
Gamma (γ)
Yousefi et al. 2022
Testicular germ cell tumors
Beta (β)
McGlynn et al. 2008

HEXACHLOROCYCLOHEXANE (HCH) 197
2. HEALTH EFFECTS
Table 2-21. Summary of Epidemiological Studies Evaluating Possible
Associations between Hexachlorocyclohexane Exposure and
Risk of Selected Cancer Types
Cancer type
Isomer
Association
a
No association
b
Thyroid cancer
Alpha (α)
Salimi et al. 2023
Beta (β)
Salimi et al. 2023
Deziel et al. 2021; Lerro et al. 2018
Gamma (γ)
Lerro et al. 2021; Salimi
et al. 2023
a
Statistically significant positive association.
b
No statistically significant positive association.
c
Follicular B-cell subtype.
d
Statistically significant inverse association.
e
Among post-menopausal women only.
The available studies provide evidence for an association between exposure to HCH and NHL, and
suggestive evidence for associations with some other cancer types (see Table 2-21). The strongest
evidence for an association between HCH exposure and NHL comes from a prospective cohort study of
54,306 pesticide applicators (the Agricultural Health Study) in Iowa and North Carolina (Alavanja et al.
2014). Cohort members were enrolled between 1993 and 1997 and followed through 2011. At
enrollment and 5 years later, the subjects filled out questionnaires about use of specific pesticides,
including frequency and duration of γ-HCH use. A total of 523 incident cases of NHL were observed
over 803,140 person-years of follow up. The risk of incident NHL was increased with total days of
γ-HCH exposure and with intensity-weighted total days of exposure after adjustment for confounders
identified in the NHL literature and for herbicide use. Analysis by subtype of NHL showed the increased
risk with HCH exposure to be limited to the follicular B-cell subtype (Alavanja et al. 2014).
Three case-control studies nested within prospective cohort studies of populations without known sources
of HCH exposure examined associations between NHL and prediagnostic blood or adipose tissue levels.
In an analysis of 167 cases and 167 controls from three prospective cohort studies (>150,000 subjects) in
Shanghai and Singapore, Bassig et al. (2020) observed a positive association between NHL and blood
levels of β-HCH measured approximately 7 years prior to diagnosis. In contrast, Cantor et al. (2003)
observed no association between NHL and exposure among 74 cases and 157 controls from a large cohort
(25,802 participants) in Maryland. In this study, serum concentrations of β-HCH were measured in 1974,
and cases were identified through 1994. Importantly, the blood concentrations of β-HCH were markedly
higher in the study by Bassig et al. (2020) (median among cases was 5,670 ng/g lipid) than in the study by
Cantor et al. (2003) (mean among cases was 139.9 ng/g lipid). Finally, Brauner et al. (2012) did not
HEXACHLOROCYCLOHEXANE (HCH) 198
2. HEALTH EFFECTS
observe a significant association between NHL and β-HCH in adipose tissue among 256 cases and
256 referents in a cohort of 57,053 participants in Denmark. Adipose samples were collected at
enrollment between 1993 and 1997 and cases were identified through 2008; the median adipose
concentration of β-HCH among cases was 59 ng/g. A large, pooled case-control study provides support
for the association between NHL and HCH exposure. Kachuri et al. (2020) pooled data across three
population-based, case-control studies in the United States and Canada (North American Pooled Project).
The odds of NHL were increased with self-reported exposure to γ-HCH in analyses of 1,690 cases and
5,131 controls (Kachuri et al. 2020). In contrast, another pooled case-control study that included
4,373 cases and 4,373 controls from studies in the United States, Canada, and Italy, found no association
between self-reported exposure to γ-HCH and increased odds of NHL or any individual subtype (De Roos
et al. 2021).
Other epidemiological studies have reported positive associations between β- or γ-HCH in blood or
qualitative exposure to γ-HCH and multiple myeloma, leukemia, colorectal cancer, female breast cancer,
prostate cancer, lung cancer, thyroid cancer, brain cancer, and hepatocellular carcinoma (see Table 2-21).
However, the evidence for these cancer types is relatively weak. In an earlier analysis of cancer incidence
in the Agricultural Health Study (cohort of 57,311 pesticide applicators), increased risks of incident
leukemia and lung cancer were observed among subjects with any self-reported use of γ-HCH compared
with those who never used it (Purdue et al. 2007). In a case-control study of children in Iran, Rafeeinia et
al. (2023) reported an association between serum levels of α-, β-, and γ-HCH and acute lymphoblastic
leukemia (ALL). Lerro et al. (2021) examined incident thyroid cancer in subjects in the Agricultural
Health Study cohort and observed an increase in the hazard ratio among subjects who reported ever use of
lindane (compared with those reporting no lindane use) after a relatively brief follow-up period of 5 years
after enrollment. Band et al. (2011) reported an association between γ-HCH exposure assessed through a
job-exposure matrix and increased risk of prostate cancer in a study of 1,153 cases and 3,999 controls.
Many of the studies reporting positive associations between multiple myeloma, colorectal cancer,
hepatocellular carcinoma, breast cancer, prostate cancer, or thyroid cancer and HCH exposures (Arrebola
et al. 2015b; Ibarluzea et al. 2004; Kumar et al. 2010; Lee et al. 2018a; Miao et al. 2021; Salimi et al.
2023; Waliszewski et al. 2005; Weber et al. 2018; Xu et al. 2010; Zhao et al. 2012) are case-control
studies in which exposure was assessed using concentrations of HCH in blood or adipose samples
collected after disease onset. The lack of temporal relationship between exposure and outcome render
these studies of uncertain utility for hazard identification.
HEXACHLOROCYCLOHEXANE (HCH) 199
2. HEALTH EFFECTS
Epidemiological studies published to date have not shown any associations between exposure to HCH
and cancers of the bladder, endometrium, pancreas, and testicular germ cells, or melanoma and soft tissue
sarcoma (see Table 2-21).
α-HCH. Increased incidences of neoplastic nodules in the liver, hepatomas, and/or hepatocellular
carcinomas were reported in several strains of mice exposed to doses between 13 and 95 mg/kg/day for
16–36 weeks (Hanada et al. 1973; Ito et al. 1973, 1976; Nagasaki et al. 1975; Tryphonas and Iverson
1983; Tsukada et al. 1979). No evidence of liver carcinogenicity was reported in Wistar rats exposed to
45 mg/kg/day α-HCH in the diet for 24 weeks (Nagasaki et al. 1975), but a dose of 70 mg/kg/day for
48 weeks resulted in liver tumors (Ito et al. 1975). Ito et al. (1975) also reported an increased incidence
of hepatocellular carcinomas in male rats exposed to α-HCH in the diet at ≥70 mg/kg/day for 72 weeks.
In studies of α-HCH tumor promotion, mixed results were observed. In rats, administration of
35 mg/kg/day of α-HCH in the diet for 65 weeks inhibited the induction of liver tumors by
0.07 mg/kg/day of aflatoxin B
1
(Angsubhakorn et al. 1981). A study of γ-glutamyltranspeptidase-positive
liver foci in rats pretreated with the tumor initiator N-nitrosomorpholine showed that administration of
α-HCH at 20 mg/kg/day in food for 49 weeks increased the volume fraction of positive foci, largely by
reducing apoptosis (Luebeck et al. 1995). Schröter et al. (1987) reported significant increases in the
number and areas of preneoplastic hepatic foci in female Wistar rats treated with doses ≥2 mg/kg/day in
the diet.
β-HCH. Animal studies of β-HCH carcinogenicity are limited by short duration of exposure, concurrent
mortality, and/or reporting limitations. β-HCH did not increase liver tumor incidences in Wistar rats
exposed to 35 or 70 mg/kg/day in the diet for 24 or 48 weeks (Ito et al. 1975), but this study was
hampered by significant mortality. No increase in liver tumor incidence was noted in dd mice exposed to
18–120 mg/kg/day in the diet for 24 or 32 weeks (Hanada et al. 1973; Ito et al. 1973). However, in a
longer study, Thorpe and Walker (1973) reported an increased incidence of hepatocellular carcinomas in
male CF1 mice and a significant increase in other (unspecified) tumors in female CF1 mice exposed to
34 mg/kg/day in the diet for 104 weeks. In this study, significant mortality occurred early in the study
(12% of males and 25% of females died within 3 months).
β-HCH, at a single oral dose of 100 mg/kg/day, did not induce an increase in the number or size of
preneoplastic hepatic foci in a two-stage study using female Wistar rats dosed with phenobarbital as a
promoting agent (Schröter et al. 1987). However, a significant increase in preneoplastic hepatic foci was
HEXACHLOROCYCLOHEXANE (HCH) 200
2. HEALTH EFFECTS
noted in rats exposed for 20 weeks to doses ≥3 mg/kg/day β-HCH in the diet after initiation with
N-nitrosomorpholine (Schröter et al. 1987).
γ-HCH (Lindane). In Wistar rats, exposure to 25 mg γ-HCH/kg/day in the diet for 24 or 48 weeks did
not result in any liver tumors (Ito et al. 1975); however, the abbreviated exposure duration and high
mortality in the control and treatment groups preclude conclusions as to carcinogenicity under this
experimental protocol. Mice (dd strain) exposed to as much as 90 mg γ-HCH/kg/day in the diet for 24
weeks did not exhibit any increased tumor incidences when compared to controls (Ito et al. 1973). An
increased incidence of malignant hepatomas was reported in male dd mice exposed to 108–120
mg/kg/day in the diet for 32 weeks (Hanada et al. 1973). In that study, survival to study end at the
carcinogenic dose was very low, so the magnitude of the effect may be underestimated.
Chronic-duration studies have shown increased incidences of tumors in mice, but not rats, exposed to γ-
HCH. No statistically significant increases in endocrine, thyroid, pituitary, adrenal gland, liver, or ovary
tumors were observed in male and female Osborne-Mendel rats fed 10.8–33 mg/kg/day in the diet for 80
weeks (NCI 1977) or in Wistar rats fed 0.07–32 mg γ-HCH/kg/day in the diet for 104 weeks (Amyes
1990); however, poor survival rates limit the significance of these results. Liver tumors have been
reported in CF1 and B6C3F1 mice exposed to 13.6–68 mg/kg/day in the diet for 80 to 104 weeks (NCI
1977; Thorpe and Walker 1973). In contrast, EPA (2000a) did not observe an increase in liver tumor
incidence in CD-1 mice exposed to doses up to 26.8 mg/kg/day for 78 weeks. Female mice, but not male
mice, in this study exhibited increased incidences of lung adenomas at the high dose (26.8 mg/kg/day)
(EPA 2000a). Increased incidences of hepatocellular adenomas and carcinomas were also observed in
obese mottled yellow A
vy
/a and lean pseudoagouti A
vy
/a (YSxVY) F1 mice exposed to 27.2 mg/kg/day in
the diet for 96 weeks, but not in lean black a/a (YSxVY) F1 mice (Wolff et al. 1987). The obese mottled
yellow and lean pseudoagouti strains have a dominant mutation at the agouti locus (Avy) that increases
their susceptibility to strain-specific neoplasms. Incidences of benign lung adenomas were also increased
in female obese mottled yellow A
vy
/a and lean pseudoagouti A
vy
/a (YSxVY)F1 mice exposed to
27.2 mg/kg/day for 24 months (Wolff et al. 1987).
In mice, dermal exposure to a 0.5% solution of γ-HCH in acetone applied twice a day for 60 days was
reported to result in no treatment-related tumors (Orr 1948). Limitations of this study include less-than-
lifetime exposure and study duration, testing of only one dose, and potential for ingestion of some of the
compound from the skin.
HEXACHLOROCYCLOHEXANE (HCH) 201
2. HEALTH EFFECTS
δ-HCH. δ-HCH did not induce a significant increase in liver tumors in male Wistar rats exposed to doses
up to 70 mg/kg/day in the diet for 48 weeks (Ito et al. 1975) or in male dd mice exposed to doses up to
90 mg/kg/day in the diet for 24 weeks (Ito et al. 1973). However, these studies were of relatively short
exposure durations, and organs other than the liver were not evaluated for histopathology.
Technical HCH or Unspecified Isomers of HCH. Ito et al. (1973) examined the carcinogenicity of pairs
of HCH isomers in dd mice exposed to 45 mg/kg/day of each isomer in the diet (total HCH dosage of
90 mg/kg/day) for 24 weeks. Exposure to β-HCH with γ- or δ-HCH, or to γ-HCH together with δ-HCH
did not result in hepatocellular carcinomas. However, when any of these isomers was administered with
α-HCH, an increased incidence of hepatocellular carcinomas was observed.
Thakore et al. (1981) reported the appearance of neoplastic nodules in the livers of Swiss mice following
dietary exposure to technical-grade HCH at 90 mg/kg/day for 6 months. Increased incidences of
hepatocellular carcinoma were reported in Swiss mice exposed to 90 mg/kg/day in the diet for 6–
8 months (Bhatt and Bano 2009; Bhatt and Nagda 2012; Trivedi et al. 2007, 2009); to 21.3–85 mg/kg/day
in the diet for 20 months (Munir et al. 1983); and to 10 or 17 mg/kg/day through gavage or the diet,
respectively, for 80 weeks (Kashyap et al. 1979). Dermal application of 2.4 mg technical-grade
HCH/kg/day by skin painting on Swiss mice for 80 weeks resulted in nonsignificant increases in the
incidences of hyperplastic and preneoplastic areas in the liver and hepatic tumors (Kashyap et al. 1979).
The EPA (IRIS 1987a) listed α-HCH as a probable human carcinogen based on sufficient evidence of
carcinogenicity in animals and inadequate data in humans. IRIS (1987b) lists β-HCH as a possible human
carcinogen based on evidence for benign liver tumors in exposed mice and inadequate data in humans.
Data on δ- and ε-HCH were considered inadequate to classify the potential human carcinogenicity (IRIS
1987d, 1987e). Although the IRIS (1987c) program did not evaluate the carcinogenicity of γ-HCH,
EPA’s Office of Pesticide Programs (EPA 2001, 2002) classified γ-HCH into the category “suggestive
evidence of carcinogenicity, but not sufficient to assess human carcinogenic potential.”
The HHS NTP determined that γ-HCH and other HCH isomers may reasonably be anticipated to cause
cancer in humans (NTP 2021). In 2018, IARC determined that there was sufficient evidence in both
humans and animals for the carcinogenicity of γ-HCH, assigning it to Group 1 (carcinogenic to humans).
IARC (2018) concluded that γ-HCH causes NHL in humans.

HEXACHLOROCYCLOHEXANE (HCH) 202
2. HEALTH EFFECTS
Mechanisms. IARC (2018) conducted an extensive review of the available data on mechanisms of HCH
carcinogenicity using the 10 key characteristics of carcinogens (Smith et al. 2016) as a framework. Their
analysis noted that the metabolism of γ-HCH yields several intermediates and metabolites, but that to
date, those involved in carcinogenesis have not yet been identified. In vitro data in rat liver microsomes
have shown the formation of a stable epoxide, indicating that γ-HCH can form electrophilic metabolites
(reviewed by IARC 2018). Based on the available in vivo and in vitro data, IARC (2018) concluded that
there was strong evidence that γ-HCH induces immunosuppression and oxidative stress, and moderate
evidence for genotoxicity and modulation of receptor-mediated effects. Section 2.14 provides details on
the in vivo evidence for immunosuppression in animals exposed orally to γ-HCH; little to no data are
available for immune system effects of other isomers. Several in vivo studies reported increased measures
of oxidative stress in the heart, liver, kidney, central nervous system, testes, and maternal or fetal tissues
after oral exposure to γ-HCH; these studies are described in the Mechanisms subsections of Sections 2.5,
2.9, 2.10, 2.15, 2.16, and 2.17. In vivo studies of estrogen-mediated effects are described under
Mechanisms in Section 2.16. Genotoxicity studies of HCH isomers are summarized in Section 2.20.
2.20 GENOTOXICITY
Numerous in vivo and in vitro studies have assessed the genotoxic potential of HCH and its isomers (α-,
β-, and γ-HCH). Genotoxicity testing results for HCH isomers are summarized below. Results of in vivo
and in vitro genotoxicity studies are presented in Tables 2-22 and 2-23, respectively.
Table 2-22. Genotoxicity of Hexachlorocyclohexane Isomers In Vivo
Species (test system)
Endpoint
Results
Isomer
Reference
Mammalian cells
Human (peripheral blood)
DNA damage
–
Alpha, beta
Varona-Uribe et al. 2016
Human (peripheral blood)
Comet assay
–
Alpha, beta
Varona-Uribe et al. 2016
Human (peripheral
lymphocytes)
Micronucleus test
–
Alpha, beta,
and gamma
Jonnalagadda et al. 2012
Mouse (bone marrow)
Micronucleus test
+
Gamma
Yaduvanshi et al. 2012
Human (peripheral
lymphocytes)
Chromosomal
aberrations
+
Alpha, beta,
and gamma
Jonnalagadda et al. 2012
Rat (bone marrow)
Chromosomal
aberrations
+
Beta
Shimazu et al. 1972
Human (peripheral
lymphocytes)
Chromosomal
aberrations
–
Gamma
Kiraly et al. 1979
Syrian hamster (bone
marrow)
Chromosomal
aberrations
–
Gamma
Dzwonkowska and
Hubner 1986

HEXACHLOROCYCLOHEXANE (HCH) 203
2. HEALTH EFFECTS
Table 2-22. Genotoxicity of Hexachlorocyclohexane Isomers In Vivo
Species (test system)
Endpoint
Results
Isomer
Reference
Mouse
Micronuclei
–
Gamma
Jenssen and Ramel 1980
Mouse (bone marrow)
Chromosomal
aberrations
+
Gamma
Kumar et al. 1995
Rat (liver)
Mitotic disturbances
+
Alpha
Hitachi et al. 1975
Mouse (liver)
DNA binding
(+)
Alpha/
gamma
Iverson et al. 1984
Mouse (germ cells)
Dominant lethal
+
Technical
Lakkad et al. 1982
Human (MGMT tumor
suppressor gene in
colorectal cancer cells)
Hypermethylation
–
Alpha, beta,
and gamma
Abolhassani et al. 2019
Human (P16 tumor
suppressor gene in
colorectal cancer cells)
Hypermethylation
–
Αlpha and
beta
Abolhassani et al. 2019
Human (P16 tumor
suppressor gene in
colorectal cancer cells)
Hypermethylation
+
Gamma
Abolhassani et al. 2019
Human (Leukocyte DNA)
DNA hypomethylation
+
Beta
Itoh et al. 2014
Human (tumor suppressor
gene E-cadherin [CDH1] in
peripheral blood
mononuclear cells)
Methylation
(+)
Beta
Lee et al. 2018b
– = negative result; + = positive result; (+) = weakly positive result; DNA = deoxyribonucleic acid
Table 2-23. Genotoxicity of Hexachlorocyclohexane Isomers In Vitro
Species (test system)
Endpoint
Results
Isomer
Reference
With
activation
Without
activation
Prokaryotic organisms
Salmonella typhimurium
TA100, TA98, TA1535,
TA1537, TA1538
(reversion assay)
Gene mutation
–
–
Gamma
Moriya et al. 1983
S. typhimurium TA98,
TA100, TA102
(Salmonella/microsome
mutagenicity assay)
Gene mutation
–
–
Gamma
Yaduvanshi et al.
2012
Escherichia coli (WP2/spot
test)
Gene mutation
NT
–
Gamma
Nagy et al. 1975
E. coli (WP2 hcr)
(reversion assay)
Gene mutation
–
–
Gamma
Moriya et al. 1983
Bacillus subtilis (rec assay)
DNA damage
NT
–
Gamma
Shirasu et al. 1976

HEXACHLOROCYCLOHEXANE (HCH) 204
2. HEALTH EFFECTS
Table 2-23. Genotoxicity of Hexachlorocyclohexane Isomers In Vitro
Species (test system)
Endpoint
Results
Isomer
Reference
With
activation
Without
activation
Eukaryotic organisms
Fungi and plant cells:
Saccharomyces cerevisiae
Gene mutation
–
–
Gamma
Shahin and von
Borstel 1977
S. cerevisiae (transformed
reporter strain HLYRGI)
DNA damage
NT
+
Gamma
Schmitt et al. 2005
Nostoc muscorum
Gene mutation
NT
–
Gamma
Kar and Singh 1979a
Allium cepa
Mitotic
disturbances
NT
+
Gamma
Nybom and Knutsson
1947
Mammalian cells
Human (peripheral
lymphocytes)
Micronuclei
NT
–
Alpha
Ennaceur 2017
Human (peripheral
lymphocytes)
Micronuclei
NT
+
Beta
Ennaceur 2017
Human (peripheral
lymphocytes)
Micronuclei
NT
+
Gamma
Ennaceur 2017
Human (mammary
carcinoma MCF-7)
Micronuclei
NT
+
Gamma
Kalantzi et al. 2004
Human (prostate carcinoma
PC-3)
Micronuclei
NT
+
Gamma
Kalantzi et al. 2004
Human (SV-40 fibroblasts)
Unscheduled
DNA synthesis
–
–
Gamma
Ahmed et al. 1977
Human (peripheral
lymphocytes)
Unscheduled
DNA synthesis
NT
+
Gamma
Rocchi et al. 1980
Rat (primary hepatocytes)
Unscheduled
DNA synthesis
NT
–
Gamma
Cifone 1990
Human (mammary
carcinoma MCF-7)
DNA damage
NT
–
Gamma
Kalantzi et al. 2004
Human (prostate carcinoma
PC-3)
DNA damage
NT
–
Gamma
Kalantzi et al. 2004
Human (ovary surface
epithelial cells)
DNA damage
NT
+
Beta
Shah et al. 2020
Human hepatocytes
DNA
fragmentation
NT
+
Alpha
Mattioli et al. 1996
Rat (primary cultures)
DNA
fragmentation
NT
+
Alpha
Mattioli et al. 1996
Mouse (hepatocytes)
DNA
fragmentation
NT
–
Alpha
Mattioli et al. 1996
Chinese hamster lung (CHL)
cells
Chromosomal
aberrations
NT
(+)
Gamma
Ishidate and
Odashima 1977
Chinese hamster ovary
(CHO) cells
Chromosomal
aberrations
NT
–
Gamma
NTP 1984

HEXACHLOROCYCLOHEXANE (HCH) 205
2. HEALTH EFFECTS
Table 2-23. Genotoxicity of Hexachlorocyclohexane Isomers In Vitro
Species (test system)
Endpoint
Results
Isomer
Reference
With
activation
Without
activation
CHO cells
Sister chromatid
exchange
NT
–
Gamma
NTP 1984
CHO cells
Chromosomal
aberrations
–
–
Gamma
Murli 1990
Human (peripheral
lymphocytes)
Sister chromatid
exchange
NT
+
Technical
Rupa et al. 1989d
Human (peripheral
lymphocytes)
Chromosomal
aberrations
NT
+
Technical
Rupa et al. 1989d
Calf (thymus DNA)
DNA binding
(+)
NT
Alpha/
gamma
Iverson et al. 1984
– = negative result; + = positive result; (+) = weakly positive result; DNA = deoxyribonucleic acid; NT = not tested
Several studies in humans evaluated the genotoxicity of HCH. Several studies examined the genotoxicity
of hexachlorocyclohexane in agricultural workers exposed to mixtures of pesticides. In rice field workers
exposed to pesticide mixtures, there was no association between peripheral blood levels of α-HCH or β-
HCH and DNA damage measured by comet assay (Varona-Uribe et al. 2016). The frequency of
micronuclei in peripheral lymphocytes in agricultural workers exposed to a complex mixture of pesticides
including HCH was not significantly different compared to unexposed workers, while the frequency of
chromosomal aberrations was significantly increased in exposed workers (Jonnalagadda et al. 2012). In
addition, a correlation between chromosomal aberrations per cell and HCH level was reported
(Jonnalagadda et al. 2012).
In workers occupationally exposed primarily to γ-HCH by inhalation in a pesticide production factory, no
appreciable increase in the frequency of chromosome aberrations was observed compared to the factory
employee control group (Kiraly et al. 1979). In colorectal cancer patients, serum levels of the α-HCH
isomer were not associated with changes in the methylation status of CpG islands of MGMT and
P16 tumor suppressor genes in colorectal cancer cells (Abolhassani et al. 2019). γ-HCH was not
associated with methylation status of the MGMT tumor suppression gene; however, hypermethylation
was found in the P16 tumor suppressor gene (Abolhassani et al. 2019). There was no significant
association between serum levels of β-HCH and methylation status of MGMT and P16 tumor suppressor
genes (Abolhassani et al. 2019); however, serum β-HCH was associated with a slight increase in
methylation of the tumor suppressor gene E-cadherin [CDH1] in peripheral blood mononuclear cells of
HEXACHLOROCYCLOHEXANE (HCH) 206
2. HEALTH EFFECTS
healthy Korean subjects (Lee et al. 2018b). There was a decreased level of global methylation associated
with β-HCH serum levels in human leukocyte DNA of Japanese women (Itoh et al. 2014).
Other studies are available regarding genotoxic effects (chromosomal aberrations, sister chromatid
exchanges) in humans exposed to a wide variety of pesticides, including HCH, when they were used on
farms (Rupa et al. 1988, 1989a, 1989b, 1989c). The specific effects of HCH, apart from the effects due to
other exposures, are not evident from these studies.
α-HCH. Both in vivo and in vitro assays for genotoxicity of α-HCH are available. α-HCH was observed
to bind to liver DNA in HPB mice following intraperitoneal administration (Iverson et al. 1984).
In human peripheral lymphocytes, in vitro exposure to α-HCH did not increase the frequency of
micronuclei in a cytokinesis-block micronucleus assay (Ennaceur 2017). Exposure to α-HCH produced
DNA fragmentation in primary cultures of rat and human hepatocytes, but not in mouse hepatocytes;
DNA repair was not induced in hepatocytes from all three species tested (Mattioli et al. 1996). α-HCH
was observed to bind to calf thymus DNA with metabolic activation (Iverson et al. 1984).
β-HCH. Limited in vivo and in vitro assays for the genotoxicity of β-HCH are available. In animals,
chromosomal aberrations were induced in bone marrow cells of Long-Evans rats following intraperitoneal
exposure to β-HCH in a study reported only as an abstract (Shimazu et al. 1972). In vitro exposure to
β-HCH increased the frequency of micronuclei at cytotoxic concentrations in human peripheral
lymphocytes in a cytokinesis-block micronucleus assay (Ennaceur 2017). β-HCH also induced DNA
damage in ovary surface epithelial cells (Shah et al. 2020).
γ-HCH (Lindane). γ-HCH has been tested in several in vivo and in vitro genotoxicity assays. The
incidence of chromosomal abnormalities (breaks and gaps with or without acentric fragments) in bone
marrow cells was increased in mice exposed to 1.6 mg γ-HCH/kg body weight/day by gavage for 7 days
(Kumar et al. 1995). In a mouse bone marrow micronucleus test, the frequency of micronucleated-
polychromatic erythrocytes was increased, and the frequency of polychromatic erythrocytes was
decreased in bone marrow cells (Yaduvanshi et al. 2012). γ-HCH was negative in a micronucleus assay
in CBA mice (Jenssen and Ramel 1980). Intraperitoneal exposure of Syrian hamsters did not induce
chromosome aberrations in bone marrow cells (Dzwonkowska and Hubner 1986). γ-HCH was observed
to bind to liver DNA in mice following intraperitoneal administration (Iverson et al. 1984).
HEXACHLOROCYCLOHEXANE (HCH) 207
2. HEALTH EFFECTS
γ-HCH did not induce gene mutations in Salmonella typhimurium (TA100, TA98, TA1535, TA1537, and
TA1538) or Escherichia coli (WP2) with or without a metabolic activation system (Moriya et al. 1983) or
in E. coli without metabolic activation in a WP2 spot test (Nagy et al. 1975). γ-HCH was also negative in
an Ames Salmonella/microsome mutagenicity assay in S. typhimurium (TA98, TA100, TA102) with and
without metabolic activation (Yaduvanshi et al. 2012). Exposure to γ-HCH did not produce DNA
damage in Bacillus subtilis in a rec assay, although a mammalian metabolic activation system was not
present (Shirasu et al. 1976). γ-HCH was not mutagenic in Nostoc muscorum algae (Kar and Singh
1979a). Mitotic disturbances (c-mitosis, which is characterized by spindle breakdown as that produced by
colchicine) and chromosome aberrations were observed in onion root tip cells exposed to commercial
γ-HCH (Nybom and Knutsson 1947). In yeast, exposure to γ-HCH did not induce mutations in
Saccharomyces cerevisiae (XV185-14C) in a reversion study (Shahin and von Borstel 1977) but did
induce DNA damage in transformed reporter strain HLYRGI (Schmitt et al. 2005).
In mammalian cells, γ-HCH induced a marginal increase in the frequency of chromosome aberrations
(including chromosomal gaps) in Chinese hamster ovary (CHO) cells (without metabolic activation),
which was interpreted by the authors of the study as providing suggestive, but not conclusive, evidence of
an effect (Ishidate and Odashima 1977). Another study reported negative results in cytogenetic tests
(chromosomal aberrations and sister chromatid exchange) in CHO cells exposed to γ-HCH without
metabolic activation (NTP 1984). In addition, γ-HCH was reported to be negative for chromosomal
aberrations in CHO cells with and without metabolic activation (Murli 1990).
In a cytokinesis-block micronucleus assay, exposure of human peripheral lymphocytes to γ-HCH induced
micronuclei and binucleated cells with micronucleus (BNMN) at a concentration of 100 µg/L, with
significant cytotoxicity at that concentration (Ennaceur 2017). γ-HCH exposure in human mammary
carcinoma MCF-7 and human prostate carcinoma PC-3 cell lines increased the frequency of micronuclei
in both cell lines in the absence of DNA damage or cytotoxicity, suggesting a clastogenic effect (Kalantzi
et al. 2004). In a microgel single cell assay, DNA damage was observed in cultures of rat nasal and
gastric mucosa cells and human nasal mucosa cells following exposure to
γ-HCH (Pool-Zobel et al. 1994).
γ-HCH was found to induce unscheduled DNA synthesis in human peripheral lymphocytes without
metabolic activation (Rocchi et al. 1980), while it was inactive for inducing unscheduled DNA synthesis
in human SV-40 fibroblasts, both with and without activation (Ahmed et al. 1977). In rat primary
hepatocytes tested without metabolic activation mammalian cells, γ-HCH did not induce unscheduled
DNA synthesis (Cifone 1990). γ-HCH was observed to bind to calf thymus DNA when tested with
exogenous metabolic activation (Iverson et al. 1984).
HEXACHLOROCYCLOHEXANE (HCH) 208
2. HEALTH EFFECTS
Technical HCH or Unspecified HCH Isomers. Technical-grade HCH has been tested for genotoxic
effects in one in vivo and one in vitro study. When male Swiss mice were exposed to technical-grade
HCH prior to mating, dominant-lethal mutations were induced, as evidenced by the number of dead
implantations per pregnant female (Lakkad et al. 1982). Cultured human lymphocytes showed a dose-
dependent increase in chromosomal aberrations (gaps, breaks, and fragments) with significant increases at
0.1 μg/mL technical-grade HCH for 48-hour treatment and at 0.05 and 0.1 μg/mL for 72-hour treatment
(Rupa et al. 1989d). In addition, sister chromatid exchanges increased in a dose-dependent manner with
the high dose (0.1 μg/mL) producing the only significant result. These results suggest mild mutagenic
activity at high doses in humans (Rupa et al. 1989d).
HEXACHLOROCYCLOHEXANE (HCH) 209
CHAPTER 3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS,
BIOMARKERS, CHEMICAL INTERACTIONS
3.1
TOXICOKINETICS
Human studies of HCH isomers provide limited quantitative information on absorption, metabolism,
distribution, and excretion. Toxicokinetics have been studied in rodents, with most quantitative
information derived from studies conducted in mice and rats. An overview of these data is provided
below.
• Absorption of HCH isomers has been demonstrated in humans by increased serum levels of the
isomers following inhalation, oral, or dermal exposure.
• N
o animal data are available from the inhalation route to quantify the extent or rate of absorption.
Technical-grade HCH has been shown to be well absorbed from the gastrointestinal tract of
animals (>90% recovery).
• The distribution of HCH isomers in humans and animals is primarily to the adipose tissue but
also to the brain, kidney, muscle, blood, and other tissues. β-HCH accumulates to a much greater
extent than other HCH isomers.
• HCH isomers have been measured in the placenta and umbilical cord blood of humans, indicating
that transplacental exposure to fetuses is likely to occur. HCH isomers have also been detected in
breast milk.
• The primary urinary metabolites are chlorophenols and 1,2,4-trichlorocyclohexane-4,5-epoxide.
The conversion occurs mainly by the action of hepatic CYP enzymes.
• HCH isomer metabolites are primarily excreted through the urine as conjugates of mercapturic
acid, glucuronide, and sulfate.
• A rat physiologically based pharmacokinetic (PBPK) model simulated the toxicokinetics of
γ-HCH. Predicted concentrations in blood, brain, muscle, and fat after a single intraperitoneal
injection and chronic-duration oral dosing compared adequately well with experimental results;
however, the model is not validated via biological evaluation of kinetic parameters.
• A human PBPK model was developed to simulate toxicokinetics of β-HCH in pregnant mothers
and infants exposed during gestation and lactation. The model was validated by comparing
predicted concentrations in breast milk, cord blood, and infant blood to concentrations measured
in mothers and infants from a Canadian Inuit population. Correlations between model-predicted
and measured values for β-HCH were relatively weak because measured concentrations were near
the limit of detection.
• A human dermal PBPK model for
γ-HCH was developed by modifying a flow-limited PBPK
model to include a skin patch compartment for the exposure location. A comparison of model
simulations in which the optimized diffusion constants were varied illustrated the importance of
HEXACHLOROCYCLOHEXANE (HCH) 210
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
considering protein binding of γ-HCH, when predicting the steady-state dermal permeability
constant (K
p
).
3.1.1 Absorption
α-, β-, γ-, and δ-HCH have been detected in the blood serum, adipose tissue, and semen of occupationally
and environmentally exposed individuals, indicating that absorption takes place following inhalation
exposure (Baumann et al. 1980; Czeglédi-Jankó and Avar 1970; Kashyap 1986; Nigam et al. 1986;
Quintana et al. 2004; Saxena et al. 1980, 1981a, 1981b). Human case reports of accidental poisoning
indicate that HCH is also absorbed following oral exposure. High blood concentrations of γ-HCH have
been demonstrated following oral exposure in these cases (Berry et al. 1987; Harris et al. 1969; Khare et
al. 1977; Munk and Nantel 1977; Nantel et al. 1977; Powell 1980; Starr and Clifford 1972).
Dermal absorption of γ-HCH has been demonstrated in several studies that examined absorption from
anti-scabies lotion or head lice shampoo (EPA 2002; Feldmann and Maibach 1974; Franz et al. 1996;
Ginsburg et al. 1977; Lange et al. 1981). Maximum serum levels in healthy volunteers and scabies
patients were reported within 4–6 hours following whole-body application (Lange et al. 1981). However,
the maximum serum levels of γ-HCH in scabies patients were greater than those reported for normal
volunteers. Studies involving a single topical application of γ-HCH to the forearm, which was left for
24 hours before washing, indicate that at least 9% of the applied dose was absorbed; maximum absorption
occurred during the first 12 hours after application of γ-HCH to the skin, but absorption continued for at
least 5 days (Feldmann and Maibach 1974). In infants and children dermally treated with 1% γ-HCH
lotion, maximum blood concentrations of γ-HCH were observed in 6 hours, and averaged 0.028 μg/mL
for the group infested with scabies and 0.024 μg/mL for the non-infested group (Ginsburg et al. 1977).
The maximum blood level measured in children aged 33–64 months treated with 1% topical γ-HCH
lotion was 64 μg/L (EPA 2002). Children aged 3.5–18 years treated for head lice with 1% γ-HCH
shampoo had a maximum γ-HCH blood level of 6.13 μg/L (EPA 2002). HCH isomers are bioavailable
from soil and can be absorbed dermally (Duff and Kissel 1996). In an in vitro study using abdominal skin
obtained from human cadavers, γ-HCH exhibited mean 24-hour dermal absorption values from 0.45 to
2.35% varying with different soil types and soil loadings of 1, 5, and 10 mg/cm
3
.
The absorption of γ-HCH through the skin was studied following application of two different preparations
to the forearm of volunteers (Dick et al. 1997a). The mean peak plasma concentrations of γ-HCH
following exposure to 120 mg γ-HCH/mL acetone and a 3 mg γ-HCH/mL formulation containing white
spirit (a petroleum-based solvent) were 0.91 and 0.47 ng/mL, respectively, although the preparation in
HEXACHLOROCYCLOHEXANE (HCH) 211
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
acetone contained a 40-fold higher concentration of γ-HCH. The proportion of the applied dose absorbed
into the systemic circulation in 6 hours was 5% for the dose applied in acetone and 60% of the applied
dose in white spirit-based formulation. Thus, the white spirit enhanced the absorption of γ-HCH relative
to acetone as the vehicle. About 30% of the applied dose for the white-spirit based formulation was
observed in the stratum corneum at 6 hours of exposure and decreased by 90% at 24 hours. Fifteen
percent of the applied dose for the acetone-based application was in the stratum corneum. The absorption
of γ-HCH through human skin was also assessed in an in vitro study (Dick et al. 1997b). γ-HCH
absorption was reported to be 15–25% in 24 hours for the two formulations that contained white spirit as
the predominant solvent, 3% in 24 hours from an aqueous spray dilution, and <1% in 24 hours for the
acetone preparation.
No information is available on the absorption of α-, β-, γ-, and δ-HCH following inhalation exposure in
experimental animals. γ-HCH is readily absorbed from the gastrointestinal tract of mice and rats (Ahdaya
et al. 1981; Turner and Shanks 1980). Ahdaya et al. (1981) demonstrated that half of the administered
dose was absorbed from the gastrointestinal tract of fasting mice approximately 14 minutes after
administration of radiolabeled γ-HCH by stomach tube. Although this study demonstrates the rapid
absorption of γ-HCH from the gastrointestinal tract, the use of fasted animals prevents an assessment of
the effect of stomach contents on the rate of absorption. Turner and Shanks (1980) studied the rate of
absorption of γ-HCH from the gastrointestinal tract and intestinal lymphatic system using rat intestinal
loop preparations. Prepared loops were injected with γ-HCH, and the blood and lymph were sampled for
30 minutes. γ-HCH was readily absorbed from the intestine into the blood; however, only a small amount
of γ-HCH entered the lymphatic system from the intestine. The extent of oral absorption of technical-
grade HCH has been estimated to be 95.8% in rats within 4 days following the administration of single
doses of the substance (Albro and Thomas 1974). Variation of the dosages from 30 to 125 mg/kg had no
effect on the percent absorption. The overall degree of absorption of technical-grade HCH administered
in the feed for 14 days was similar (94.9%), but the average absorption values of α-, β-, γ-, and δ-HCH
were 97.4, 90.7, 99.4, and 91.9%, respectively (Albro and Thomas 1974). HCH isomers in contaminated
soil were shown to be bioaccessible in an in vitro gastrointestinal model (Tao et al. 2009).
Dermal absorption of γ-HCH was demonstrated in rats and rabbits (Bosch 1987a, 1987b). Male rats
treated dermally with radiolabeled γ-HCH (20% emulsifiable concentrate) on a 4.9 cm
2
shaved dorsal
area exhibited absorption of radiolabel, which increased with time of exposure (Bosch 1987a). After
4 hours, 10.1, 5.3, and 2.0% were absorbed from doses of 0.06, 0.6, and 6 mg/cm
2
/kg, respectively. After
24 hours, 27.7, 20.9, and 5.1% were absorbed from doses of 0.06, 0.6, and 6 mg/cm
2
/kg, respectively.
HEXACHLOROCYCLOHEXANE (HCH) 212
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Male rabbits treated dermally with radiolabeled γ-HCH (20% emulsifiable concentrate) in a 28.3-cm
2
shaved dorsal area absorbed, after 4 hours, 29.6, 18.3, and 7.3% radiolabel from doses of 0.005, 0.05, and
0.5 mg/cm
2
/kg, respectively, and, after 24 hours, 55.7, 40.0, and 16.6% from the same respective doses
(Bosch 1987b). In weanling rabbits, levels of γ-HCH in the blood after a single application of a 1%
solution (60 mg γ-HCH/kg) were 1.67 and 2.48 μg/mL in two rabbits that had been shaved and depilated,
then stripped to remove the keratin layer (Hanig et al. 1976). In contrast, a blood level of only
0.67 μg/mL was seen in a rabbit that had only been shaved and depilated, indicating that absorption
increases with loss of skin integrity.
Dermal absorption of γ-HCH was evaluated in human skin grafted onto a nude mouse model and the
results were compared to an in vivo rat model and in vitro rat and human models (Capt et al. 2007). The
maximum percent absorbed, which included the amount directly absorbed and present in the skin and
stratum corneum, was comparable for the human skin grafted onto a nude mouse (20.7% of applied dose)
and the in vitro human skin model (24.5%). Data for the rat in vivo and in vitro models appeared to
overestimate the potential human absorption. The maximum percent absorbed was 39.8% in the rat in
vivo model and 62.5% in the rat in vitro model.
3.1.2 Distribution
Occupational studies provide information on the distribution of HCH isomers following inhalation
exposure in humans. Air concentrations of α-HCH (0.002–1.99 mg/m
3
), β-HCH (0.001–0.38 mg/m
3
), and
γ-HCH (0.004–0.15 mg/m
3
) were associated with concurrent mean blood serum levels in workers of 69.6,
190.3, and 36.9 μg/L, respectively (Baumann et al. 1980). Serum total HCH concentrations of 0.14–
0.60 ppm were found in workers with unknown levels of exposure to technical-grade HCH (Nigam et al.
1986). HCH isomers have also been detected in adipose tissues of occupational workers and the general
population (Arrebola et al. 2013, 2014; Baumann et al. 1980; Kim et al. 2014; Mustieles et al. 2017;
Pestana et al. 2011; Ploteau et al. 2017; Quintana et al. 2004; Siddiqui et al. 1981). Accumulation of
β-HCH has been shown to increase approximately linearly with time of exposure (Baumann et al. 1980).
In a national EPA survey, adipose tissue samples collected from surgical procedures or autopsies between
1969 and 1983 showed β-HCH concentrations >0.37 ppm lipid in the highest quartile (Quintana et al.
2004).
Case reports of poisoning confirm that γ-HCH is distributed to the central nervous system. γ-HCH was
detected in the cerebrospinal fluid of a young boy following ingestion of an unknown quantity of γ-HCH
HEXACHLOROCYCLOHEXANE (HCH) 213
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
(Davies et al. 1983). γ-HCH was also detected in brain tissue (110 ppb) and heart blood (33.3 ppb)
collected during the autopsy of an infant who was treated with a whole-body application of a 1% γ-HCH
lotion after a hot bath (Davies et al. 1983).
HCH isomers have been measured in the placenta and/or umbilical cord blood of humans, indicating that
transplacental exposure to fetuses is likely to occur (Alvarado-Hernandez et al. 2013; Anand and Taneja
2020; Anand and Taneja 2020; Anand et al. 2019; Dewan et al. 2013; Fukata et al. 2005; Hernik et al.
2016; Herrero-Mercado et al. 2010, 2011; Junque et al. 2020; Lopez-Espinosa et al. 2007; Morello-Frosch
et al. 2016; Saxena et al. 1981a; Shen et al. 2007; Siddiqui et al. 2003; Vizcaino et al. 2011; Yin et al.
2019; Yu et al. 2013). Placental transfer of HCH isomers was analyzed using matched maternal serum,
cord serum, and placenta samples in mother-infant pairs (Yin et al. 2019; Zhang et al. 2018). β-HCH was
the predominant isomer measured in each sample type. An analysis of concentration ratios for all HCH
isomers suggests that the rate of transfer from the placenta to cord blood is slower than for maternal
serum to the placenta (Yin et al. 2019). Transfer data for the two enantiomers of α-HCH suggests that
placental transfer may involve both simple diffusion and active transport (Yin et al. 2019). Experiments
using an in vitro placenta model (human choriocarcinoma derived BeWo cells in a confluent, polarized
monolayer) confirm that multiple mechanisms are likely involved in transplacental transfer of HCH
isomers (Yin et al. 2020). Maternal serum concentrations of β-HCH were shown to increase between the
first trimester of pregnancy and delivery, possibly due to mobilization of fat stores or changes in blood
volume at different stages of pregnancy (Junque et al. 2020). Concentrations in cord blood serum were
correlated with maternal serum concentrations at delivery in this study.
HCH isomers have also been detected in human breast milk (Bedi et al. 2013; Chen et al. 2018; Dewan et
al. 2013; Dimitriadou et al. 2016; Elserougy et al. 2013; Fytianos et al. 1985; Hernik et al. 2016; Kao et
al. 2019; Minh et al. 2004; Shen et al. 2007; Yalcin et al. 2015). α-, β-, and γ-HCH have been found to be
bioconcentrated and excreted in breast milk of women who have been exposed to technical-grade HCH in
pesticide residues (Nair et al. 1996). All four of the HCH isomers (α, β, γ, and δ) discussed in this profile
have been detected in human semen following environmental exposure (Szymczynski and Waliszewski
1981).
In a study of Wistar rats exposed to air concentrations of 0.02–5 mg/m
3
γ-HCH for 90 days, male rats
exhibited higher serum γ-HCH levels than females, but females had higher liver, brain, and fat levels
(Oldiges et al. 1983). The organ levels of γ-HCH were dose-dependent but had returned to baseline levels
after a 4-week recovery period. Oral animal studies provide more detailed information on the distribution
HEXACHLOROCYCLOHEXANE (HCH) 214
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
of HCH or its isomers (Chand and Ramachandran 1980; Eichler et al. 1983; Srinivasan and
Radhakrishnamurty 1983). γ- and β-HCH are primarily stored in the fat of rats acutely exposed for 5, 10,
or 15 days (Srinivasan and Radhakrishnamurty 1983). The overall distribution of γ-HCH was greatest in
fat, followed by brain, kidney, muscle, lungs, heart, spleen, liver, and blood. γ-HCH has also been found
in the adrenal glands of rats (Lahiri et al. 1990; Sulik et al. 1988). In an experiment lasting 12 days, the
accumulation of γ-HCH in the brain of rats dosed with 5 or 12 mg/kg/day by gavage began to decline
after 8 days. This reduction was not observed in rats given 20 mg/kg/day (Tusell et al. 1988).
In the brain of rats, α-HCH has been found to accumulate preferentially in the white matter, an area
containing lipid-rich myelin, as opposed to gray matter (Portig et al. 1989). However, the same brain
distribution pattern was not noted for γ-HCH in mice, even though α- and γ-HCH are equally lipophilic.
Differences in distribution of γ- and α-HCH are most likely due to stereospecificity, because only the
+-enantiomer of α-HCH was shown to accumulate in white matter (Portig et al. 1989). A comparison of
the enantiomeric fractions in the blood, liver, and brain of mice following administration of a single
gavage dose of α-HCH, demonstrated enrichment of the +-enantiomer in the brain, but not in the liver or
blood (Yang et al. 2010). Enantioselective transport across the blood-brain barrier was also demonstrated
in rabbits exposed orally or dermally to α-HCH (Xue et al. 2010). Toxicokinetic modeling from this
study suggested that enrichment of the +-enantiomer in blood was due to a faster elimination rate for the
–-enantiomer in rabbits (Xue et al. 2010). In mice, gavage exposure to 5.9 mg/kg/day γ-HCH for 3 days
was shown to increase the permeability of the blood-brain barrier, as measured by increased fluorescein
dye uptake (Sinha and Shukla 2003). Similar effects were not observed in rats given 8.8 mg/kg/day for
3 days (Sinha and Shukla 2003).
The distribution pattern for β-HCH was found to be in the following order: fat > kidney > lungs > liver >
muscle > heart > spleen > brain > blood. β-HCH accumulates in tissues to a greater degree than γ-HCH
except in the brain, where the γ-HCH accumulates at a higher concentration (Srinivasan and
Radhakrishnamurty 1983). This accumulation increases with increasing dose and treatment period for
β-HCH more so than for γ-HCH. The greater accumulation of β-HCH in tissues is expected since this
isomer is known to be metabolized more slowly. In addition, γ-HCH is known to induce the liver
cytochrome P-450 mixed-function oxygenase system (CYP), and thus, self-induced metabolism is an
important factor that minimizes the accumulation of γ-HCH residues in animal tissues.
The preferential accumulation of HCH in fatty tissues is also observed following intermediate- and
chronic-duration exposure of rats to HCH isomers in the diet (overall distribution: fat > liver > serum)
HEXACHLOROCYCLOHEXANE (HCH) 215
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
(Amyes 1990; Chand and Ramachandran 1980; Dikshith et al. 1991c; Fitzhugh et al. 1950) or exposure to
α- or γ-HCH by gavage (overall distribution: fat > kidney > liver > brain > blood) (Eichler et al. 1983).
HCH has been shown to accumulate in amniotic fluid, placenta, and fetal tissues after oral treatment of
pregnant mice (Srivastava and Raizada 2000). In rats gavaged with γ-HCH on LDs 9 or 14, γ-HCH levels
were higher in their milk than plasma (Dalsenter et al. 1997b). Levels of γ-HCH in the offspring of those
rats were approximately twice as high in kidneys and liver than in brain and testes.
Some information on the distribution of γ-HCH is available from studies in which laboratory animals
were exposed by dermal application (Bosch 1987a, 1987b; Hanig et al. 1976; Solomon et al. 1977a,
1977b). A study on the distribution of γ-HCH in guinea pigs following acute dermal exposure indicates
that accumulation of γ-HCH in the brain is greater than in the blood after single and multiple topical
applications (Solomon et al. 1977a, 1977b); the levels in both tissues increased with the number of
applications. Following dermal treatment of rats with 50 or 100 mg/kg/day technical-grade HCH for
120 days, α-, β-, γ-, and δ-HCH were accumulated in testicular tissue and sperm in a dose-related manner
(Prasad et al. 1995). β-HCH was present at the highest concentration in testicular tissue and sperm.
3.1.3 Metabolism
The metabolism of γ-HCH is illustrated in Figure 3-1. Angerer et al. (1983) determined that
chlorophenols were the primary urinary metabolites of γ-HCH excreted by workers involved in γ-HCH
production. In the study, glucuronides and sulfates of chlorophenols were cleaved by acidic hydrolysis of
urine samples. The metabolites 2,3,5-, 2,4,6-, and 2,4,5-trichlorophenol accounted for almost 57.7% of
the γ-HCH metabolites identified in the urine collected during the last 2 hours of the workers' shifts.
Other urinary metabolites identified included other trichlorophenols, dichlorophenols, tetrachlorophenols,
and dihydroxychlorobenzenes. Pentachlorophenol has also been identified as a urinary metabolite in
humans following occupational exposure (Engst et al. 1979). In vitro investigations indicate that human
liver microsomes convert γ-HCH by dechlorination, dehydrogenation, dehydrochlorination, and
hydroxylation to five primary metabolites: 3,6/4,5-hexachlorocyclohexene, pentachlorocyclohexene,
2,4,6-trichlorophenol, 2,3,4,6-tetrachlorophenol, and pentachlorobenzene (Fitzloff et al. 1982). Similar in
vitro studies have demonstrated that an epoxide forms during the metabolism of pentachlorocyclohexene.
This stable halogenated hydrocarbon epoxide metabolite may be responsible for the mutagenic and
carcinogenic effects of γ-HCH (Fitzloff and Pan 1984).

HEXACHLOROCYCLOHEXANE (HCH) 216
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Figure 3-1. The Proposed Metabolism of Hexachlorocyclohexane
RS
(Cl)n
OH
OH
O
O
OH
SR
(Cl)n
OH
OH
OH
OH
OH
OH
OH
OH
O
OH
OH
3,4,5-PCCOL
3,4,6,5-PCCH
1,2,3,4-TTCB
cis B-PCCH
Oxide
1,2,4-TCB
2,4,5-TCB
3,6,4,5-PCCH
1,2,4-TCB
2,3,5-TCB
Glucuronide and Sulfate Conjugates
Mercapturic Acid Conjugates
3,4,6,5-TCCH
1,2,4,6-TCCOL
2,4,5,6-TCCOL
2,4,5,6-TCCOL
CO
2
HCH
PCCHA
2,4,6-TCP
3,6/4,5-HCCH
HCCOL
+
2,3,4,5-TTCP
2,3,4,5-TTCP
1,2,4-TCB
PCB
HCB
HCCHD
PCCOL
1,2,4-TCB-5,b-epoxide
1,2,4-TCB-
5,b-epoxide
3,6/4,5-HCCH = 3,6/4,5-hexachlorocyclohexene; HCB = hexachlorobenzene; HCCHD = hexachlorocyclohexadiene;
HCCOL = hexachlorocyclohexenol; HCH = hexachlorocyclohexane; PCB = pentachlorobenzene;
PCCH = pentachlorocyclohexene; PCCHA = pentachlorocyclohexane; PCCOL = pentachlorocyclohexenol;
TCB = trichlorobenzene; TCCH = tetrachlorobenzene; TCCOL = tetrachlorocyclohexenol; TCP = trichlorophenol;
TTCP = tetrachlorophenol
Sources: Chadwick et al. 1985; Fitzloff and Pan 1984; Fitzloff et al. 1982
In animals, γ-HCH appears to be transformed by hepatic enzymes to form chlorophenols, chlorobenzenes,
chlorocyclohexanes, chlorocyclohexenes, chlorocyclohexenols, and conjugates of mercapturic acid,
glucuronide, and sulfate (Chadwick and Freal 1972a; Chadwick et al. 1978a; Engst et al. 1979; Kujawa et
al. 1977). These metabolites have been identified in various tissues and in the urine of laboratory
animals. Metabolites found in the liver of rats following intermediate exposure to γ-HCH via gavage or
diet include di-, tri-, tetra-, and pentachlorobenzenes; pentachlorocyclohexenes; and pentachloro-
2-cyclohexen-1-ol (Chadwick and Freal 1972a; Kujawa et al. 1977). Metabolites identified in the blood
of these rats include di-, tri-, tetra-, and pentachlorophenols and pentachloro-2-cyclohexen-1-ol (Kujawa
et al. 1977). Di-, tri-, and tetrachlorophenols; pentachlorocyclohexenes; and pentachloro-2-cyclohexen-
1-ol have been identified in samples of kidney, spleen, heart, and brain tissue from rats fed γ-HCH
HEXACHLOROCYCLOHEXANE (HCH) 217
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
(Kujawa et al. 1977). Metabolites found in the urine include tri-, tetra-, and pentachlorophenol;
pentachloro-2-cyclohexen-1-ol; and isomers of tetrachloro-2-cyclohexen-1-ol (Chadwick and
Freal 1972a; Chadwick et al. 1978c; Kujawa et al. 1977). The metabolism of γ-HCH in the intestine was
reported to be very minor, or the metabolites were completely absorbed. No metabolites were detected in
the feces or in the adrenal gland (Kujawa et al. 1977). In vitro preparations using rat liver slices have also
found that γ-HCH is converted to hexachlorobenzene (Gopalaswamy and Aiyar 1984). However, these
findings have not been confirmed in in vivo experiments.
The major urinary metabolites formed in rats, following intermediate oral exposure to α- or β-HCH, were
identified as tri- and tetrachlorophenols; pentachlorocyclohexene was also identified as a metabolite of
γ-HCH in kidney tissue (Macholz et al. 1982a, 1982b).
The toxicity of γ-HCH appears to be dependent on CYP enzymes. Intermediate exposure to γ-HCH
resulted in greater toxicity in DBA/2 (D2) mice than in C57BL/6 (B6) mice; the DBA/2 (D2) mice are
considered unresponsive to microsomal enzyme induction by aromatic hydrocarbons (Liu and Morgan
1986). Increased toxicity was associated with higher blood and brain concentrations in D2 mice than in
B6 mice at the time of sacrifice. In addition, D2 mice were found to have more 2,4,6-trichlorophenol in
the liver, kidney, and spleen than the less-susceptible B6 mice. The inability of D2 mice to undergo
enzyme induction to increase the rate of detoxification led to γ-HCH’s enhanced toxicity in this strain.
Other investigators have demonstrated the importance of the hepatic microsomal enzymes in the toxicity
of γ-HCH (Baker et al. 1985; Chadwick and Freal 1972a; Chadwick et al. 1981; Chand and
Ramachandran 1980; Tanaka et al. 1979). Chadwick et al. (1981) demonstrated that pretreatment of rats
with inducers of hepatic enzymes significantly influenced the metabolism and excretion of γ-HCH and its
metabolites by altering specific metabolic pathways; excretion of γ-HCH metabolites in the urine
increased nearly 4-fold following pretreatment with Aroclor 1254 or phenobarbital. Following
pretreatment with Aroclor 1254, a 7-fold increase in expired metabolites was observed. Naphthoflavone
had no effect on the excretion rate.
3.1.4 Excretion
Humans excrete HCH isomers and their metabolites in urine, breast milk, sweat, and semen (Angerer et
al. 1981; Genuis et al. 2016). Analysis of urine from humans occupationally exposed to HCH showed the
presence of chlorinated phenols and all isomers of di-, tri-, and tetrachlorophenol (Angerer et al. 1981).
In another study, the elimination of β-HCH (a byproduct of γ-HCH production studied due to its long
HEXACHLOROCYCLOHEXANE (HCH) 218
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
half-life in humans) was investigated in a group of 40 former workers of a γ-HCH-producing plant by
analyzing at least two blood specimens from different time points between 1952 and 1980. The median
half-life of β-HCH was 7.2 years, calculated by concentrations in whole blood, and 7.6 years, calculated
by concentrations in extractable lipids (Jung et al. 1997), assuming first-order kinetics for excretion.
Nonmetabolized γ-HCH was excreted in the urine and feces of healthy volunteers and scabies patients
acutely exposed to a 0.3% γ-HCH emulsion by whole-body application. The cumulative excretion of
nonmetabolized γ-HCH was almost the same in the healthy volunteers and the scabies patients (Zesch et
al. 1982). The elimination of γ-HCH was studied following application of two different preparations to
the forearm of volunteers (Dick et al. 1997a). The elimination half-life was between 50 and 111 hours for
the acetone-based application, and 25–58 hours for the white-spirit based formulation. Absorbed γ-HCH
was excreted in the urine as conjugates of 2,4,6-, 2,3,5-, and 2,4,5-trichlorophenol. Only 0.01–0.15% of
the dose was excreted in the urine in 72 hours following dermal exposure for 6 hours. In a study in which
children infested with scabies and their noninfested siblings were treated dermally with 1% γ-HCH lotion,
the blood level was found to diminish rapidly after application, with a half-life of 17.9 hours in infested
children and 21.4 hours in noninfested children (Ginsburg et al. 1977).
The excretion kinetics of β-HCH into breast milk were studied by monitoring breast milk concentrations
in lactating mothers after birth (Song et al. 2018; Waliszewski et al. 2009). In 40 lactating women, breast
milk concentrations of β-HCH decreased by approximately 30%, from 95 µg/kg fat on the 4
th
day after
birth to 66 µg/kg fat on PND 30 (Waliszewski et al. 2009). Song et al. (2018) monitored monthly breast
milk concentrations in 40 lactating women during the first 6 months after birth. The average breast milk
concentrations of β-HCH decreased from 127 µg/kg lipid 1 month after birth to 84.8 µg/kg lipid 6 months
after birth, representing a 32% reduction over this time period. The excretion profile for β-HCH in milk
lipids followed zero-order kinetics and the mean excretion rate was approximately 7% per month.
Excretion of γ-HCH and its metabolites in laboratory animals has been well documented. Data indicate
that the major route of elimination is via the urine following intermediate- and chronic-duration oral
feeding in mice (Chadwick et al. 1985). Very little is eliminated in exhaled air (Ahdaya et al. 1981;
Chadwick et al. 1985) or in feces (Chadwick et al. 1985) following acute-, intermediate-, and chronic-
duration oral administration in rodents. Because of its high lipid solubility, γ-HCH is excreted through
the dam’s milk (Dalsenter et al. 1997b).
HEXACHLOROCYCLOHEXANE (HCH) 219
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Very little γ-HCH is excreted unaltered. Various phenylmercapturic acid derivatives have been detected
in the urine of rats, formed by the conjugation of γ-HCH metabolites with glutathione subsequent to
dechlorinations and dehydrochlorinations (Allsup and Walsh 1982; Kurihara et al. 1979). In vitro
investigations using rat liver cells indicate that glutathione conjugation is lower for β-HCH compared to
γ- and α-HCH, which are readily conjugated (Fitzloff and Pan 1984; Fitzloff et al. 1982). Γ-HCH
metabolites are excreted in the form of phenylmercapturic acids and glucuronide and sulfate conjugates
(Chadwick et al. 1978a).
In male rats treated dermally with radiolabeled γ-HCH, 0.28, 0.08, and 0.02% radiolabel was excreted in
urine within 4 hours after doses of 0.06, 0.6, and 6 mg/cm
2
/kg, respectively (Bosch 1987a). After
24 hours, 4.4, 3.2, and 0.6% radiolabel had been excreted in urine from the same respective doses. In a
similar study with male rabbits, 3.8, 2.6, and 1.3% radiolabel was excreted in urine within 4 hours after
doses of 0.005, 0.05, and 0.5 mg/cm
2
/kg, respectively (Bosch 1987b). After 24 hours, 25.5, 11.6, and
6.8% radiolabel had been excreted in urine from the same respective doses.
3.1.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
Models are simplified representations of a system with the intent of reproducing or simulating its
structure, function, and behavior. PBPK models are more firmly grounded in principles of biology and
biochemistry. They use mathematical descriptions of the processes determining uptake and disposition of
chemical substances as a function of their physicochemical, biochemical, and physiological
characteristics (Andersen and Krishnan 1994; Clewell 1995; Mumtaz et al. 2012a; Sweeney and Gearhart
2020). PBPK models have been developed for both organic and inorganic pollutants (Ruiz et al. 2011)
and are increasingly used in risk assessments, primarily to predict the concentration of potentially toxic
moieties of a chemical that will be delivered to any given target tissue following various combinations of
route, dose level, and test species (Mumtaz et al. 2012b; Ruiz et al. 2011; Sweeney and Gearhart 2020;
Tan et al. 2020). PBPK models can also be used to more accurately extrapolate from animal to human,
high dose to low dose, route to route, and various exposure scenarios and to study pollutant mixtures (El-
Masri et al. 2004). Physiologically based pharmacodynamic (PBPD) models use mathematical
descriptions of the dose-response function to quantitatively describe the relationship between target tissue
dose and toxic endpoints (Clewell 1995).
DeJongh and Blaauboer (1997) simulated the toxicokinetics of γ-HCH in rats with a PBPK model. A
five-compartment model was constructed: (1) the liver, serving as the metabolizing organ; (2) blood;
HEXACHLOROCYCLOHEXANE (HCH) 220
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
(3) fat; (4) brain; and (5) a lumped compartment representing all other tissues, consisting mainly of
muscle tissue. Values for the physiological parameters and tissue-blood partition coefficients were
obtained from the literature. The model was calibrated on a dataset from the literature on the disposition
of γ-HCH from blood in vivo after single oral dosage and first-order biotransformation and
gastrointestinal absorption constants for γ-HCH were obtained.
The model was validated by simulating the disposition of γ-HCH in vivo after single intraperitoneal and
chronic-duration oral dosing and comparing these simulations with experimental results. Simulated
γ-HCH concentrations in fat, brain, and muscle compared well with measured values obtained after single
intraperitoneal exposure in rats. Simulated levels in blood were slightly higher than measured levels after
oral and intraperitoneal exposure.
A human PBPK model was developed to assess pre- and postnatal exposure to β-HCH and other neutral
persistent organic pollutants (Verner et al. 2009). The infant portion of the model was added to a
previously published maternal model (Verner et al. 2008) that consisted of nine compartments (liver,
brain, adipose tissue, richly perfused tissues, poorly perfused tissues, mammary tissue, uterus, placenta,
and fetus). Β-HCH was assumed to be completely absorbed from contaminated food and absorption was
entered as a direct input to the maternal liver. Excretion into breast milk was modeled as an output from
mammary tissue. The infant portion of the model consisted of five compartments, including liver, brain,
adipose tissue, richly perfused tissues, and poorly perfused tissues. The infant liver was modeled as
receiving β-HCH directly from breast milk for the first year of life (100% absorption was assumed) with
subsequent first-pass metabolism. The brain was included as a potential target organ of toxicity and
adipose tissue was considered the primary site for β-HCH storage. The initial body burden in the infant
was equivalent to lipid-adjusted levels of β-HCH in maternal blood at delivery. Distribution between
compartments in both the maternal and infant models was derived using blood flow and tissue:blood
partition coefficients. Metabolism was parameterized by transforming a published physiological half-life
of 7.6 years into a liver volume-adjusted intrinsic clearance value. Intrinsic clearance values based on
hepatic metabolism were assumed to be the same in mothers and infants. Model parameters described by
Verner et al. (2008) for the maternal model were adjusted for blood lipids during pregnancy, breast milk
lipid content, and excreted volume in breast milk. Parameters for the infant model described infant
physiology as a function of sex, age, body weight, and body height, and included sex-specific organ
volumes and blood flows and tissue:blood and milk:blood partition coefficients.
HEXACHLOROCYCLOHEXANE (HCH) 221
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
The model was validated by comparing predicted concentrations in breast milk, cord blood, and infant
blood to concentrations measured in mothers and infants from a Canadian Inuit population. Correlations
between model predictions and measured values for β-HCH were relatively weak (r=0.35 for cord blood;
r=0.7 for breast milk; r=0.62 for infant blood) because measured concentrations were near the limit of
detection. A sensitivity analysis was performed using Monte Carlo simulations based on variability in
breast milk consumption, fraction of lipids in breast milk, and fraction of lipids in infant adipose tissue for
PCB-153 and p,p’-DDT. Variability was estimated to be approximately 2.5-fold between the 5
th
and
95
th
percentiles. Predicted blood concentrations were highly variable for β-HCH, with only 66% of
individual values falling within the 2.5-fold range of variation.
A human dermal PBPK model for γ-HCH was developed by modifying a flow-limited PBPK model to
include a skin patch compartment for the exposure location (Sawyer et al. 2016). Optimized dermal
absorption parameters were calculated for γ-HCH by adjusting diffusion equations for binding to protein
and lipids and these parameters were included in the PBPK model to describe in vivo toxicokinetics.
Model simulations were run using the time course data from Dick et al. (1997a) where volunteers were
exposed to a 3 mg γ-HCH/mL formulation containing white-spirit for 6 hours and blood samples were
analyzed for γ-HCH for up to 80 hours after exposure. A comparison of model simulations in which the
optimized diffusion constants were varied illustrated the importance of considering protein binding of
γ-HCH, when predicting the steady-state dermal permeability constant (K
p
).
3.1.6 Animal-to-Human Extrapolations
Extrapolating animal toxicity data to predict human risk from HCH exposure appears to be reasonable
since similar effects are seen in both species.
CYP metabolism of HCH isomers occurs in both humans and rodents. The presence of chlorophenols
and chlorobenzenes in urine of workers occupationally exposed to γ-HCH (Angerer et al. 1983; Engst et
al. 1979) was similar to observations of rats experimentally exposed to γ-HCH (Chadwick and Freal
1972a; Chadwick et al. 1978a; Engst et al. 1976; Kujawa et al. 1977). In vitro investigations indicate that
human liver microsomes convert γ-HCH to chlorocyclohexenes, chlorophenols, and chlorobenzenes
(Fitzloff et al. 1982). Both human and rat microsomes have been shown to form an identical epoxide in
vitro following γ-HCH exposure (Fitzloff and Pan 1984). An important difference in interspecies
metabolism of γ-HCH is the production of α-2μ-globulin in the male rat (Dietrich and Swenberg 1990,
1991), a protein not present in humans, which is well known for its role in renal toxicity.
HEXACHLOROCYCLOHEXANE (HCH) 222
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Similar clinical toxic effects resulting from HCH exposure have been observed in laboratory animals
dosed experimentally and humans experiencing occupational, therapeutic, and accidental domestic
exposures to HCH. These include neurological, hepatic, hematological, and dermatological effects.
Though reproductive, immunological, and carcinogenic effects have been reported in occupationally
exposed humans and in animals, the human studies lack both quantitative exposure data and strong causal
associations and also involve concurrent exposures to other chemicals. While rodents appear to be
adequate models for a variety of human effects of HCH exposure, care must be taken in interpreting data
from reproductive toxicity feeding studies in sheep (Beard and Rawlings 1999; Beard et al. 1999a) since
significant differences exist in the gastrointestinal physiology of ruminants and humans.
3.2 CHILDREN AND OTHER POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
This section discusses potential health effects from exposures during the period from conception to
maturity at 18 years of age in humans. Potential effects on offspring resulting from exposures of parental
germ cells are considered, as well as any indirect effects on the fetus and neonate resulting from maternal
exposure during gestation and lactation. Children may be more or less susceptible than adults to health
effects from exposure to hazardous substances and the relationship may change with developmental age.
This section also discusses unusually susceptible populations. A susceptible population may exhibit
different or enhanced responses to certain chemicals than most persons exposed to the same level of these
chemicals in the environment. Factors involved with increased susceptibility may include genetic
makeup, age, health and nutritional status, and exposure to other toxic substances (e.g., cigarette smoke).
These parameters can reduce detoxification or excretion or compromise organ function.
Populations at greater exposure risk to unusually high exposure levels of HCH are discussed in
Section 5.7, Populations with Potentially High Exposures.
Several human studies suggest that the developing fetus may be susceptible to health effects of prenatal
exposure to HCH isomers, with reports of decreased birth weight or increased risk of fetal growth
restriction associated with higher maternal or fetal concentrations of HCH isomers (see Section 2.17).
Individuals with genetic polymorphisms that alter the metabolism and excretion of HCH isomers, may be
at increased risk of these effects. For example, polymorphism of glutathione S-transferase mu 1
(GSTM1) was shown to contribute to the risk of preterm birth or fetal growth restriction following
HEXACHLOROCYCLOHEXANE (HCH) 223
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
exposure to β-HCH (Mustafa et al. 2013; Sharma et al. 2012). A significant interaction was also observed
between polymorphism of the CYP17 gene (A1A2) and
γ-HCH in maternal blood and the risk of preterm
birth (Sharma et al. 2013).
In human studies, serum cord blood levels of β-HCH were associated with increased serum levels of TSH
(see Section 2.17 and Table 2-16); altered thyroid hormone status may affect neurodevelopment of
infants. In addition, adverse neurodevelopmental outcomes have been associated with elevated
concentrations of total HCH or specific HCH isomers in maternal breast milk or children’s blood (Lenters
et al. 2019; Sisto et al. 2015).
Serious neurological effects and occasional deaths have been reported in children following exposure to
γ-HCH by accidental ingestion or by topical application (see Sections 2.2 and 2.15).
Studies of animals exposed to γ-HCH by oral administration demonstrate that the developing organism is
exquisitely sensitive to the toxic effects of this isomer. Developmental effects of γ-HCH observed in
studies of rats, mice, and mink include reduced viability and pup body weight; perturbation of male and
female reproductive tract development; alterations in the developing liver, thymus, spleen, and heart; and
developmental neurotoxicity (see Section 2.17). Little to no data are available for the other isomers of
HCH.
Few studies have compared effects in young and aged animals exposed by the same regimen to HCH
isomers. Weanling rabbits were more sensitive to γ-HCH treatment than young adults, as seen by higher
mortality rates accompanied by excitement and convulsions after a single whole-body treatment with a
1% solution at a dose of 60 mg/kg γ-HCH (Hanig et al. 1976). As discussed in Section 2.17, there is
evidence that γ-HCH causes functional impairment of the developing blood brain barrier in young rats
(Gupta et al. 1999). The brain uptake of fluorescein was significantly increased in 10-day-old pups
treated with a single 2 mg/kg dose, as well as in those treated with 2 mg/kg/day for 8 days. The effect
appeared to be age-related because the brain uptake index was lower when rats were administered a single
2 mg/kg dose at 15 days of age, and there was no effect on brain permeability at a higher dose of
4 mg/kg/day when administered for 3 days to adults (Gupta et al. 1999).
Following intraperitoneal dosing of dams with γ-HCH on GDs 12–17, GABAA receptors in rat fetuses
were studied with radiolabeled t-butylbicyclophosphorothionate (TBPS), a ligand that binds to the
GABAA receptor (Brannen et al. 1998). Treatment with γ-HCH significantly reduced the TBPS binding
HEXACHLOROCYCLOHEXANE (HCH) 224
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
affinity in fetal brainstems and it was concluded that the effect could potentially lead to abnormal brain
activity, increased susceptibility to seizures, and behavioral effects. TBPS binding in brains of fetuses
was reduced when compared to adults (Brannen et al. 1998).
Effects of γ-HCH on the levels of reproductive hormones in blood of male animals appear to be more
significant in younger animals compared with older animals. Male Wistar rats treated with γ-HCH
beginning at 9 weeks of age exhibited significantly decreased serum testosterone and growth hormone
and increased serum LH and FSH (Agrahari et al. 2019). Similar results were observed in another group
exposed at 18 weeks of age, but treatment at 27 weeks of age resulted in smaller decreases in serum
testosterone and growth hormone, and no significant effect on serum LH or FSH (Agrahari et al. 2019).
Differences in oxidative effects have been observed in the testes of young (15-day-old ) versus mature
(90-day-old) rats following intraperitoneal injection with 10 or 20 mg/kg technical-grade HCH (Samanta
and Chainy 1997). Lipid peroxidation occurred to a greater extent in mature rats. However, the percent
decrease in cytosolic superoxide dismutase activity was greater in young rats, which have increased
baseline activity of the enzyme. Based on the findings of this study, it does not appear that young rats are
at increased risk of oxidative testicular damage.
Although it is unknown whether the ability to metabolize HCH specifically differs between children and
adults, some enzymes, which belong to the enzyme superfamilies involved in phase II HCH metabolism,
are developmentally regulated in humans. The development of uridine 5’-diphospho-glucuronosyl-
transferase (UDP-glucuronosyltransferase; responsible for glucuronide conjugation) depends on the
enzyme isoform, but, in general, adult activity is attained by 6–18 months of age (Leeder and Kearns
1997). Development of sulfotransferase (responsible for sulfate conjugates) activity is also substrate-
specific and is usually earlier than UDP-glucuronosyltransferase. In fact, levels of some sulfotransferases
may be greater during infancy and early childhood than during adulthood (Leeder and Kearns 1997). A
series of enzymes are involved in the production of mercapturic acid conjugates: γ-glutamyltrans-
peptidase, glutathione S-transferase, cysteinyl glycinase, and N-acetyl transferase (Sipes and Gandolfi
1991). There are two superfamilies of N-acetyltransferase, and the N-acetyltransferase 2 superfamily has
members that are developmentally regulated in humans. There is some N-acetyltransferase 2 activity in
fetuses by 16 weeks of gestation. Infants up to 2 months of age have the slow metabolizer phenotype of
this gene; the adult distribution of slow and fast metabolizer phenotypes is reached by 4–6 months of age
and full adult activity is achieved at 1–3 years of age (Leeder and Kearns 1997).
HEXACHLOROCYCLOHEXANE (HCH) 225
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Case-control studies have evaluated the interactive effect of exposure to HCH isomers and genetic
polymorphisms on the increased risk of cancer or diabetes (Li et al. 2013, 2016; McCready et al. 2004;
Sharma et al. 2013, 2019). The risk of breast cancer associated with higher serum levels of HCH isomers
was increased by the presence of a polymorphism in the GSTM1 gene (GSTM1 null) (Li et al. 2013;
McCready et al. 2004). Genetic polymorphisms of CYP1A1 did not influence the breast cancer risk
associated with β-HCH exposure (McCready et al. 2004). A significant interaction was demonstrated
between the GSTM1 null polymorphism and β-HCH blood levels on risk of urinary bladder cancer
(Sharma et al. 2013). Mortazavi et al. (2019) did not show a similar correlation between GSTM1 or
GSTT1 polymorphisms and bladder cancer risk associated with HCH isomers. Sharma et al. (2019)
evaluated the influence of CYP1A1, GSTM1, and GSTT1 polymorphisms on the increased risk of
epithelial ovarian cancer risk associated with HCH isomers. Significant interactions between β-HCH
blood levels and CYP1A1m1 and GSTM1 and GSTT1 null genotypes were observed for increased risk of
ovarian cancer (measured as increased cancer antigen-125 or CA-125 levels). The risk of type 2 diabetes
associated with exposure to β-HCH was elevated in individuals carrying a single-nucleotide
polymorphism in the gene encoding adiponectin (ADIPOQ) (Li et al. 2016). Serum adiponectin levels
were reduced in individuals with this polymorphism.
People with lowered convulsion thresholds due to epilepsy (treated or untreated), cerebrovascular
accidents, or head injuries may be at greater risk of the central nervous system effects of γ-HCH toxicity
and may suffer increased risk of or severity of seizures (Kramer et al. 1980; Matsuoka 1981). Exposure
to β-HCH may increase the risk of hypertension in individuals with an elevated BMI (Arrebola et al.
2015a).
3.3 BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as biomarkers of exposure, biomarkers of effect, and biomarkers of susceptibility
(NAS/NRC 2006).
The National Report on Human Exposure to Environmental Chemicals provides an ongoing assessment
of the exposure of a generalizable sample of the U.S. population to environmental chemicals using
biomonitoring (see http://www.cdc.gov/exposurereport/). If available, biomonitoring data for HCH from
this report are discussed in Section 5.6, General Population Exposure.
HEXACHLOROCYCLOHEXANE (HCH) 226
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
A biomarker of exposure is a xenobiotic substance or its metabolite(s) or the product of an interaction
between a xenobiotic agent and some target molecule(s) or cell(s) that is measured within a compartment
of an organism (NAS/NRC 2006). The preferred biomarkers of exposure are generally the substance
itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. Biomarkers of
exposure to HCH are discussed in Section 3.3.1.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an
organism that (depending on magnitude) can be recognized as an established or potential health
impairment or disease (NAS/NRC 2006). This definition encompasses biochemical or cellular signals of
tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial
cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung
capacity. Note that these markers are not often substance specific. They also may not be directly
adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effect caused
by HCH are discussed in Section 3.3.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability
to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or
other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the
biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are
discussed in Section 3.2, Children and Other Populations that are Unusually Susceptible.
3.3.1 Biomarkers of Exposure
HCH isomers measured in human serum are generally normalized by total lipid content, based on total
cholesterol and triglycerides (e.g., ng/g lipid) (Bradman et al. 2007; Curren et al. 2014; Everett and
Matheson 2010; Kaur et al. 2020; Mørck et al. 2014). Porta et al. (2009) suggested that adjustment of
serum concentrations by total cholesterol may be more appropriate than total lipid in studies of patients
with severe disease (e.g., pancreatic cancer). Concentrations of HCH isomers have also been measured
using whole blood (Sexton and Ryan 2012; Sexton et al. 2011).
Urinary concentrations of 2,4,5- and 2,4,6-trichlorophenol (in units of µg/g creatinine) were measured as
indicators of γ-HCH exposure in the NHANES III survey (1998–2004) (Allen et al. 2006).
Pentachlorophenol was also included as a γ-HCH metabolite in some studies (Naeher et al. 2009). The
use of these phenolic urinary metabolites as exposure biomarkers is limited because these are not specific
HEXACHLOROCYCLOHEXANE (HCH) 227
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
to γ-HCH and may result from exposure to other chlorinated benzenes or phenols (Angerer et al. 1981;
Naeher et al. 2009).
Measurement of HCH isomers in hair has also been used as an exposure biomarker and was suggested to
be a better measure of chronic exposure than blood or serum concentrations (Michalakis et al. 2012;
Tsatsakis et al. 2008). A linear relationship between exposure level and hair concentration was observed
in a 90-day gavage study in rats exposed to a mixture of pesticide including γ- and β-HCH (Appenzeller
et al. 2017).
HCH isomers have been detected in adipose tissue samples taken by biopsy or following surgical
procedures (Aulakh et al. 2007; Ociepa-Zawal et al. 2010).
There are few quantitative data to correlate levels of any of the HCH isomers in human tissue or fluids
with past exposure. A study in which children infested with scabies and their non-infested siblings were
treated dermally with 1% γ-HCH lotion found no correlation between the dose applied and the subsequent
level of γ-HCH in blood (Ginsburg et al. 1977). The blood level was also seen to diminish rapidly after
application, with a half-life of 17.9 hours in infested children and 21.4 hours in non-infested children.
In contrast, β-HCH persists in the blood for a longer period of time than the other isomers. A study of
workers in a γ-HCH -producing factory found that levels of β-HCH in blood serum were higher than
those of other isomers, and there was a significant correlation between serum levels of β-HCH and length
of employment (Baumann et al. 1980). Studies of populations with general HCH exposure have
consistently found the level of the β-isomer to be higher than those of the other isomers (Kashyap 1986;
Nigam et al. 1986; Ramachandran et al. 1984). This is probably due to the greater tendency of β-HCH to
persist and accumulate in the body, while the other isomers are more rapidly metabolized or excreted. A
survey of epidemiological studies involving workers occupationally exposed to “crude benzene
hexachloride” as much as 10–15 years prior to sampling reported serum levels of 20–348 μg/L β-HCH
(Morgan and Lin 1978). Unfortunately, none of the above studies specified exposure levels, so it is still
questionable whether blood HCH levels can be used as biomarkers to quantify exposure.
There is also a direct correlation between HCH levels in the blood and human adipose tissue and semen
(Baumann et al. 1980; Radomski et al. 1971a, 1971b; Szymczynski and Waliszewski 1981);
concentrations of β-HCH in subcutaneous adipose tissues were found to be 300 times higher than blood
levels (Baumann et al. 1980). Levels of β-HCH detected in skin lipids correlated with those found in
HEXACHLOROCYCLOHEXANE (HCH) 228
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
human adipose tissue (Sasaki et al. 1991). Although exposure levels were not known, the results of this
study indicate that measuring β-HCH in skin lipids can be an easy means of determining relative levels or
times of individual exposure. The method of collecting the skin lipid samples was noninvasive, involving
washing the face with soap and wiping 3–4 hours later with fat-free cotton soaked in 70% ethanol. Β- and
γ-HCH have also been found in samples of human maternal adipose tissue, maternal blood, cord blood,
and breast milk in women who were exposed to unknown levels of various organochlorine pesticides in
Kenya (Kanja et al. 1992).
3.3.2 Biomarkers of Effect
No biomarkers of effect, specific for HCH isomers, have been identified in the literature. Several studies
have demonstrated increases in lipid peroxidation and depletion of antioxidants in the central nervous
system, liver, kidney, male reproductive tract, and maternal or fetal tissues in animals exposed to γ-HCH;
however, these are nonspecific effects induced by a wide range of compounds.
3.4 INTERACTIONS WITH OTHER CHEMICALS
Toxicokinetics. The metabolism of γ-HCH can be altered by exposure to other chlorinated hydrocarbon
insecticides such as DDT. Exposure to various chlorinated hydrocarbon insecticides, including γ-HCH, is
thought to produce generalized nonspecific induction of microsomal enzymes, including cytochrome
P450s. Induction of these enzymes could affect the toxicokinetics of a variety of xenobiotics that are
metabolized through microsomal oxidation. Induction of mixed-function oxidase activity by other
chlorinated hydrocarbon insecticides stimulates the oxidative degradation of γ-HCH to the
tetrachlorophenols and enhances its elimination in the urine (Chadwick and Freal 1972b). Guinea pigs
maintained on diets deficient in vitamin C and protein showed altered γ-HCH metabolism and excretion.
Vitamin C deficiency decreased the amount of γ-HCH and its metabolites excreted in the urine and
increased the amount stored in the kidney (Chadwick et al. 1972). Cadmium, which is known to inhibit
hepatic drug-metabolizing enzymes in mammals, also inhibited the metabolism of γ-HCH in adult male
Wistar rats exposed to the compound after short- and long-term pretreatment with cadmium (Chadwick et
al. 1978b). Cadmium may inhibit γ-HCH metabolism indirectly by increasing levels of zinc and reducing
levels of copper in the liver (Chadwick et al. 1978b). The addition of cadmium to the diet also increased
the concentration of γ-HCH measured in the plasma and liver (Khanna et al. 1988).
HEXACHLOROCYCLOHEXANE (HCH) 229
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Liver Effects. Pretreatment with γ-HCH reduced the clearance and exacerbated the liver toxicity (i.e.,
increased ALT and AST levels) of a single oral dose of 500 mg/kg acetaminophen in rats (Akhlaq et al.
2006). Acute exposure to ethanol was shown to increase the hepatotoxicity of
γ-HCH in an
intraperitoneal injection study as measured by increased ALT and AST activity (Radosavljević et al.
2008). The increase in liver weight produced by oral subchronic exposure to commercial HCH was
exacerbated by concurrent exposure to phenobarbital or carbon tetrachloride (Khanna et al. 2002).
A low-protein diet potentiated the effects of γ-HCH on reducing the weights of various organs in male
rats (Khanna et al. 1990). Serum and liver lipid content and cholesterol levels were increased in animals
fed low-protein diets. The low-protein diet increased the levels of γ-HCH found in the various organ
tissues. Histopathological changes in the liver, kidneys, and muscles following dietary exposure to a
pesticide mixture containing monocrotophos, endosulfan, and HCH were exacerbated in protein-
malnourished and diabetic rats (Benjamin et al. 2006).
Natural plant extracts (i.e., ajwain extract, Hyrtios aff. Erectus sponge extract) have been shown to reduce
rodent liver toxicity of HCH isomers administered individually (Anilakumar et al. 2009) or as a mixture
with other organochlorine compounds (Abd El-Moneam et al. 2017). The mechanisms by which these
plant extracts mitigate HCH toxicity may include antioxidant activity and/or alterations in HCH
absorption, distribution, metabolism, or elimination. Oral administration of aloe vera extract prevented
the liver toxicity of
γ-HCH in rats (measured by serum enzymes), when administered concurrently for
4 weeks (Etim et al. 2006). Intravenous administration of gadolinium chloride to hyperthyroid rats
resulted in Kupffer cell depletion and a reduction in oxidative stress and liver injury following
intraperitoneal injection of
γ-HCH (Simon-Giavarotti et al. 2002). Administration of the antioxidant plant
extract andrographolide (from Andrographis paniculate) was shown to exert a hepatoprotective effect in
mice chronically exposed to technical-grade HCH (Trivedi et al. 2007, 2009). Liver toxicity measured by
serum enzymes and histopathology and liver tumor formation occurring in mice exposed to HCH in the
diet were not seen in mice given the combined exposure to andrographolide and HCH. Measures of
oxidative stress in the mouse liver associated with HCH were also ameliorated by the combined treatment
(Trivedi et al. 2007).
Γ-HCH was shown to be an aryl hydrocarbon receptor (AhR) antagonist in rat and human hepatoma cells
(DR-H4IIE and DR-Hep-G2, respectively) and mammary gland carcinoma DR-T47-D cells. When
administered as a mixture with other organochlorine compounds, an additive response on AhR
antagonism was observed (Doan et al. 2019).
HEXACHLOROCYCLOHEXANE (HCH) 230
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Gupta et al. (2011) showed that
γ-HCH promoted the formation of preneoplastic lesions (glutathione-
S-transferase placental [GST-P] positive foci) in the rat liver following initiation by diethylnitrosamine.
Simultaneous treatment with the dietary flavonoid quercetin appeared to reverse this promotion resulting
in decreased apoptosis, reduced incidence of GST-P positive foci and lower expression of p53.
Immunological Effects.
Γ-HCH produced apoptosis and necrotic cell death in isolated mouse
thymocytes in vitro and a greater-than-additive effect was observed when
γ-HCH was administered in
combination with malathion or permethrin (Olgun et al. 2004). Each of these pesticides produced
oxidative stress in mouse thymocytes (increased superoxide anion and hydrogen peroxide) with a greater-
than-additive effect on superoxide anion production observed when
γ-HCH and malathion were
administered together (Olgun and Misra 2006). Hydrogen peroxide production was not significantly
higher if pesticides were given in combination.
Γ-HCH given in combination with malathion or
permethrin increased superoxide dismutase activity and decreased the activity of glutathione-peroxidase
and glutathione-reductase in mouse thymocytes, suggesting a role for oxidative stress in cytotoxicity
(Olgun and Misra 2006).
Ocimum sanctum seed oil (OSSO) antagonized the immunotoxic effects of
γ-HCH on humoral immunity
(i.e., anti-SRBC response) and delayed-type hypersensitivity (i.e., footpad thickness) (Mediratta et al.
2008).
Neurological Effects. Γ-HCH is a central nervous system stimulant, whereas the α-, β-, and δ-isomers of
HCH are mainly depressants (McNamara and Krop 1948; Smith 1991). Isomeric interactions can occur,
such that α-, β-, and δ-HCH counteract the effects of
γ-HCH; neurotoxicity is reduced when a dose of
δ-HCH is accompanied by an equal or higher dose of the other isomers. These interactions likely account
for differences in the neurotoxicity of γ-HCH and technical-grade HCH, the majority of which is
comprised of isomers other than γ-HCH (60–70% α-HCH, 5–12% β-HCH, 10–15% γ-HCH, 6–10%
δ-HCH, and 3–4% ε-HCH [Baumann et al. 1980; Kutz et al. 1991]).
Γ-HCH and dieldrin, given in combination, produced a greater-than-additive effect on reactive oxygen
species generation, caspase activation, reduced mitochondrial membrane potential, and enhanced
cytotoxicity in immortalized rat dopaminergic neuronal cells in vitro (Sharma et al. 2010). Pretreatment
with an antioxidant plant extract (Decalepis hamiltonii) for 7 days was shown to prevent lipid
peroxidation, glutathione depletion, and altered activity of antioxidant enzymes in major rat brain regions
HEXACHLOROCYCLOHEXANE (HCH) 231
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
induced by a single dose of technical-grade HCH (Srivastava and Shivanandappa 2014). The natural
product Chaetoglobosin K (ChK) was shown to prevent or reverse the
γ-HCH-induced inhibition of gap
junction-mediated communication in rat RG-2 astroglial cells in vitro (Sidorova and Matesic 2008).
Ethanol acted as an antagonist to
γ-HCH in the central nervous system by decreasing the seizure
incidence and intensity of convulsions and prolonging the duration of the latency period in rats
(Mladenović et al. 2007). Anticonvulsant medications (e.g., diazepam, clonazepam, phenobarbital) were
also effective in reducing seizures and lethality induced by
γ-HCH in mice (Tochman et al. 2000).
Reproductive Effects. Vitamin A supplements decreased HCH-induced toxicity in the rat testes, while
deficiencies in vitamin A potentiated the toxicity (Pius et al. 1990). Combined antioxidant treatment with
vitamin C, vitamin E, and α-lipoic acid was shown to reduce
γ-HCH -induced testicular toxicity in mice
(i.e., testes weight and histopathology) (Nagda and Bhatt 2011). Daily injection of garlic extracts
following oral exposure to
γ-HCH reversed the observed male reproductive toxicity of γ-HCH alone in
rats (decreased weights of testes, epididymis, seminal vesicles and prostate, sperm effects, and altered
serum hormone levels). Injection of garlic extracts also reduced measures of oxidative stress in the rat
testes and brain (Hfaiedh et al. 2011). Oral administration of the antioxidant, curcumin, either before,
concurrently, or after oral
γ-HCH dosing for 14 or 28 days, protected against male reproductive toxicity
including decreased testes and epididymis weight and effects on sperm count, morphology, and motility
(Sharma and Singh 2010). Curcumin also reduced the testicular levels of superoxide dismutase, catalase,
and glutathione-transferase; however, testicular glutathione content was not affected.
Soy isoflavones in the diet have been shown to alter uterine morphology (i.e., increased hyperplasia) and
expression of Erα in the rat (Yang et al. 2014; Zhang et al. 2016). These effects were reduced by
simultaneous gavage administration of
γ-HCH.
Developmental Effects. Cadmium interacts with γ-HCH to cause significant embryotoxic and teratogenic
effects in the developing rat fetus when administered together at a dosage that, for either toxin alone, is
insufficient to cause any deleterious effects on development (Saxena et al. 1986).

HEXACHLOROCYCLOHEXANE (HCH) 232
CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION
4.1
CHEMICAL IDENTITY
HCH consists of eight isomers (Safe 1993). Only γ-HCH, α-HCH, β-HCH, and δ-HCH are of
commercial significance and considered in this profile. The pesticide lindane refers to products that
contain >99% γ-HCH. The α-, β-, and δ-isomers, as well as technical-grade HCH are not synonymous
with γ-HCH (Farm Chemicals Handbook 1993). Technical-grade HCH (CAS Registry Number
608-73-1) is not an isomer of HCH, but rather a mixture of several isomers; it consists of approximately
60–70% α-HCH, 5–12% β-HCH, 10–15% γ-HCH, 6–10% δ-HCH, and 3–4% ε-HCH (Kutz et al. 1991).
Information regarding the chemical identities of α-, β-, γ-, and δ-HCH is located in Table 4-1.
Table 4-1. Chemical Identity of Hexachlorocyclohexane Isomers
a
Characteristic
α-Hexachlorocyclohexane
β-Hexachlorocyclohexane
Synonym(s) and
registered trade name(s)
1-alpha, 2-alpha, 3-beta, 4-alpha,
5-beta, 6-beta-benzene-trans-
hexachloride; alpha-1,2,3,4,5,6-hexa-
chlorocyclohexane; alpha-benzene
hexachloride; alpha-BHC; alpha-
HCH; alpha-hexachloran; alpha-
hexachlorane; alpha-hexachloro-
cyclohexane; alpha-lindane;
benzenehexachloride-alpha-isomer;
cyclohexane 1,2,3,4,5,6-(alpha, DL);
cyclohexane 1,2,3,4,5,6-hexachloro,
alpha-; cyclohexane
1,2,3,4,5,6-hexachloro-, alpha-
isomer; cyclohexane, alpha-
1,2,3,4,5,6-hexachloro; ENT 9232
1-alpha, 2-beta, 3-alpha, 4-beta,
5-aplha, 6-beta-hexachlorocyclo-
hexane; beta 1,2,3,4,5,6-hexachloro-
cyclohexane; beta-benzenehexa-
chloride; beta-BHC; beta HCH; beta-
hexachloran; beta-hexachloro
benzene;
beta-lindane; cyclohexane,
1,2,3,4,5,6-hexachloro-, beta-; cyclo-
hexane, 1,2,3,4,5,6-hexachloro-, beta-
isomer; cyclohexane, 1,2,3,4,5,6-hexa-
chloro-, trans-; cyclohexane, beta-
1,2,3,4,5,6-hexachloro-; ENT 9233;
trans-alpha-benzenehexachloride
Chemical formula
C
6
H
6
Cl
6
C
6
H
6
Cl
6
SMILES
C1(C(C(C(C(C1Cl)Cl)Cl)Cl)Cl)Cl
C1(C(C(C(C(C1Cl)Cl)Cl)Cl)Cl)Cl
Chemical structure
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
CAS Registry Number
319-84-6
319-85-7

HEXACHLOROCYCLOHEXANE (HCH) 233
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-1. Chemical Identity of Hexachlorocyclohexane Isomers
a
Characteristic
γ-Hexachlorocyclohexane
δ-Hexachlorocyclohexane
Synonym(s) and registered
trade name(s)
Lindane; 1-alpha, 2-alpha, 3-beta,
4-alpha, 5-alpha, 6-beta-hexachloro-
cyclohexane; benzene hexachloride-
gamma-isomer; BHC; cyclohexane
1,2,3,4,5,6-hexachloro-gamma-
isomer; ENT 7796; gamma-benzene
hexachloride; gamma-BHC; gamma-
hexachlorocyclohexane; gamma-
1,2,3,4,5,6-hexachlorocyclohexane;
gamma-HCH; gamma-
lindane; HCH;
HCCH; hexachlorocyclohexane,
gamma-isomer; 1,2,3,4,5,6-hexa-
chlorocyclohexane, gamma-isomer,
Etan 3G (Diachem S.P.A.); Forlin;
Gamaphex; Isotox (Chevron
Chemical Co.); Germate Plus
(Gustafson Inc.); Gamma-Mean 400
and Gamma Mean L. (Oregon-
California Chemicals, Inc.); Hammer
(Exsin Industries); Lindagam;
Novigam; Silvanol; Kwell
1-alpha,2-alpha,3-alpha, 4-beta,
5-alpha, 6-beta-hexachlorocyclo-
hexane; cyclohexane,
1,2,3,4,5,6-hexachloro-, delta-
isomer; cyclohexane, delta-
1,2,3,4,5,6-hexachloro-; delta-
(AEEEEE)- 1,2,3,4,5,6-hexachloro-
cyclohexane; delta-benzenehexa-
chloride; delta-BHC; delta-HCH;
delta-1,2,3,4,5,6-hexachlorocyclo-
hexane; delta-lindane; ENT 9234
Chemical formula
C
6
H
6
Cl
6
C
6
H
6
Cl
6
SMILES
C1(C(C(C(C(C1Cl)Cl)Cl)Cl)Cl)Cl
C1(C(C(C(C(C1Cl)Cl)Cl)Cl)Cl)Cl
Chemical structure
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
CAS Registry Number
58-89-9
319-86-8
a
All information obtained from NLM 2021.
CAS = Chemical Abstracts Service; SMILES = Simplified molecular-input line-entry system
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Information regarding the physical and chemical properties of α-, β-, γ-, and δ-HCH is located in
Table 4-2.

HEXACHLOROCYCLOHEXANE (HCH) 234
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Hexachlorocyclohexane Isomers
Property
α-Hexachloro-
cyclohexane
(CAS 319-84-6)
β-Hexachloro-
cyclohexane
(CAS 319-85-7)
γ-Hexachlorocyclo-
hexane
(CAS 58-89-9)
δ-Hexachloro-
cyclohexane
(CAS 319-86-8)
Molecular weight
290.83
a
290.83
a
290.83
a
290.83
a
Color
Brownish to white
b
No data
White
c
No data
Physical state
Crystalline solid
b
;
monoclinic prisms
a
Crystalline solid
a,d
Crystalline solid
d
;
monoclinic prisms
b
Fine plates
a,c
Melting point
159–160°C
a
314–315°C
a
112.5°C
a,e
141–142°C
a
Boiling point
288°C at
760 mmHg
b
60°C at 0.5 mmHg
a
323.4°C at
760 mmHg
b
60°C at 0.36 mmHg
a
Density (g/cm
3
)
1.87 at 20°C
a
1.89 at 19°C
a
1.89 at 19°C
f
No data
Odor
Phosgene-like odor
b
No data
Slightly musty odor
b
No data
Odor threshold:
Water
0.88 ppm for
unspecified purity
g
0.00032 mg/kg
h
12 mg/kg
h
No data
Air
No data
No data
No data
No data
Solubility:
Water
10 ppm
i
; 69.5 mg/L
at 28°C
j
5 ppm
k
17 ppm
k
; 7.3 mg/L at
25°C
b
10 ppm
j
Organic
solvents
Soluble in alcohol
j
;
1.8 g/100 g in
ethanol
i
; 6.2 g/100 g
in ether
j
1.1 g/100 g in
ethanol; 1.8 g/100 g
in ether; 1.9 g/100 g
in benzene
i
6.4 g/100 g in
ethanol; 20.8 g/100 g
in ether; 28.9 g/100 g
in benzene
i
24.4 g/100 g in
ethanol;
35.4 g/100 g in
ether; 41.4 g/100 g
in benzene
i
Partition coefficients:
Log K
ow
3.8
l
3.78
l
3.72
l
4.14
l
Log K
oc
3.57
f
3.57
m
3.0
m
; 3.57
f
3.8
f
Vapor pressure
4.5x10
-5
mmHg at
25°C
b
3.6x10
-7
mmHg at
20°C
b
4.2x10
-5
mmHg at
20°C
b
;
9.4x10
-6
mmHg at
20°C
b
3.5x10
-5
mmHg at
25°C
b
Henry’s law
constant
6.86x10
-6b
4.5x10
-7m,n
3.5x10
-6b
2.1x10
-7o,p
Autoignition
temperature
No data
No data
Not flammable
b
No data
Flashpoint
No data
No data
Approximately 150°F
(closed cup)
b
No data

HEXACHLOROCYCLOHEXANE (HCH) 235
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Hexachlorocyclohexane Isomers
Property
α-Hexachloro-
cyclohexane
(CAS 319-84-6)
β-Hexachloro-
cyclohexane
(CAS 319-85-7)
γ-Hexachlorocyclo-
hexane
(CAS 58-89-9)
δ-Hexachloro-
cyclohexane
(CAS 319-86-8)
Flammability
limits
No data
No data
Not flammable
b
No data
Conversion
factors
q
ppm to mg/m
3
in air (20°C): ppm x 4.96 = mg/m
3
;
mg/m
3
to ppm in air (20°C): mg/m
3
x 0.20 = ppm
Explosive limits
No data
No data
No data
No data
CAS = Chemical Abstracts Service
a
Lide 1991.
b
NLM 2021.
c
Kirk and Othmer 1985.
d
IARC 1979.
e
Budavari et al. 1989.
f
Weiss 1986.
g
Fazzalari 1978.
h
Verschueren 1983.
i
Clayton and Clayton 1981.
j
Kurihara et al. 1973.
k
Hollifield 1979.
l
Hansch and Leo 1995.
m
Rippen et al. 1982.
n
Veith et al. 1979.
o
Pankow et al. 1984.
p
EPA 1982a.
q
Same for all isomers.

HEXACHLOROCYCLOHEXANE (HCH) 236
CHAPTER 5. POTENTIAL FOR HUMAN EXPOSURE
5.1 OVERVIEW
HCH isomers have been identified in at least 312 of the 1,868 hazardous waste sites that have been
proposed for inclusion on the EPA National Priorities List (NPL) (ATSDR 2022). However, the number
of sites in which HCH isomers have been evaluated is not known. The number of sites in each state is
shown in Figure 5-1. Of these sites, 308 are located within the United States, 1 is located in the Virgin
Islands, 1 is in Guam, and 2 are in Puerto Rico (not shown).
Figure 5-1. Number of NPL Sites with Hexachlorocyclohexane Contamination
• Reg
istrations of γ-HCH agricultural products have been cancelled since 2006, significantly
reducing most consumer exposure routes. The primary route of exposure is through dermal
contact from medicinal use. One percent γ-HCH shampoos or lotions are registered with the
FDA and are used for prescription treatment of lice and scabies.
• Individuals who are at risk of higher exposures to γ-HCH include those who live near
contaminated sites and anyone who improperly uses γ-HCH prescription medications .

HEXACHLOROCYCLOHEXANE (HCH) 237
5. POTENTIAL FOR HUMAN EXPOSURE
• Historically, HCH has been released to the environment during its formulation process and
through its use. HCH isomers are persistent and have been recently detected in air, water, and
soil. The general public may be exposed to low levels of HCH through inhalation of
contaminated ambient air, consumption of contaminated drinking water, or incidental ingestion of
or dermal contact with contaminated soils. HCH has not been found to be a major contaminant of
drinking water supplies.
• Once released to the environment, HCH can partition to all environmental media. HCH can exist
in the vapor and particulate phase in the atmosphere. HCH can volatilize from soils but is not
expected to volatilize significantly from water. HCH has low to moderate mobility in soils and
may leach to groundwater. HCH has low to moderate potential to bioaccumulate and has been
detected in aquatic organisms in the United States.
• HCH has a long atmospheric lifetime but can be removed by photodegradation with hydroxyl
radicals or wet and dry deposition. Biodegradation is believed to be the dominant decomposition
process for HCH in soil and water, although hydrolysis and photolysis may occur to a lesser
extent. The rates of degradation depend on the ambient environmental conditions.
5.2 PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
5.2.1 Production
Table 5-1 summarizes information on companies that reported the production, import, or use of γ-HCH
for the Toxics Release Inventory (TRI) in 2021 (TRI21 2022). Most of the uses by these facilities are
considered to be ancillary, indicating purposes other than chemical processing or manufacturing.
Examples of ancillary uses as defined under TRI include cleaners, degreasers, lubricants, fuels, and waste
treatment uses. TRI data should be used with caution since only certain types of industrial facilities are
required to report. This is not an exhaustive list.
Table 5-1. Facilities that Produce, Process, or Use γ-Hexachlorocyclohexane
State
a
Number of facilities
Minimum amount on
site in pounds
b
Maximum amount on
site in pounds
b
Activities and uses
c
AR
1
1,000
9,999
9, 12
NE
1
10,000
99,999
9, 12
OH
2
1,000
99,999
12

HEXACHLOROCYCLOHEXANE (HCH) 238
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-1. Facilities that Produce, Process, or Use γ-Hexachlorocyclohexane
State
a
Number of facilities
Minimum amount on
site in pounds
b
Maximum amount on
site in pounds
b
Activities and uses
c
TX
2
1,000
99,999
9, 12
UT
1
10,000
99,999
9, 12
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
2. Import
3. Used Processing
4. Sale/Distribution
5. Byproduct
6. Reactant
7. Formulation Component
8. Article Component
9. Repackaging
10. Chemical Processing Aid
11. Manufacture Aid
12. Ancillary
13. Manufacture Impurity
14. Process Impurity
Source: TRI21 2022 (Data are from 2021)
HCH does not occur as a natural substance. The manufacturing of technical-grade HCH involves the
photochlorination of benzene, which yields an isomeric mixture consisting of α-HCH, β-HCH, γ-HCH,
δ-HCH, ε-HCH, and inerts (IARC 1979); this reaction can be started by free-radical initiators such as
visual or ultraviolet light, X-rays, or γ-rays (Kirk and Othmer 1985). Treatment with methanol or acetic
acid, followed by fractional crystallization, concentrates γ-HCH to the 99.9% required in the technical-
grade of γ-HCH (IARC 1979); nitric acid is used to remove odor (NLM 2021). None of the isomers or
technical-grade HCH are currently produced in the United States. The production of γ-HCH exceeded
2.27x10
6
g in 1976 (NLM 2021); commercial γ-HCH production in the United States reportedly ended in
that year (EPA 1989a).
γ-HCH was available in emulsifiable and flowable concentrates, soluble concentrates/liquids, wettable
powders, dusts, ready-to-use liquids, pressurized liquids and impregnated materials, oil base and aerosol
sprays, and granules, as well as a smoke generator (Berg 1988; EPA 1985a). γ-HCH was sold separately
or in combination with fungicides, fertilizers, other insecticides, or wood preservatives (Hayes 1982).
5.2.2 Import/Export
Current data on the importation of γ-HCH were not located. Γ-HCH was not included in import/export
information submitted by manufacturers under EPA’s Chemical Data Reporting (CDR) database in the
2020 (covering 2016–2019) reporting cycle (EPA 2022a). The reporting threshold was 2,500 pounds.
HEXACHLOROCYCLOHEXANE (HCH) 239
5. POTENTIAL FOR HUMAN EXPOSURE
Γ-HCH historically was imported from France, Germany, Spain, Japan, and China (EPA 1985a).
Currently, India is the only country where γ-HCH is reportedly produced; thus, India may be the current
supplier to the United States (Vijgen et al. 2011). The U.S. imports of γ-HCH declined from 1.52x10
5
kg
in 1977 to 8.53x10
4
kg in 1982 (NLM 2021). In 2002, it was estimated that 90 metric tons (9.0x10
4
kg)
of γ-HCH were imported into the United States (Hauzenberger et al. 2002). Up until 2001, it was
estimated that 500 metric tons of γ-HCH-containing pesticide products were exported annually by the
United States (primarily to Canada) (Hauzenberger et al. 2002). That export volume dropped to 25 metric
tons in 2001 and continued to decline significantly as other countries and the United States ceased the
usage of γ-HCH containing pesticides.
5.2.3 Use
γ-HCH was initially registered by the U.S. Department of Agriculture (USDA) in the 1940s and over the
years, was approved for use on a wide variety of fruit and vegetable crops (including seed treatment),
tobacco, greenhouse vegetables and ornamentals, forestry (including Christmas tree plantations), farm
animal premises, and other uses. In February 1977, EPA issued a notice of Rebuttal Presumption Against
Registration (RPAR), now called a Special Review, and continued registration of pesticide products
containing γ-HCH. EPA took this action in response to indications of γ-HCH’s potential carcinogenic
effect, possible developmental and reproductive effects, possible blood dyscrasias, and delayed toxic
effects, as well as its acute toxic effects seen in aquatic wildlife (IARC 1979). In October of 1983, EPA
issued a “Notice of Intent to Cancel Pesticide Products Containing γ-HCH.” The contentions concerning
developmental and reproductive effects were successfully challenged by industry. EPA no longer permits
the use of γ-HCH for purposes involving direct aerial application (EPA 1985b). The notice restricted
certain applications of γ-HCH on livestock, structures, and domestic pets to certified applicators or
persons under their direct supervision (EPA 1985b). In November 1993, EPA issued a “Notice of Receipt
of a Request for Amendments to Delete Uses” for several formulations of γ-HCH powder, 99.5%
technical-grade HCH, and dust concentrate, which would delete from the pesticide label most uses of
γ-HCH for agricultural crops and use on animals and humans (EPA 1993). According to the EPA’s last
Registration Eligibility Decision (RED), the last approved food/feed use of γ-HCH that was supported for
re-registration was seed treatment on barley, corn, oats, rye, sorghum, and wheat (EPA 2002). Since the
1998 and 1999 use deletions, the registrants were no longer interested in supporting the seed treatment
use on broccoli, Brussel sprouts, celery, cabbage, cauliflower, collards, kale, kohlrabi, mustard greens,
lettuce, radishes, spinach, or Swiss Chard (EPA 2002). Based on EPA estimates from 1996 to 2001,
about 233,000 pounds of γ-HCH were used annually as a seed treatment (EPA 2002). In August 2006,
HEXACHLOROCYCLOHEXANE (HCH) 240
5. POTENTIAL FOR HUMAN EXPOSURE
EPA issued “Notice of Receipt of Requests to Voluntarily Cancel Lindane Pesticide Registrations,”
which would end the use of γ-HCH as seed treatments, and notice of final orders of cancellation was
issued in December of 2006 (EPA 2006a, 2006b).
γ-HCH is available and regulated by the FDA, for the pharmaceutical treatment of scabies and head lice
(EPA 2002). A 1% γ-HCH lotion is available for the treatment of scabies, and a 1% shampoo is available
for the treatment of head lice; both are prescription use only. Both uses have been on the market since
1947, but they were labeled as a second line therapy in 1995 after a review by the FDA. The FDA has
issued revised labels for 1% γ-HCH lotion and 1% γ-HCH shampoo to be used with caution for infants,
children, and the elderly or anyone who weighs <110 pounds (50 kg), and people with other skin
conditions (FDA 2015). The products are contraindicated in premature infants and people with disorders
that cause seizures (FDA 2015). In the past, γ-HCH was used in veterinary products to control mites and
other pests, but recent data suggest that no products are currently registered in the United States for this
use (Hauzenberger et al. 2002).
5.2.4 Disposal
HCH is listed as a toxic substance under Section 313 of the Emergency Planning and Community Right to
Know Act (EPCRA) under Title III of the Superfund Amendments and Reauthorization Act (SARA)
(EPA 2020c). Disposal of wastes containing HCH is controlled by a number of federal regulations.
The recommended disposal technique for γ-HCH is incineration, at 400–500 ℃ in the presence of a
catalytic mixture of 5–10% metal chloride (copper, iron, zinc, or aluminum) on activated carbon (EPA
1975). Residence times based on this method were not reported. Other effective waste disposal methods
include treatment with strongly alkaline solution or oxidation. In a laboratory-scale study, 98.5% γ-HCH
was removed after a 6.5-hour treatment at pH 11.5 (EPA 1975). Γ-HCH can be effectively oxidized by
ozone and somewhat effectively oxidized by potassium permanganate; oxidation with chlorine or
hydrogen peroxide was ineffective (EPA 1975). EPA standard treatment for hazardous wastes containing
α-, β-, δ-, and γ-HCH is through either incineration or removal from liquid wastes by adsorption, prior to
land disposal (EPA 2014).
While current disposal techniques may be adequate, chemical and biological degradation methods may
provide increased efficiency and quality of disposal at a greatly reduced cost. Some methods utilize
various absorbents, which can be natural (ex. Agricultural solid wastes, biomass) or engineered materials
HEXACHLOROCYCLOHEXANE (HCH) 241
5. POTENTIAL FOR HUMAN EXPOSURE
(e.g., nanomaterials, activated carbon absorbents), to remove pesticides from wastewater prior to
treatment of the absorbent solid waste via microbial degradation or incineration (Saleh et al. 2020).
Microbial strains enriched from HCH-contaminated media can be employed for direct treatment of
wastewater. One laboratory-scale study was able to degrade HCH in wastewater with a consortium of
Pseudomonas, Burkholderia, Flavobacterium, and Vibrio microbial strains (Saleh et al. 2020). Another
method successfully degraded γ-HCH via UV-Fenton treatment (Saleh et al. 2020). This process is cost
effective, around $28.70 per kilogram of contaminants treated, and has shown a 90% chemical oxygen
demand (COD) reduction in studies with other organochlorine pesticides.
It’s unclear how frequently chemical or biological degradation methods are employed, but there are clear
benefits of these disposal techniques. They can be cost-effective, reliable, and can be adapted to the
variety of disposal challenges presented by the multitude of pesticides that are currently used. The use of
microbial consortia ensures that each pesticide will be degraded rapidly. These methods can also be used
for pesticide mixtures (Saleh et al. 2020).
5.3 RELEASES TO THE ENVIRONMENT
The Toxics Release Inventory (TRI) data should be used with caution because only certain types of
facilities are required to report (EPA 2022b). This is not an exhaustive list. Manufacturing and
processing facilities are required to report information to the TRI only if they employ ≥10 full-time
employees; if their facility's North American Industry Classification System (NAICS) codes is covered
under EPCRA Section 313 or is a federal facility; and if their facility manufactures (defined to include
importing) or processes any TRI chemical in excess of 25,000 pounds, or otherwise uses any TRI
chemical in excess of 10,000 pounds, in a calendar year (EPA 2022b).
5.3.1 Air
Estimated releases of 189 pounds (~0.086 metric tons) of γ-HCH to the atmosphere from 7 domestic
manufacturing and processing facilities in 2021 accounted for about 69% of the estimated total
environmental releases from facilities required to report to the TRI (TRI21 2022). These releases are
summarized in Table 5-2.

HEXACHLOROCYCLOHEXANE (HCH) 242
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-2. Releases to the Environment from Facilities that Produce, Process, or
Use γ-Hexachlorocyclohexane
a
Reported amounts released in pounds per year
b
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
Total release
On-site
j
Off-site
k
On- and off-site
γ-HCH
AR
1
0
0
0
35
0
0
35
35
NE
1
180
0
0
20
0
180
20
200
OH
2
0
0
0
1
0
0
1
1
TX
2
8
0
0
0
0
8
0
8
UT
1
0
0
0
0
28
0
28
28
Total
7
189
0
0
56
28
189
84
273
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, wastewater treatment-(metals only), and publicly owned treatment works (
POTWs) (metal
and metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for
disposal, unknown.
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF = reporting facilities; UI = underground injection
Source: TRI21 2022 (Data are from 2021)
All isomers of HCH are considered hazardous air pollutants (HAPs) known to cause or suspected of
causing cancer or other serious human health effects or harmful environmental effects (EPA 2020a), as
regulated under the Clean Air Act. EPA's National Emission Inventory (NEI) database contains
comprehensive and detailed estimates regarding sectors part of the emissions inventory system (EIS)
which emit criteria air pollutants and HAPs for the 50 United States, Washington D.C., Puerto Rico, and
the U.S. Virgin Islands. The NEI database includes point and non-point source emissions, onroad
sources, non-road sources, and event sources such as emissions from wildfires or prescribed burning.
According to data from the 2017 NEI, 62.40 pounds of γ-HCH were released from waste disposal,
industrial solvent use, industrial processes, and fuel combustion (EPA 2020b). These data are
summarized in Table 5-3.

HEXACHLOROCYCLOHEXANE (HCH) 243
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-3. γ-HCH Emissions as Reported by the 2017 National Emission Inventory
Release sector
Emissions (pounds)
Waste disposal
54.607700078
Solvent; industrial surface coating and solvent use
5.91946
Industrial processes; ferrous metals
1.819
Industrial processes; NEC
0.04
Fuel combustion; commercial/institutional oil
0.01
NEC = not elsewhere classified
Source: EPA 2020b
Historically, the largest source of γ-HCH releases to the air resulted from agricultural use of the pesticide
γ-HCH, from aerial pesticide application or wind erosion of contaminated soils. γ-HCH may have also
been released to the atmosphere via volatilization from treated agricultural soils and plant foliage (Lewis
and Lee 1976). Evaporative loss of γ-HCH from water is not considered a significant potential source of
atmospheric γ-HCH because of its relatively high water solubility (Mackay and Leinonen 1975).
Quantitative historical estimates of the amount of γ-HCH released from these sources were not located in
the literature. Aerial applications of γ-HCH were prohibited in the United States as its use as a pesticide
was continuously restricted and eventually prohibited (EPA 1985b, 2006a, 2006b), and atmospheric
releases from agricultural sources today are not expected.
5.3.2 Water
No releases of γ-HCH to water from 7 domestic manufacturing and processing facilities required to report
to the TRI were reported in 2021 (TRI21 2022). This estimate includes releases to wastewater treatment
and publicly owned treatment works (POTWs) (TRI21 2022). These releases are summarized in
Table 5-2.
γ-HCH can be released to surface water via “down-the-drain” releases from consumer wash-off of
treatments for lice and scabies (EPA 2002). These releases would be treated by wastewater treatment and
POTWs, which did not report releases of γ-HCH to the TRI in 2021. Average γ-HCH concentrations in
wastewater treated in Los Angeles County, California, have been reported to range from 3.6x10
-5
to
0.03 μg/L (EPA 2002; Humphreys et al. 2008). α-HCH was not detected in 165 wastewater and
wastewater treatment effluent samples collected between 2001 and 2010 (WQP 2021). One wastewater
sample collected in 2003 contained 0.014 μg/L α-HCH (WQP 2021). β-HCH was not detected in
HEXACHLOROCYCLOHEXANE (HCH) 244
5. POTENTIAL FOR HUMAN EXPOSURE
24 wastewater samples collected between 2003 and 2009 (WQP 2021). Wastewater may be a negligible
source of hexachlorocyclohexanes to surface water and groundwater.
After soil or aerial application for agricultural use, γ-HCH could be released to surface water via surface
runoff (as the dissolved chemical or adsorbed to particulates) or via wet deposition of rain and snow
(Tanabe et al. 1982; Wheatley and Hardman 1965). Lake Ontario received 7 kg/year of α-HCH and
<2 kg/year of γ-HCH due to suspended sediment loading from the Niagara River between 1979 and 1981
(Kuntz and Warry 1983). Historically, the Great Lakes in general received 0.77–3.3 metric tons/year of
α-HCH and 3.7–15.9 metric tons/year of γ-HCH from atmospheric deposition of these contaminants
(Eisenreich et al. 1981). Because γ-HCH is no longer allowed to be used for agricultural purposes, these
are not expected to be significant sources of releases today.
Further from agricultural areas, urban stormwater runoff has historically resulted in releases of HCH to
water in the range of parts per billion to parts per trillion. In 1982, α- and γ-HCH were detected in
samples of urban stormwater runoff from Denver, Colorado, and Washington, D.C., at 0.0027–0.1 and
0.052–0.1 μg/L in 20 and 11%, respectively, of the 86 samples collected; β-HCH was detected only in
runoff from Washington, D.C., in 5% of the samples at a concentration of 0.1 μg/L (Cole et al. 1984). In
urban runoff samples collected in the Canadian Great Lakes Basin, γ-HCH was detected at mean
concentrations of 0.0065 μg/L and 0.0035 mg/kg in the aqueous and sediment portions, respectively; the
mean annual loading of the compound in runoff in the basin was reported to be 4.1 kg/year (Marsalek and
Schroeter 1988). Stormwater samples collected in 2007, just after the ban on γ-HCH for agricultural use,
showed concentrations between 6.0x10
-5
and 0.14 μg/L α-HCH and 3.8x10
-4
and 0.0976 μg/L β-HCH
(WQP 2021).
γ-HCH could be released to groundwater via soil leachate after agricultural application or from improper
disposal at contaminated sites. Available adsorption data indicate that γ-HCH has a low to moderate
mobility in soils, and the results of monitoring studies suggest that γ-HCH does migrate to groundwater
(Page 1981; Sandhu et al. 1978). Groundwater samples were collected from a packaging and
reformulating pesticide facility in Florida, which had disposed of γ-HCH wastes in unlined trenches until
1996. Ground water concentrations for the site ranged from 30 to 420 μg/L for α-, γ-, and δ-HCH
(Chartrand et al. 2015).
HEXACHLOROCYCLOHEXANE (HCH) 245
5. POTENTIAL FOR HUMAN EXPOSURE
5.3.3 Soil
Estimated releases of 56 pounds (~0.025 metric tons) of γ-HCH to soil from 7 domestic manufacturing
and processing facilities in 2021, constituting about 20% of the estimated total environmental releases
from facilities required to report to the TRI (TRI21 2022). No additional quantities were released via
underground injection (TRI21 2022). These releases are summarized in Table 5-2.
γ-HCH has historically been released to the soil by direct application of the pesticide to soil, and can be
released by direct or indirect releases during formulation, storage, and/or disposal. Hazardous waste sites
where γ-HCH has been disposed of in the past are sources of γ-HCH in soils. However, the application of
γ-HCH to laboratory refuse columns simulating municipal landfills indicated that γ-HCH did not
volatilize or leach from the refuse surface, and movement through the column was slight, suggesting that
codisposal of γ-HCH with municipal refuse will result in minimal releases (Reinhart and Pohland 1991;
Reinhart et al. 1991).
5.4 ENVIRONMENTAL FATE
5.4.1 Transport and Partitioning
Air. HCH isomers are volatile, relatively persistent substances in the atmosphere and are expected to be
capable of long-range transportation. HCH can exist in the vapor and particulate phases based on the
reported vapor pressures of the isomers (NLM 2021). Volatilization of γ-HCH used as a seed treatment
was confirmed, with 12–30% of the applied pesticide volatilizing within 6 weeks of planting the seed
(Waite et al. 2001, 2007). Correspondingly, atmospheric concentrations of γ-HCH were variable and
increased when pesticide usage occurred; α-HCH concentrations were less variable throughout the year
(Hoff et al. 1992a). During the winter, higher ratios of α-HCH to γ-HCH reflected the movement of air
containing the more persistent α-HCH isomer from the colder Arctic regions to the south, while the lower
ratios in the summer reflected both increased γ-HCH usage in the region and the lack of movement of
Arctic air (Hoff et al. 1992a). γ-HCH was also seen to move with warm air during the summer months
from the lower United States (or areas even further to the south) to the Great Lakes region, although a
similar trajectory could not be identified for the more ubiquitous α-HCH. Levels of α-HCH in air are not
dominated by volatilization or partitioning to surfaces, but are dependent on local temperature changes
(Hoff et al. 1992b). α-HCH appears to have a long residence time in the atmosphere and is controlled
primarily by transport. Long-range transport potentials were estimated for α- and γ-HCH based on North
HEXACHLOROCYCLOHEXANE (HCH) 246
5. POTENTIAL FOR HUMAN EXPOSURE
American monitoring data, and were reported to be 11,151 miles (17,946 km) and 6,047 miles
(9,732 km), respectively (Shen et al. 2004). The potential for widespread global distribution has been
reported in several studies (Hargrave et al. 1988; Knap and Binkley 1991; Tanabe et al. 1982; Wittlinger
and Ballschmiter 1990). α- and γ-HCH have been measured in arctic air as recently as 2018, but
concentrations have been declining due to declining global emissions and limited use of lindane (Wong et
al. 2021).
HCH isomers in the atmosphere are likely to be subject to rain-out and dry deposition, which may result
in the contamination of surface soil and water. Air-sea exchange fluxes of -0.95 and -17 ng/m
2
/day were
observed over the northwest Pacific Ocean for α- and γ-HCH, respectively, supporting the oceans as an
important sink for atmospheric HCH (Wu et al. 2020). γ-HCH removal rates were 2.5%/week by rainfall
and 3.3%/week by dry deposition, and the estimated residence time of γ-HCH in the atmosphere was
17 weeks (Atkins and Eggleton 1971). The dry deposition flux rate of α-HCH ranged from 0.1 to
5.1 ng/m
2
/day in deposition samples collected in June–August 1997 near the southern Baltic Sea (Wiberg
et al. 2001). The flux rate of γ-HCH was 0.9–32.6 ng/m
2
/day over the same time frame. Seasonal
variation resulted in lower dry deposition rates during the winter months. In samples collected between
February and March 1998, the flux rate for α-HCH ranged from 0.25 to 0.54 ng/m
2
/day, and the dry
deposition flux rate for γ-HCH was 3.4–14.1 ng/m
2
/day (Wiberg et al. 2001). The dry deposition flux rate
of γ-HCH in south central Saskatchewan in 1998 where it had been used as a seed treatment in a canola
field ranged from <29 to 2,203 ng/m
2
/day, and the amount in rainfall over the same period ranged from
<10 to 200 ng/L (Waite et al. 2001). Uptake by plants may be another removal pathway, as observed for
α- and γ-HCH under experimental conditions with lettuce, romaine, and garlic leaf (Yang et al. 2007).
Removal was controlled by plant-air equilibration and correlated strongly with the reported log octanol-
air partition coefficients (K
OA
), 7.44 and 7.72 α- and γ-HCH, respectively (Yang et al. 2007).
Water. In surface waters, HCH has a slight tendency to dissolve and remain in the water column based
on the water solubilities and octanol-water partition coefficients (K
ow
) of the isomers (Clayton and
Clayton 1981; Hansch and Leo 1995; Hollifield 1979; Kurihara et al. 1973). Although γ-HCH has a
relatively high vapor pressure and Henry’s law constant compared with many other organochlorine
insecticides, evaporative loss of γ-HCH from water is not considered to be significant. Mackay and
Leinonen (1975) calculated theoretical losses of several pesticides from saturated water solutions and
predicted a volatilization half-life of 191 days for γ-HCH. Evidence of increased flux from the Arctic
Ocean of α-HCH due to increased temperatures and decreased sea ice coverage may suggest this as
pathway of growing significance as global warming continues (Wong et al. 2021).
HEXACHLOROCYCLOHEXANE (HCH) 247
5. POTENTIAL FOR HUMAN EXPOSURE
γ-HCH released to water may undergo adsorption/desorption with sediments and other materials in the
water. Adsorption and desorption studies of γ-HCH in natural water-sediment systems performed by
Saleh et al. (1982) indicate that a diversity of the natural water-sediment characteristics may affect the
sorption-desorption behavior of γ-HCH in addition to the organic carbon content of the sediments.
γ-HCH is sorbed to silt solutions with a slow desorption rate, indicating that transport through the
environment is most likely to be particle mediated (Noegrohati and Hammers 1992). Biosorption of
γ-HCH was seen for the fungus Rhizopus arrhizus and activated sludge, with equilibrium being reached
within 1 and 4 hours, respectively. Death of the sludge biomass resulted in rapid desorption with zero-
order kinetics, suggesting that adsorbed γ-HCH can be released back into the environment (Tsezos and
Wang 1991a). The sorption of γ-HCH from water using wood charcoal has been described
(Keerthinarayana and Bandyopadhyay 1998); it was found to be a good sorbent for γ-HCH from water.
Sediment and Soil. HCH present in soil can leach to groundwater, sorb to soil particulates, or
volatilize to the atmosphere. In general, the leaching of organic chemicals through soil is governed by the
water solubility of the chemicals and their propensity to bind to soil. Based on the results of a number of
laboratory soil column leaching studies that used soils of both high and low organic carbon content as
well as municipal refuse, γ-HCH generally has low to moderate mobility in soils, with K
oc
values ranging
from 641 to 3,362; log K
oc
range of 2.810–3.5266 (EPA 1982b; Melancon et al. 1986; Reinhart et al.
1991). Adsorption of γ-HCH to soil particulates is generally a more important partitioning process than
leaching to groundwater. However, groundwater sediments, which have low organic carbon content
(<0.1%), are not sufficient to adsorb γ-HCH to the extent that groundwater contamination is prevented
(Nordmeyer et al. 1992). The presence of black carbon in soils from incomplete combustion may impact
sorption affinity. HCH isomers showed varying preference for partitioning to black carbon (α-HCH >
β-HCH > δ-HCH) in soils with 0.82–2.26% organic carbon and 0.04–0.5% black carbon (Ali et al. 2016).
Sorption was observed to be a limiting factor in bioavailability of γ-HCH in soil to earthworms (Šmídová
et al. 2012).
Using sediment (0.44% organic carbon) obtained from a sugarcane growing region of Australia, the K
oc
of γ-HCH was measured as 2,164 (Just et al. 1990). The K
oc
of γ-HCH in a mineral soil containing 1.26%
organic carbon content was measured as 832 (Chiou et al. 1998). In a sandy soil (0.105% organic carbon)
γ-HCH had a measured K
oc
of 3,362, and a desorption K
d
of 3.53 (Melancon et al. 1986). The partition
coefficient (K
p
) of γ-HCH in a laboratory column experiment with municipal solid waste was
853 (Reinhart et al. 1991). In a study involving a laboratory sediment/water system (pH 7.42; 2.18%
HEXACHLOROCYCLOHEXANE (HCH) 248
5. POTENTIAL FOR HUMAN EXPOSURE
organic carbon), α- and γ-HCH isomers were adsorbed on sediments under both aerobic and anaerobic
conditions and few differences were noted in the adsorption behavior of each isomer. Under aerobic and
anaerobic conditions, the K
oc
values of α-HCH were 681 and 617, respectively, while the K
oc
values for
γ-HCH were 641 and 694, respectively (Wu et al. 1997). A mixture of HCH isomers (α-, β-, γ-, and
δ-HCH) sorbed very strongly to a soil from Nagpur, India (pH 7.6, 0.387% organic carbon), with a K
oc
value of 54,000. Some desorption was observed, believed to be due to the water solubility of HCH
(Wadaskar et al. 2006). Desorption experiments with a sandy loam soil slurry showed isomeric
differences in desorption capacity, α- ≥ γ- > δ- > β-HCH (Quintero et al. 2005).
γ-HCH sorbed to the soil can partition to the atmosphere by wind erosion of surface soil particulates
(Stanley et al. 1971) and via volatilization from treated agricultural soils and plant foliage (Lewis and Lee
1976). In tests conducted in a model laboratory system at 10 and 20°C, volatilization half-lives of γ-HCH
from soil and oat plant surfaces of 2.3–24.8 and 0.29–0.73 days, respectively, were reported (Dorfler et al.
1991a); half-lives were greater on dry, sandy soils versus peat soils; however, when moisture was added
to the soils, the half-life was greater for the peat soil, while warmer temperatures decreased the half-life
under all soil and moisture conditions (Dorfler et al. 1991b). In tests performed with a wind tunnel, a
volatilization rate of >20% for γ-HCH from soil surfaces within a 24-hour period was determined (Rüdel
1997). A 6-fold increase in γ-HCH volatilization from soil was seen in laboratory experiments when the
temperature increased from 15 to 45°C; flooding the soil also increased the volatilization (Samuel and
Pillai 1990). A field study conducted in south central Saskatchewan, Canada in 1997–1998 in which
γ-HCH was applied as a seed treatment to canola, determined that between 12 and 30% of the initial
amount applied volatilized to the atmosphere (Waite et al. 2001); a follow-up study determined
volatilization rates of 190 mg/hectare/week at 1 week after application and 420 mg/hectare/week 2 weeks
after application (Waite et al. 2007). The volatilization rate from plant surfaces was 55% for γ-HCH.
Application of γ-HCH to fields of sunflowers and sugarbeets resulted in a 54% evaporative loss of the
pesticide within 24 hours (Neururer and Womastek 1991).
Other Media. γ-HCH has a low to moderate potential to bioaccumulate. A summary of some of the
bioconcentration factors (BCFs) from experimental studies with γ-HCH are provided in Table 5-4.
γ-HCH shows limited uptake from soils and bioconcentration by plants and terrestrial organisms and does
not appear to biomagnify to a great extent.

HEXACHLOROCYCLOHEXANE (HCH) 249
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-4. Results of Experimental Bioaccumulation
Studies with γ-Hexachlorocyclohexane
Species
Exposure route
Bioconcentration factor
Reference
Brine shrimp
Water
183
Matsumura and Benezet 1973
Rainbow trout (fry)
Water
319
Ramamoorthy 1985
Pink shrimp
Water
84
Schimmel et al. 1977
Pinfish
Water
218
Grass shrimp
Water
63
Sheepshead minnow
Water
490
Brine shrimp
Sand
95
Matsumura and Benezet 1973
Northern brook silverside
fish
Sand
1,613
A BCF of 1,273 (lipid basis) in prawns (crustacean) was seen to be 0.58 times the γ-HCH concentration in
the underlying sediment, indicating that aquatic organisms may accumulate γ-HCH from the water
column, and uptake from contaminated sediment alone may not be extensive (Just et al. 1990). BCFs for
the isomers of HCH, using zebra fish under steady-state conditions, were 1,100 for α-HCH, 1,460 for
β-HCH, 850 for γ-HCH, and 1,770 for δ-HCH; BCFs determined by uptake and clearance rate constants
were slightly lower (Butte et al. 1991). Elimination of γ-HCH occurred rapidly in zebra mussels (BCF of
10) and metabolism of γ-HCH was not observed (Berny et al. 2002). BCFs on a wet weight basis for
γ-HCH in different fish species were positively correlated with their lipid content (Geyer et al. 1997).
The bioaccumulation of γ-HCH by tubificide oligochaetes from a static system consisting of sediment and
water has been reported (Egeler et al. 1997). Microalgae Scenedesmus quadricauda and Coccomyxa
subellipsoidea were exposed for 24 hours to mine dump effluent containing α-, β-, γ-, and δ-HCH,
resulting in bioaccumulation factors (BAFs) of 74.6, 60.5, 29.4, and 107.2 for α-, β-, γ-, and δ-HCH,
respectively, in S. quadricauda, and BAFs of 50.8, 47.6, 21.5, and 56.3 for α-, β-, γ-, and δ-HCH,
respectively, in C. subellipsoidea (
Kováčik et al. 2018).
γ-HCH applied to an aquatic mesocosm (i.e., a small, artificial ecosystem) at 61.3 μg/L was reduced by
50% at 24 hours post-application, while at 19 weeks post-application, the concentration in the water was
only 0.2%; no γ-HCH was detected at 21 weeks. The biological half-life was estimated to be 16.7 days.
Movement through the water column was shown by increasing sediment concentrations up to a maximum
of 75.4 μg/kg at 96 hours post-application; however, sediment concentrations decreased to below the
detection limit at 23 weeks to give a half-life in sediment of 48.1 days. Rooted aquatic macrophytes have
a BCF of 56 at a maximum concentration of 1.7 mg/kg at 24 hours post-application; however, at
14 weeks, all residues were below the detection limit for a half-disappearance time of 18 days.
HEXACHLOROCYCLOHEXANE (HCH) 250
5. POTENTIAL FOR HUMAN EXPOSURE
Gastropods in the system had a maximum γ-HCH concentration of 7.2 mg/kg at 24 hours post-treatment,
yielding a BCF of 232.4 and a half-disappearance time of 13.7 days with all residues eliminated by
13 weeks (Caquet et al. 1992).
Trophic transfer of γ-HCH may occur in the foodweb. A study assessed the potential of transfer between
zebra mussels and their predators, tufted ducks (Aythia fugigula) at the birds’ wintering grounds in Lake
Geneva, bordering Switzerland and France. γ-HCH concentrations in the mussels ranged from
approximately 5 to 500 ng/g wet weight, while γ-HCH in the liver of the tufted ducks ranged from 8.9 to
175.6 ng/g wet weight (Bemy et al. 2003).
In tests with radiolabeled γ-HCH, grain, maize, and rice plants accumulated 0.95, 0.11, and 0.04%,
respectively, of the amount of bound residues following 14–20 days growth in a sandy loam soil.
Bioconcentration increased by 4–10 times when the plants were grown in test soils containing both bound
and extractable residues of γ-HCH (Verma and Pillai 1991). Plants and grains grown on soil treated with
γ-HCH showed α-HCH as the predominant isomer, although all isomers were found to some extent;
amounts decreased with increasing time after application (Singh et al. 1991). A different trend in isomer
uptake was observed in garlic. Garlic (Allium sativum L.) was planted in pots containing soil treated with
α-, β-, γ-, and δ-HCH isomers. The BCFs of the underground parts were in the range of 0.48–0.90, 1.60–
1.84, 1.40–2.34, and 2.60–3.64 for α-, β-, γ-, and δ-HCH, respectively, and above-ground parts were
1.50–2.26, 4.50–6.79, 5.16–6.81, and 9.30–12.18, respectively (Chen et al. 2013). The phytoavailability
of the isomers was observed to be δ- > γ- ≥ β- > α-HCH, which generally agreed with the isomer’s water
solubility and vapor pressure (Chen et al. 2013). Evidence of plant uptake from air has been reported.
Lettuce, romaine, and garlic leaf were maintained in air chambers that exposed them to air polluted with
α- and γ-HCH for 5 days. Measured accumulation factors of α-HCH in the crops were 77.25, 190.8, and
95.23 for lettuce, romaine, and garlic leaf, respectively, and accumulation factors of γ-HCH were 187.6,
321.9, and 124.5, respectively (Yang et al. 2007).
Uptake of γ-HCH by earthworms from a treated soil has also been reported. Eisenia andrei exposed to
grassland soil spiked with γ-HCH for 24 hours had measured BAFs ranging from 5 to 35 (
Šmídová et al.
2015)
. Following exposure to 5 ppm of the compound for up to 8 weeks, earthworms bioconcentrated
γ-HCH by a factor of 2.5. The earthworms biotransformed more than 50% of the accumulated γ-HCH;
the main degradation product was γ-2,3,4,5,6-pentachlorocyclohex-1-ene (Viswanathan et al. 1988).
HEXACHLOROCYCLOHEXANE (HCH) 251
5. POTENTIAL FOR HUMAN EXPOSURE
γ-HCH and the other isomers of HCH do not appear to undergo biomagnification in terrestrial food chains
to a great extent, although there is a moderate potential for transfer of γ-HCH to animal tissue as a result
of soil ingestion or ingestion of contaminated foliage (Wild and Jones 1992). Clark et al. (1974) found
that γ-HCH levels in the adipose tissue of cattle were 10 times higher than in the feed (0.002 mg/kg).
Szokolay et al. (1977) examined relative accumulation of HCH isomers including γ-HCH and various
components in the food chain in Czechoslovakia. Lower γ-HCH residues were found in tissues of
animals (chickens, sheep, pigeons) feeding entirely on plant material, whereas carnivores had higher
concentrations.
γ-HCH that is adsorbed to sediments may be recycled to the atmosphere as gas bubbles that are formed in
the sediment by the methanogenesis and denitrification processes of bacteria. In one case studied, it was
estimated that 85% of the γ-HCH associated with the sediment gas bubbles would be released to the
atmosphere, with the remaining 15% being dissolved in the water column as the bubble rises toward the
surface (Fendinger et al. 1992).
5.4.2 Transformation and Degradation
Air. HCH is degraded in the atmosphere by reacting with photochemically produced hydroxyl radicals.
The rate of this reaction is not very rapid however, and all of the HCH isomers have rather long
atmospheric lifetimes. The rate constants for the reaction of α- and γ-HCH with hydroxyl radicals were
measured as 1.4x10
-13
and 1.9x10
-13
cm
3
/molecule-second, respectively (Brubaker and Hites 1998).
Using an average hydroxyl radical concentration of 5x10
5
molecule/cm
3
, the corresponding half-lives are
about 115 and 84 days for α- and γ-HCH, respectively. In locations where the atmospheric hydroxyl
radical concentration is very low, the persistence times of these compounds are much longer. Cortes and
Hites (2000) estimated that the average half-life of α- and γ-HCH around the Great Lakes region ranged
from about 3 to 4 years. Calculated half-lives based on artic air measurements ranged from 4.6 to
8.9 years for α-HCH, and from 4.4 to 10 years for γ-HCH; one arctic station reported a half-life
of -28 years for γ-HCH, possibly due to the shorter monitoring period and the lack of change in
concentrations over this period (Wong et al. 2021). Since HCH does not absorb light >290 nm, direct
photolysis in the atmosphere is not expected to be an important environmental fate process. However,
Chen et al. (1984) reported photodegradation half-lives of 91, 152, 104, and 154 hours for thin films of
α-HCH, β-HCH, γ-HCH, and δ-HCH, respectively, when irradiated with light of wavelength 295–
305 nm. No absorption bands were observed in this spectral region, however, for any of the HCH
isomers, and the mechanism of photodegradation and its environmental significance are uncertain. A

HEXACHLOROCYCLOHEXANE (HCH) 252
5. POTENTIAL FOR HUMAN EXPOSURE
direct photolysis study of α-HCH showed maximum absorption in the middle ultraviolet (UV) range at
252 nm, with a half-life around 2 hours (pseudo-first-order rate constant of 0.34/hour) (Zhang et al. 2014).
The environmental relevance of this is unclear, since the middle UV range wavelengths are filtered out by
the stratosphere. Similar indirect photolysis studies were conducted at ≥280 nm with α-HCH in the
presence of H
2
O
2
at a molar ratio of 100:1 (H
2
O
2
:α-HCH). The indirect photolysis half-life was around
4.3 hours (pseudo-first-order rate constant 0.16/hour). A proposed final photolysis product is
2,4,6-trichlorophenol (Zhang et al. 2014).
Water. Biodegradation is believed to be the dominant degradative process for γ-HCH in aquatic systems,
although hydrolysis and indirect photolysis may also occur. Sharom et al. (1980) found that <30% of the
applied γ-HCH remained in unsterilized natural waters in capped bottles after 16 weeks. Biodegradation
was concluded to be responsible for these results, although it was unclear to what extent hydrolysis or
adsorption to the glass bottles may have contributed. Zoeteman et al. (1980) estimated river, lake, and
groundwater half-lives for γ-HCH from degradation data in these environments to be 3–30, 30–300, and
>300 days, respectively. In natural lake water with a pH of 9.0 and a hardness of >600 mg calcium
carbonate/L, the half-life of γ-HCH was estimated to be 65 hours (Ferrando et al. 1992). γ-HCH, applied
at concentrations of 50 or 500 μg/L to aerobic batch cultures of microorganisms with sodium acetate as a
carbon source, was initially removed by adsorption and followed by desorption onto the biomass with
subsequent decomposition (McTernan and Pereira 1991). Approximately 56–62% of the γ-HCH was
removed from the water column in 23 days, with 26% removal by adsorption onto the biological solids
produced in these batch reactors. Microbial growth, using γ-HCH in the absence of sodium acetate,
increased as the microorganisms became acclimated; the pesticide still showed toxic properties, as
evidenced by a concurrent increase in microbial death rates. Evidence of biodegradation of HCH isomers
in groundwater has also been reported. In an in situ study of a former pesticide formulating plant, the
biodegradation half-lives of α-, β-, and δ-HCH isomers were determined in groundwater below the site by
compound-specific stable carbon isotope analysis respectively. Half-lives were determined based on
isotopic depletion from samples collected over 3 years at various wells spreading out from the
contaminant source. Half-lives were 223, 62–287, and 120–632 days for α-, β-, and δ-HCH isomers,
respectively (Bashir et al. 2015).
It has been shown that γ-HCH is degraded by nitrogen-fixing blue-green algae. These algae reduce the
toxic effects of γ-HCH following repeated inoculations (Kar and Singh 1979b). The degradation of
γ-HCH became more efficient with time, thus reducing the pesticide's toxicity in cultures of nitrogen-
HEXACHLOROCYCLOHEXANE (HCH) 253
5. POTENTIAL FOR HUMAN EXPOSURE
fixing blue-green algae. Dechlorination of γ-HCH to γ-pentachlorocyclohexene was also shown to occur
with fungi in aqueous suspensions (Macholz and Kujawa 1985) and in algal cultures (Sweeney 1969).
Hydrolysis is not considered an important degradation process for HCH in aquatic environments under
neutral pH conditions. However, under alkaline conditions, γ-HCH is hydrolyzed fairly rapidly. Saleh et
al. (1982) tested rates of hydrolysis of γ-HCH in sterilized natural waters at 25°C and found that
hydrolysis of γ-HCH followed first-order kinetics with half-lives of 92 hours at pH 9.3, 648 hours at
pH 7.8, and 771 hours at pH 7.3. EPA (1989b) reported a hydrolysis half-life of 207 days at pH 7 and
25°C using distilled water. Alkaline hydrolysis (pH 9.78) of α-HCH was observed with a calculated half-
life of 1,083 hours (based on pseudo-first-order rate constant of 0.0064/hour), giving
1,3,4,5,6-pentachlorocyclohexane, 1,2,4-trichlorobenzene, and 1,2,3-trichlorobenzene as the major
products (Zhang et al. 2014).
Somewhat conflicting information is available on the rate of photolysis of γ-HCH in water. Since HCH
does not contain chromophores that absorb light >290 nm, direct photolysis is not expected to occur.
However indirect photolysis, whereby a photosensitizing agent may absorb light and then transfer its
excitation energy to HCH, may occur. Humic and fulvic acids are well-known photosensitizing agents
and are practically ubiquitous in natural waters. In the study by Saleh et al. (1982), the authors reported
γ-HCH first-order photolysis half-lives of 169, 1,791, and 1,540 hours in pond water, lake water, and
water from a quarry at pH 9.3, 7.3, and 7.8, respectively, when solutions were exposed to direct sunlight.
However, the rapid rate of degradation at pH 9.3 may have been enhanced by hydrolysis reactions rather
than by photolysis. In another study, α- and γ-HCH were shown to undergo enhanced photolysis when
aqueous solutions were spiked with 5 and 25 ppm of soil fulvic acid, and irradiated with natural sunlight
(Malaiyandi et al. 1982). Hamada et al. (1981) found that γ-HCH underwent photodegradation to form
two isomers of tetrachlorohexene and pentachlorohexene in propanol solution when irradiated with UV
light produced by a low-pressure mercury lamp. Oxidants commonly found in natural waters, such as
peroxy radicals, hydroxyl radicals, and singlet oxygen species, can degrade HCH in water. Mill (1999)
estimated that the indirect photolysis half-life of HCH in natural waters is about 270 days, and the
dominant oxidant for HCH was the hydroxyl radical. Photolysis of γ-HCH in aqueous solution in the
presence of polyoxomethallate, a strong oxidizing agent, has also been demonstrated (Hiskia et al. 1997).
Sediment and Soil. γ-HCH in soil or sediment is degraded primarily by biodegradation, although
hydrolysis may occur in moist soils under alkaline conditions. Tu (1976) reported that 71 of
147 microorganisms isolated from a loamy sand soil were able to utilize a γ-HCH solution as the sole
HEXACHLOROCYCLOHEXANE (HCH) 254
5. POTENTIAL FOR HUMAN EXPOSURE
carbon source. White rot fungus degraded radiolabeled γ-HCH in aerobic pure culture laboratory tests.
In a silt loam soil/corncob test matrix, 34.7% of the compound was degraded over a 60-day test period,
whereas 53.5% degradation was observed in liquid cultures over a 30-day test period (Kennedy et al.
1990). The results of this study have been confirmed by more recent studies (Mougin et al. 1996, 1997).
The isolation of γ-HCH-degrading bacteria, classified as Sphingomonas paucimobilis, from contaminated
soils has been reported (Thomas et al. 1996). A Pseudomonas species has also been isolated from
pretreated soil that is able to degrade γ- and α-HCH, but not β-HCH, within 10–20 days under both
flooded (anaerobic) and unflooded (aerobic) conditions; greater degradation rates were observed under
aerobic conditions (Sahu et al. 1993). Under aerobic conditions, actinobacteria strains of Streptomyces
sp. isolated from a polluted site were able to utilize α-HCH as its only carbon source, and some were able
to utilize β-HCH when supplemented to the α-HCH system; no growth was observed in the presence of
δ-HCH. At pH 7 and 30°C, the actinobacteria were able to degrade up to 100% α-HCH and 55% β-HCH
after 7 days (Sineli et al. 2014). The concentrations and persistence of γ-HCH in soil may be dependent
on soil types. An analysis of two soil types, loamy sand (approximately 1–2% organic matter) and muck
(approximately 27–56% organic matter), for γ-HCH residues showed that mean residues in the loamy
sand soil had decreased from 95 ppb dry weight in 1971 to below the detection limit of 10 ppb in 1989;
however, in muck, residues had decreased from 426 ppb in 1971 to 168 ppb in 1989 (Szeto and Price
1991). The presence of crops on the soils also affects the persistence of HCH residues, with half-lives of
58.8 and 83.8 days for cropped and uncropped plots, respectively. β-HCH was the most persistent
isomer, with half-lives of 184 and 100 days, respectively, on cropped and uncropped plots; γ-HCH was
next at 107 and 62.1 days, followed by α-HCH at 54.4 and 56.1 days, and finally, δ-HCH at 33.9 and
23.4 days. Only trace amounts of the isomers were found to leach below 20 cm soil depth (Singh et al.
1991). The β-HCH isomer comprised 80–100% of the total HCH residues found in soil or vegetation on
land surrounding an industrial landfill in Germany 10 years after the final HCH input (Heinisch et al.
1993). Biodegradation was observed to be a limiting factor in uptake of γ-HCH by earthworms (Šmídová
et al. 2012).
Most available information suggests that γ-HCH transformation is favored in biologically rich, anaerobic
environments (EPA 1979; Haider 1979; Kalsch et al. 1998). In bench-scale anaerobic digestion tests
designed to assess the fate of semivolatile organic pollutants in primary and secondary sludges, γ-HCH
was found to undergo 98% degradation at 120 days. Sorption of the compound to the digester solids
accounted for 2% of the initial feed; none of the compound was lost by volatilization. The digesters were
operated at 35°C with a 30-day solids retention time (Govind et al. 1991). Similar results were seen with
live activated sludge where initially reversible biosorption dominates the removal process followed by an
HEXACHLOROCYCLOHEXANE (HCH) 255
5. POTENTIAL FOR HUMAN EXPOSURE
increased aerobic biodegradation after approximately 10 hours of acclimation. The biodegradation
process includes hydrolytic dechlorination with subsequent ring cleavage and finally, partial or total
mineralization (Tsezos and Wang 1991b). Adaptation of sewage sludge is slow and may take 1–
2 months; however, once acclimation occurs, 70–80% biodegradation of γ-HCH may occur, with the
percentage of degradation decreasing with increasing sludge age (Nyholm et al. 1992). Co-oxidation and
reductive dechlorination are the probable degradation mechanisms (Jacobsen et al. 1991; Nyholm et al.
1992).
Numerous diverse studies on biological degradation have shown that γ-HCH was transformed to
tetrachlorohexene; tri-, tetra-, and pentachlorinated benzenes; penta- and tetracyclohexanes; other isomers
of HCH; and other related chemicals. The products varied depending on the organisms present, analytical
methods applied, and when the sample was analyzed relative to its collection date (EPA 1979).
Laboratory studies have demonstrated the bioisomerization of γ-HCH to α-, β-, and δ-HCH but
bioisomerization in the environment was considered to be nonsignificant by an investigator who
conducted a field study (Waliszewski 1993). Levels of individual isomers were approximately 0.1–
1.4 and 0.8–4.0% of the γ-HCH concentrations at 3–31 and 34–46 weeks, respectively, following γ-HCH
treatment of soil. The study authors suggested that their inability to simulate all environmental conditions
in the laboratory could explain differences between laboratory and field results.
Abiotic transformation and degradation processes of γ-HCH in soil/sediment are not thought to be
significant pathways. As discussed earlier for water, photolysis or hydrolysis are not considered
important degradation pathways of γ-HCH and other isomers; the exception being hydrolysis under
alkaline conditions.
Other Media. Several Organisation for Economic Cooperation and Development (OECD) and
European Union standardized tests exist to quantify potential for biodegradation in a wastewater
treatment facility. A closed bottle test, conducted according to EC directive 92/69/EEC, was initiated
with 2 mg/L of γ-HCH in a 25 mg/L slurry of activated sludge in mineral nutrient medium, under aerobic
conditions. γ-HCH achieved 100% degradation based on theoretical oxygen uptake after 9 days (Lapertot
and Pulgarin 2006).

HEXACHLOROCYCLOHEXANE (HCH) 256
5. POTENTIAL FOR HUMAN EXPOSURE
5.5 LEVELS IN THE ENVIRONMENT
Reliable evaluation of the potential for human exposure to HCH depends, in part, on the reliability of
supporting analytical data from environmental samples and biological specimens. Concentrations of
HCH in unpolluted atmospheres and in pristine surface waters are often so low as to be near the limits of
current analytical methods. In reviewing data on HCH levels monitored or estimated in the environment,
it should also be noted that the amount of chemical identified analytically is not necessarily equivalent to
the amount that is bioavailable.
Table 5-5 shows the lowest limit of detections that are achieved by analytical analysis in environmental
media. An overview summary of the range of concentrations detected in environmental media, based on
the most recent data available, post-cancellation of γ-HCH as a pesticide, is presented in Table 5-6.
Table 5-5. Lowest Limit of Detection Based on Standards
a
Media
Detection limit
Reference
Air
0.2 pg/m
3
– 200 ng/m
3
(α-, β-, γ-HCH)
EPA 1999d
Drinking water
0.0053 μg/L (α-HCH)
0.0036 μg/L (β-HCH)
0.0060 μg/L (γ-HCH)
0.0020 μg/L (δ-HCH)
EPA 1995
Surface water and groundwater
7 pg/L – 0.0053 μg/L (α-HCH)
6 pg/L – 0.0036 μg/L (β-HCH)
9 pg/L – 0.0060 μg/L (γ-HCH)
5 pg/L – 0.0020 μg/L (δ-HCH)
EPA 1995, 2007
Soil
6 ng/L; 1.3 ng/kg (α-HCH)
7 ng/L; 0.6 ng/kg (β-HCH)
11 ng/L; 0.7 ng/kg (γ-HCH)
5 ng/L; 2.0 ng/kg (δ-HCH)
EPA 2000b, 2007
Sediment
0.500 μg/kg; 6 ng/L (α-HCH)
0.221; 7 ng/L (β-HCH)
0.200 μg/kg; 11 ng/L (γ-HCH)
5 ng/L (δ-HCH)
EPA 2000b; USGS 2003
Whole blood
1 ppb (α-, β-, γ-HCH)
1.3 ng/g lipid (β-HCH)
0.92 ng/g lipid (γ-HCH)
CDC 2019; EPA 1980
a
Detection limits based on using appropriate preparation and analytics. These limits may not be possible in all
situations.

HEXACHLOROCYCLOHEXANE (HCH) 257
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-6. Summary of Ambient Environmental Levels of HCH
Media
Low
High
Reference
α-HCH
Outdoor air (ng/m
3
)
0.00016
0.017
WQP 2023
Indoor air
No data
Surface water (ng/L)
0.1
1.7
WQP 2023
Groundwater
Not detected
WQP 2023
Drinking water
No data
Food
Not detected
FDA 2020b
Soil and sediment
Not detected
WQP 2023
β-HCH
Outdoor air (ng/m
3
)
0.00034
0.064
WQP 2023
Indoor air
No data
Surface water (ng/L)
0.11
1.2
WQP 2023
Groundwater
Not detected
WQP 2023
Drinking water
No data
Food
Not detected
FDA 2020b
Soil and sediment (μg/kg)
0.032
0.39
WQP 2023
γ-HCH
Outdoor air (ng/m
3
)
<1.7
No data
EPA 2021
Indoor air
No data
Surface water
No data
Groundwater
No data
Drinking water
No data
Food
Not detected
FDA 2020b
Soil and sediment
No data
δ-HCH
Outdoor air
Not detected
WQP 2023
Indoor air
No data
Surface water (ng/L)
0.44
3.7
WQP 2023
Groundwater
Not detected
WQP 2023
Drinking water
No data
Food
Not detected
FDA 2020b
Soil and sediment
Not detected
WQP 2023
HCH, technical grade
Outdoor air
No data
Indoor air
No data
Surface water
No data
Groundwater
No data
Drinking water
No data

HEXACHLOROCYCLOHEXANE (HCH) 258
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-6. Summary of Ambient Environmental Levels of HCH
Media
Low
High
Reference
Food
No data
Soil and sediment (μg/kg)
0.47
1.26
WQP 2023
Detections of HCH in air, water, and soil at NPL sites are summarized in Table 5-7.
Table 5-7. Hexachlorocyclohexanes (HCHs); Levels in Water, Soil, and Air of
National Priorities List (NPL) Sites
Medium
Median
a
Geometric
mean
a
Geometric
standard
deviation
a
Number of
quantitative
measurements
NPL sites
α-HCH
Water (ppb)
0.987
1.08
45.2
43
29
Soil (ppb)
3,390
2,970
156
52
28
Air (ppbv)
0.0016
0.0010
11
6
5
β-HCH
Water (ppb)
0.68
1.09
18.7
34
22
Soil (ppb)
950
911
61.7
44
33
Air (ppbv)
0.0017
0.00080
7.1
2
2
γ-HCH
Water (ppb)
0.515
0.819
49.2
64
38
Soil (ppb)
2,800
2,090
127
66
42
Air (ppbv)
0.0044
0.0038
29
7
7
δ-HCH
Water (ppb)
0.57
1.18
34.5
29
19
Soil (ppb)
1,100
392
71.9
31
22
Air (ppbv)
0.0002
0.00016
1.5
3
2
HCH, technical grade
Water (ppb)
0.86
3.0
24
6
5
Soil (ppb)
8,700
2,300
81
10
6
Air (ppbv)
0.000017
0.000017
1
2
1
a
Concentrations found in ATSDR site documents from 1981 to 2022 for 1,868 NPL sites (ATSDR 2022). Maximum
concentrations were abstracted for types of environmental media for which exposure is likely. Pathways do not
necessarily involve exposure or levels of concern.
HEXACHLOROCYCLOHEXANE (HCH) 259
5. POTENTIAL FOR HUMAN EXPOSURE
5.5.1 Air
HCH isomers have been detected in ambient air. The highest concentrations were found prior to γ-HCH
agricultural use restrictions; available monitoring data after the restriction and ban of γ-HCH pesticides
showed a gradual decrease and the results of the most recent monitoring studies are below the parts per
billion range. The results of outdoor air monitoring studies are presented in Table 5-8. Precipitation
samples, if available, are included in Table 5-8 because they reflect removal of atmospheric HCH.
One reference to indoor air monitoring was located in the literature search. In a study of preschool
children’s potential exposure to pesticides in North Carolina, indoor air samples from 13 daycare centers
and 129 homes of the preschool children (ages 20–66 months) were collected between 2000 and 2001
(Morgan et al. 2014). γ-HCH was detected in ranges of below the limit of detection (<0.09 ng/m
3
) to
18.5 ng/m
3
in the children’s homes and <0.09–8.97 ng/m
3
in the preschools. Detection frequencies were
13 and 20%, respectively. Seventy-four percent of the homeowners reported applying insecticides at their
homes, and 90% of these had applied an insecticide in the past year before sampling. Among the daycare
centers, 62% reported using insecticides, and 88% of those reported usage within a year of sample
collection (Morgan et al. 2014).
5.5.2 Water
Water monitoring data are presented in Table 5-9. HCH isomers have been detected in surface water,
groundwater, and drinking water. The highest concentrations were found in groundwater below a facility
that processed pesticides and stored wastes in unlined trenches until 1996 (Law et al. 2004). A study of
the same site some years later still detected HCH isomers (Chartrand et al. 2015). Generally, surface
water concentrations are lower than those detected in groundwater. Data from the EPA’s Water Quality
Portal (WQP), a system that maintains water monitoring data from stations across the United States, have
been divided into two categories: prior to γ-HCH pesticide cancellation (years up to and including 2006)
and recent years post-cancellation (WQP 2023). Although recent data are limited, a decrease in surface
and groundwater concentrations of α-, β-, and δ-HCH can be seen in this dataset; data for γ-HCH were not
reported. From other studies, a decrease of γ-HCH in surface water can be observed, possibly due to use
limitations; trends for drinking water and groundwater are not as clear. Most recent monitoring data
report concentrations below the parts per billion range for surface water and groundwater.

HEXACHLOROCYCLOHEXANE (HCH) 260
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-8. Outdoor Air Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
α-HCH
Alabama
Not reported
January–October 1996
and May 1997
0.092 ng/m
3
Jantunen et
al. 2000
Eagle Harbor,
Michigan; Sleeping
Bear Dunes State
Park, Michigan;
Sturgeon Point,
New York
Rural
1990–1997
0.110–
0.140 ng/m
3
Cortes and
Hites 2000
Chesapeake Bay
Rural and
agricultural
April 2000–September
2003; excluding winter
months
0.002–
0.142 ng/m
3
0.026 ng/m
3
Gas phase; average of
averages at three sites;
detection frequency 99–100%
Goel et al.
2010
Chesapeake Bay
Rural and
agricultural
April 2000 –September
2003; excluding winter
months
0.0012 ng/m
3
Particulate phase; detection
frequency 1%
Goel et al.
2010
Chesapeake Bay
Rural and
agricultural
April 2000–September
2003; excluding winter
months
0.2–11 ng/L
1.7 ng/L
Rainwater; average of
averages at three sites;
detection frequency 3–18%
Goel et al.
2010
Youngstown, Ohio
Urban/suburban
2000–2001
0.051 ng/m
3
Shen et al.
2004
Solomons, Maryland
Rural
2000–2001
0.091 ng/m
3
Shen et al.
2004
Wilmington, North
Carolina
Urban/suburban
2000–2001
0.015 ng/m
3
Shen et al.
2004
Turkey Point,
Florida
Rural
2000–2001
0.029 ng/m
3
Shen et al.
2004
Muscle Shoals
Suburban/rural
2000–2001
0.056 ng/m
3
Shen et al.
2004
United States
Ambient air
2015–2019
0.00016–
0.017 ng/m
3
0.0042 ng/m
3
Detected in 83% of
481 samples; no data reported
for 2020–2023
b
WQP 2023
c

HEXACHLOROCYCLOHEXANE (HCH) 261
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-8. Outdoor Air Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
β-HCH
United States
Ambient air
2015–2018
0.00034–
0.064 ng/m
3
0.005 ng/m
3
Detected in 28% of
481 samples; no data reported
for 2020–2023
b
WQP 2023
c
γ-HCH
College Station,
Texas
Rural
1979–1980
0.01–
1.60 ng/m
3
0.23 ng/m
3
Ground level, ambient air
Atlas and
Giam 1988
College Station,
Texas
Rural
1979–1980
0.30–
7.8 ng/L
2.81 ng/L
Rainwater samples
Atlas and
Giam 1988
Adirondack
Mountains, New
York
Not reported
1985
0.509 ng/m
3
Troposphere samples
Knap and
Binkley 1991
Newport News,
Virginia
Not reported
1988
0.021 ng/m
3
Troposphere samples
Knap and
Binkley 1991
Alabama
Not reported
January–October 1996
and May 1997
0.050 ng/m
3
Jantunen et
al. 2000
Eagle Harbor,
Michigan; Sleeping
Bear Dunes State
Park, Michigan;
Sturgeon Point,
New York
Rural
1990–1997
0.024–
0.062 ng/m
3
Cortes and
Hites 2000
Lake Superior
Not reported
1984; wetfall season
3.0 ng/L
Rainwater samples, annual
loading rate of 2.0 μg/m
2
/year
Strachan
1988
Portland, Oregon
Urban
1982
0.45–1 ng/L
Rain and snow water
Pankow et al.
1984
Hawaii
Not reported
1970–1971
1–19 ng/L
5 ng/L
Rainwater
Bevenue et
al. 1972
Youngstown, Ohio
Urban/suburban
2000–2001
0.049 ng/m
3
Shen et al.
2004
Solomons, Maryland
Rural
2000–2001
0.072 ng/m
3
Shen et al.
2004

HEXACHLOROCYCLOHEXANE (HCH) 262
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-8. Outdoor Air Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
Wilmington, North
Carolina
Urban/suburban
2000–2001
0.026 ng/m
3
Shen et al.
2004
Turkey Point,
Florida
Rural
2000–2001
0.031 ng/m
3
Shen et al.
2004
Muscle Shoals
Suburban/
agricultural
2000–2001
0.055 ng/m
3
Shen et al.
2004
North Carolina
Not reported
2000–2001
<0.09–
0.11 ng/m
3
Air samples collected outside
daycare centers; detection
frequency 8%
Morgan et al.
2014
North Carolina
Not reported
2000–2001
<0.09–
6.15 ng/m
3
Air samples collected outside
students; homes; detection
frequency 12%
Morgan et al.
2014
Chesapeake Bay
Rural and
agricultural
April 2000–September
2003; excluding winter
months
0.0012–
0.382 ng/m
3
0.049 ng/m
3
Gas phase; average of
averages at three sites;
detection frequency 81–100%
Goel et al.
2010
Chesapeake Bay
Rural and
agricultural
April 2000–September
2003; excluding winter
months
0.0013–
0.027 ng/m
3
0.024 ng/m
3
Particulate phase; average of
averages at two sites; detection
frequency 2–7%
Goel et al.
2010
Chesapeake Bay
Rural and
agricultural
April 2000–September
2003; excluding winter
months
0–35 ng/L
4.0 ng/L
Rainwater; average of
averages at two sites; detection
frequency 1–61%
Goel et al.
2010
Texas
Various ambient air
monitoring sites
January–December
2007
0.005 ng/m
3
Detected in 20 samples; below
the limit of detection in
345 samples
EPA 2021
Texas
Various ambient air
monitoring sites
January–December
2008
<1.7 ng/m
3
Below the limit of detection in
488 samples
EPA 2021
Texas
Various ambient air
monitoring sites
January–June 2009
<1.7 ng/m
3
Below the limit of detection in
120 samples
EPA 2021

HEXACHLOROCYCLOHEXANE (HCH) 263
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-8. Outdoor Air Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
δ-HCH
United States
Ambient air
2015–2019
ND
Not detected in 26 samples; no
data reported for 2020–2023
b
WQP 2023
c
a
Liquid unit conversion: 1 ng/L = 1 ppt = 0.001 ppb; gaseous unit conversion: ppbv = ([concentration ng/m
3
] x 0.001) / 11.89, assuming standard temperature and
pressure.
b
As of June 2023.
c
Data collected by USGS monitoring stations across the United States; mean and ranges do not reflect samples reported as not detected/below detection limit.
ND = not detected; USGS = U.S. Geological Survey

HEXACHLOROCYCLOHEXANE (HCH) 264
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
α-HCH
Lake
Superior
Surface water
Spring 1997
2.8 ng/L
Marvin et al.
2004
Lake Erie
Surface water
Spring 1998
0.41 ng/L
Marvin et al.
2004
Lake Ontario
Surface water
Spring 1998
0.40 ng/L
Marvin et al.
2004
York River
estuary
Surface water
June 1998–
April 1999
~0.025–
0.175 ng/L
Concentrations were lower in freshwater
areas than areas with higher salinity
Padma and
Dickhut 2002
United States
Surface water
1978–2006
2.93x10
-5
–
55,000 ng/L
150 ng/L
Detected in 0.5% of 35,766 samples
WQP 2023
b
United States
Surface water
2020
ND
Not detected in 775 samples
WQP 2023
b
United States
Surface water
2021
0.11–
0.976 ng/L
0.54 ng/L
Detected in 0.2% of 902 samples
WQP 2023
b
United States
Surface water
2022
0.1–1.7 ng/L
0.28 ng/L
Detected in 2% of 945 samples
WQP 2023
b
United States
Surface water
2023
d
ND
Not detected in 9 samples
WQP 2023
b
United States
Surface water at
Portland Harbor
Superfund Site
2003–2006
4.2x10
-5
–
0.002 ng/L
0.026
Detected in 45% of 193 samples
WQP 2023
b
United States
Groundwater
1981–2006
1.1–5,000
ng/L
153 ng/L
Detected in 0.3% of 16,493 samples
WQP 2023
b
United States
Groundwater
2020, 2022
ND
Not detected in 125 samples; no data
reported for 2021 or 2023
c
WQP 2023
b
United States
Groundwater at
EPA Region 10
Superfund Sites
1987–2002
ND
Not detected in 396 samples
WQP 2023
b
United States
Groundwater at
Boomsnub
Superfund Site
1995
ND
Not detected in 18 samples
WQP 2023
b

HEXACHLOROCYCLOHEXANE (HCH) 265
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
58–
5.0x10
5
ng/L
51,000 ng/L
Samples collected from 19 shallow wells;
not detected (<20 ng/L) in 6 wells; mean
and ranges do not reflect samples reported
as not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
30–
4.2x10
5
ng/L
44,000 ng/L
Samples collected from 15 deep wells; not
detected (<20 ng/L) in 5 wells; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Surface water near
active pesticide
reformulating and
packaging facility
2000
660–680 ng/L
670 ng/L
Three samples collected from creek
adjacent to the site; not detected
(<20 ng/L) in one sample; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
β-HCH
United States
Surface water
1980–2006
0.0385–
80,000 ng/L
1,260 ng/L
Detected in 1% of 7,198 samples
WQP 2023
b
United States
Surface water
2020
ND
Not detected in 248 samples
WQP 2023
b
United States
Surface water
2021
0.18–0.84 ng/L
0.49 ng/L
Detected in 4% of 221 samples
WQP 2023
b
United States
Surface water
2022
0.11–1.2 ng/L
0.42 ng/L
Detected in 8% of 454 samples
WQP 2023
b
United States
Surface water
2023
d
ND
Not detected in 5 samples
WQP 2023
b
United States
Surface water
Portland Harbor
Superfund Site
2004–2006
0.00017–
0.0337
0.0060
Detected in 25% of 190 samples
WQP 2023
b
United States
Groundwater
1981–2006
10–300
ng/L
48 ng/L
Detected in 1% of 2,478 samples
WQP 2023
b
United States
Groundwater
2020
ND
Not detected in 3 samples; no data
reported for 2021–2023
c
WQP 2023
b
United States
Groundwater at
EPA Region 10
Superfund Sites
1987–2002
ND
Not detected in 395 samples
WQP 2023
b
United States
Groundwater at
Boomsnub
Superfund Site
1995
ND
Not detected in 18 samples
WQP 2023
b

HEXACHLOROCYCLOHEXANE (HCH) 266
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
30–43,000 ng/L
12,000 ng/L
Samples collected from 19 shallow wells;
not detected (<20 ng/L) in 8 wells; mean
and ranges do not reflect samples reported
as not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
82–820 ng/L
340 ng/L
Samples collected from 15 deep wells; not
detected (<20 ng/L) in 8 wells; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Surface water near
active pesticide
reformulating and
packaging facility
2000
38–440 ng/L
300 ng/L
Three samples collected from creek
adjacent to the site
Law et al. 2004
γ-HCH
New Jersey
Wells
Not reported
(1981 or
earlier)
NR–900 ng/L
1,076 wells, not detected in around half of
samples
Page 1981
Chesterfield
County,
South
Carolina
Rural drinking
water
Not reported
(1978 or
earlier)
0–93 ng/L
23 ng/L
Sandhu et al.
1978
Hampton,
South
Carolina
Rural drinking
water
Not reported
(1978 or
earlier)
0–319 ng/L
147 ng/L
Sandhu et al.
1978
Cincinnati,
Ohio
Drinking water
Not reported
(1976 or
earlier)
0.01 ng/L
Keith et al. 1976
Oahu, Hawaii
Drinking water
1970–1971
0.2 ng/L
Bevenue et al.
1972

HEXACHLOROCYCLOHEXANE (HCH) 267
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
Ozark
Plateaus
Province of
Arkansas,
Kansas,
Missouri, and
Oklahoma
Groundwater
April–
September
1993
28 and 32 ng/L
Detected in two samples from domestic
wells
Adamski and
Pugh 1996
Connecticut
Drinking water well
Not reported
(1999 or
earlier)
60 ng/L
Eitzer and
Chevalier 1999
United States
Drinking water
1998–2005
1–690 ng/L
62 ng/L
Samples collected from 44 states across
the United States; ranges and averages do
not include samples reported to be below
the method reporting level
EPA 2010
Washington,
D.C. and
Denver,
Colorado
Surface water
Not reported
(1984 or
earlier)
52–100 ng/L
Cole et al. 1984
Niagara
River
Surface water
1980–1981
2.1 ng/L
Mean of 99% samples
Kuntz and Warry
1983
Lake
Michigan
tributary
streams
Surface water
Not reported
(1974 or
earlier)
ND–150 ng/L
EPA 1974
United States
Surface water
Not reported
(1985 or
earlier)
Median: 20 ng/L
Detected in 27% of 4,505 samples
Staples et al.
1985
Lake Ontario
Surface water
1983
0.806–1.85
ng/L
Biberhofer and
Stevens 1987
Patuxent
River
Surface water
1995
1.0 ng/L
Harman-Fetcho
et al. 1999
Lake
Superior
Surface water
Spring 1997
0.38 ng/L
Marvin et al.
2004

HEXACHLOROCYCLOHEXANE (HCH) 268
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
Lake Erie
Surface water
Spring 1998
0.32 ng/L
Marvin et al.
2004
Lake Ontario
Surface water
Spring 1998
0.24 ng/L
Marvin et al.
2004
York River
estuary
Surface water
June 1998–
April 1999
~0.04–
0.21 ng/L
Concentrations were higher in freshwater
areas than areas with higher salinity
Padma and
Dickhut 2002
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
110–
6.6x10
5
ng/L
99,000 ng/L
Samples collected from 19 shallow wells;
not detected (<20 ng/L) in 11 wells; mean
and ranges do not reflect samples reported
as not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
120–
3.6x10
5
ng/L
73,000 ng/L
Samples collected from 15 deep wells; not
detected (<20 ng/L) in 10 wells; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Surface water near
active pesticide
reformulating and
packaging facility
2000
440–470 ng/L
460 ng/L
Three samples collected from creek
adjacent to the site; not detected
(<20 ng/L) in one sample; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
δ-HCH
United States
Surface water
1980–2006
0.00339–
75,000 ng/L
1.56 ng/L
Detected in 0.8% of 7,106 samples
WQP 2023
b
United States
Surface water
2020
ND
Not detected in 248 samples
WQP 2023
b
United States
Surface water
2021
0.48–1 ng/L
0.72 ng/L
Detected in 3% of 221 samples
WQP 2023
b
United States
Surface water
2022
0.44–3.7 ng/L
0.97 ng/L
Detected in 5% of 545 samples
WQP 2023
b
United States
Surface water
2023
d
ND
Not detected in 5 samples
WQP 2023
b
United States
Surface water
Portland Harbor
Superfund Site
2004–2006
0.000508–1.67
0.14
Detected in 14% of 190 samples
WQP 2023
b
United States
Ground water
1980–2006
20–300 ng/L
4.8 ng/L
Detected in 1% of 2,417 samples
WQP 2023
b
United States
Ground water
2020
ND
Not detected in three samples; no data
reported for 2021–2023
c
WQP 2023
b

HEXACHLOROCYCLOHEXANE (HCH) 269
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Water Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic type
Date(s)
Range
a
Mean
a
Notes
Reference
United States
Groundwater at
EPA Region
10 Superfund Sites
1987–2002
ND
Not detected in 391 samples
WQP 2023
b
United States
Groundwater at
Boomsnub
Superfund Site
1995
ND
Not detected in 18 samples
WQP 2023
b
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
40–
5.7x10
5
ng/L
74,000 ng/L
Samples collected from 19 shallow wells;
not detected (<20 ng/L) in 8 wells; mean
and ranges do not reflect samples reported
as not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Groundwater below
active pesticide
reformulating and
packaging facility
2000
36–
2.9x10
5
ng/L
26,000 ng/L
Samples collected from 15 deep wells; not
detected (<20 ng/L) in 3 wells; mean and
ranges do not reflect samples reported as
not detected/below detection limit
Law et al. 2004
Northeastern
Florida
Surface water near
active pesticide
reformulating and
packaging facility
2000
55–970 ng/L
640 ng/L
Three samples collected from creek
adjacent to the site
Law et al. 2004
HCH, mixture
Northeastern
Florida
Groundwater below
contaminated site
Not reported
(2015 or
earlier)
30,000–
4.2x10
5
ng/L
Range reported for α-, γ-, and δ-HCH
Chartrand et al.
2015
a
Liquid unit conversion: 1 ng/L = 1 ppt = 0.001 ppb.
b
Data collected by USGS monitoring stations across the United States; mean and ranges do not reflect samples reported as not detected/below detection limit.
c
As of June 2023.
ND = not detected; USGS = U.S. Geological Survey
HEXACHLOROCYCLOHEXANE (HCH) 270
5. POTENTIAL FOR HUMAN EXPOSURE
5.5.3 Sediment and Soil
HCH has been detected in soil and sediment as a result of agricultural use. Soil and sediment monitoring
data are presented in Table 5-10. Data from the EPA’s WQP has been divided into two categories to
reflect potential detection decreases as a result of the γ-HCH pesticide cancellation in 2006 (WQP 2021).
A clear trend could not be discerned from the data, possibly due to the large ranges reflecting differences
in land use. Most recent monitoring data report concentrations at the parts per billion range.
5.5.4 Other Media
HCH isomers have been detected in aquatic organisms; the results of these monitoring studies are
summarized in Table 5-11. Data on terrestrial organism monitoring were not located. Schmitt et al.
(1985) reported the results of a monitoring study of fish tissues from 107 freshwater stations in the United
States from 1976 to 1981, which supported a decline in tissue occurrence of detectable α- and γ-HCH
residues in aquatic organisms. α- and β-HCH have been detected in organisms as recently as 2018,
however (WQP 2021). The most recent monitoring studies have detected HCH isomers in aquatic
organisms in the parts per billion range.
Historically, as a result of pesticide use, γ-HCH was detected in meat, vegetables, and other food items,
both imported to and produced in the United States. Due to the discontinued agricultural use of γ-HCH
by the United States and many other countries, residues are typically no longer detected in food products.
γ-HCH was detected in 5 out of 612 imported rice samples at a maximum concentration of 0.03 ppm
during an FDA pesticide monitoring study conducted in 1993–1994 (Roy et al. 1997). A 10-year (1982–
1991) FDA study of ready-to-eat foods commonly consumed in the United States showed that α-, β-, δ-,
and γ-HCH were frequently detected (Rogers et al. 1995). The results of this study reported average
concentrations of 0.0010, 0.0027, 0.0030, and 0.0012 ppm for α-, β-, δ-, and γ-HCH isomers,
respectively, in 243 ready-to-eat foods. HCH isomers were also detected in the following feed types
formulated for infants and toddlers and in adult diet foodstuffs: whole milk and other dairy products;
meat, fish, and poultry; oils and fats; vegetables; and sugars and adjuncts (Gartrell et al. 1986a, 1986b).
γ-HCH residues were detected in fat samples of domestic farm animals collected in Ontario, Canada, in
1986–1988. Mean concentrations in fat from chickens, turkeys, beef, lamb, and pork ranged from
0.012 to 0.032 ppm; the mean concentration in hen eggs was 0.008 ppm (Frank et al. 1990). A pesticide

HEXACHLOROCYCLOHEXANE (HCH) 271
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-10. Soil and Sediment Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic
type
Date(s)
Range
a
Mean
a
Notes
Reference
α-HCH
Alabama
Soil
Not
reported
(1999 or
earlier)
0–
0.269 µg/kg
Detected in 26 of 39 soils from
6 regions
Harner et al. 1999
Sequoia National Park, Rocky
Mountain National Park, Mt.
Rainier National Park, Denali
National Park, Noatak
National Preserve, and Gates
of the Arctic National Park
and Preserve
Sediment core
from deepest
point in several
lakes
2003–
2005
<0.8 µg/kg
Not detected
Genualdi et al. 2011
United States
Soil and
sediment
1978–2006
4.0x10
-5
–
879 µg/kg
3.0 µg/kg
Detected in 8% of 9,360 samples
WQP 2023
b
United States
Soil and
sediment
2020–2021
ND
Not detected in 178 samples; no
data reported for 2022–2023
c
WQP 2023
b
United States
Soil and
sediment at
Lower
Duwamish
Waterway
Superfund Site
1991–2006
0.14–
1.8 µg/kg
0.59 µg/kg
Detected in 2% of 335 samples
WQP 2023
b
United States
Soil/sediment
at Portland
Harbor
Superfund Site
1997–2006
0.00238–
120 µg/kg
1.6 µg/kg
Detected in 15% of 1,772 samples
WQP 2023
b
United States
Soil at Delta
400 West
Plume
2004
ND
Not detected in 7 samples
WQP 2023
b

HEXACHLOROCYCLOHEXANE (HCH) 272
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-10. Soil and Sediment Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic
type
Date(s)
Range
a
Mean
a
Notes
Reference
β-HCH
United States
Soil and
sediment
1983–2006
4.0x10
-5
–
4,230 µg/kg
18 µg/kg
Detected in 8% of 8,106 samples
WQP 2023
b
United States
Soil and
sediment
2020
0.032–
0.39 µg/kg
0.17 µg/kg
Detected in 4% of 142 samples
WQP 2023
b
United States
Soil and
sediment
2021
ND
Not detected in 8 samples; no
data reported for 2022–2023
c
WQP 2023
b
United States
Soil and
sediment at
Lower
Duwamish
Waterway
Superfund Site
1991–2006
0.087–
13 µg/kg
4.1 µg/kg
Detected in 1% of 327 samples
WQP 2023
b
United States
Soil/sediment
at Portland
Harbor
Superfund Site
1997–2006
0.00138–
318 µg/kg
3.9 µg/kg
Detected in 42% of 1,819 samples
WQP 2023
b
United States
Soil at Delta
400 West
Plume
2004
ND
Not detected in 7 samples
WQP 2023
b
γ-HCH
Alabama, Arkansas, Georgia,
Illinois, Iowa
Soil
Not
reported
(1974 or
earlier)
10–
150 µg/kg
52 µg/kg
Crockett et al. 1974
Alabama
Soil
Not
reported
(1999 or
earlier)
0–1.07 µg/kg
Detected in 26 of 39 soils from
6 regions
Harner et al. 1999
HEXACHLOROCYCLOHEXANE (HCH) 273
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-10. Soil and Sediment Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic
type
Date(s)
Range
a
Mean
a
Notes
Reference
Niagara River
Suspended
sediment
Not
reported
(1983 or
earlier)
2 µg/kg
Detection frequency 33%
Kuntz and Warry 1983
Lake Ontario
Settling
particulates
1982
2.4 µg/kg
Oliver and Charlton
1984
James River, Virginia
Creek
sediments
1976
7.3–
8.5 µg/kg
Saleh et al. 1978
Gulf of Mexico
Sediment
1987
<0.02–
1.74 µg/kg
0.07 µg/kg
Detection frequency 19%
Sericano et al. 1990
Around the Great Lakes
Sediment
May 1989
<0.10–
0.99 µg/kg
wet weight
Verbrugge et al. 1991
Indian River Lagoon, Florida
Sediment from
impoundments
along the river
Not
reported
(1992 or
earlier)
9.4–
34.4 µg/kg
33 sediment samples from
11 impoundment
Wang et al. 1992
δ-HCH
United States
Soil and
sediment
1983–2006
0.0078–
89.8 µg/kg
3.4 µg/kg
Detected in 6% of 5,747 samples
WQP 2023
b
United States
Soil and
sediment
2020–2021
ND
Not detected in 162 samples; no
data reported for 2022–2023
c
WQP 2023
b
United States
Soil and
sediment at
Lower
Duwamish
Waterway
Superfund Site
1991–2006
0.081–
1,100 µg/kg
160 µg/kg
Detected in 3% of 254 samples
WQP 2023
b
United States
Soil/sediment
at Portland
Harbor
Superfund Site
1997–2006
0.00216–
45.4 µg/kg
1.3 µg/kg
Detected in 9% of 1,797 samples
WQP 2023
b

HEXACHLOROCYCLOHEXANE (HCH) 274
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-10. Soil and Sediment Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Geographic
type
Date(s)
Range
a
Mean
a
Notes
Reference
United States
Soil at Delta
400 West
Plume
2004
ND
Not detected in 7 samples
WQP 2023
b
HCH, mixture
South Carolina
0–10 cm
surface soils
from cotton
fields
November
1999
0.1–0.54
µg/kg dry
weight
0.27 µg/kg
dry weight
Reported as sum of α-, β-, and
γ-isomers; not detected
(<0.1 µg/kg dry weight) in 10 of
16 samples; mean and ranges do
not reflect samples reported as not
detected/below detection limit
Kannan et al. 2003
Georgia
0–10 cm
surface soils
from cotton
fields
December
1999
0.16–
0.49 µg/kg
dry weight
0.33 µg/kg
dry weight
Reported as sum of α-, β-, and
γ-isomers; not detected
(<0.1 µg/kg dry weight) in 14 of
16 samples; mean and ranges do
not reflect samples reported as not
detected/below detection limit
Kannan et al. 2003
United States
Sediment
2017
0.47–
1.26 µg/kg
0.75 µg/kg
HCH isomer or mixture not
specified; detected in 19% of
42 samples
WQP 2023
b
a
Solid unit conversion: 1 µg/kg = 1 ppb.
b
Data collected by USGS monitoring stations across the United States; mean and ranges do not reflect samples reported as not detected/below detection limit.
c
As of July 2023.
ND = not detected; USGS = U.S. Geological Survey

HEXACHLOROCYCLOHEXANE (HCH) 275
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
α-HCH
Southwestern and
Midwestern United
States
Freshwater Fish
1980–
1981
0.03–
0.04 ng/g
Highest concentrations detected
from 107 monitoring stations
across the United States
Schmitt et al.
1985
United States
Freshwater fish
1984
NR–
10 ng/g
<10 ng/g
Schmitt et al.
1990
Louisiana section of
the Mississippi River
Blue crab, cobia, flathead
catfish, freshwater drum, long-
nose gar, red drum, red snapper,
river shrimp, small-mouth
buffalo, spotted gar
1990–
1994
ND
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Bigmouth buffalo
1990–
1994
2.4 ng/g
Average detection in 1 of
3 sampling years; not detected in
other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Blue catfish
1990–
1994
0.333–
26.3 ng/g
Range of average detections in
3 of 4 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Carp
1990–
1994
31.1 ng/g
Range of average detections in
3 of 4 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Channel catfish
1990–
1994
1.83–
7.23 ng/g
Range of average detections in
2 of 3 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Crawfish
1990–
1994
4.25 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Largemouth bass
1990–
1994
1.00 ng/g
Average detection in 1 of
2 sampling years; not detected in
other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Striped bass
1990–
1994
2.88 ng/g
Average detection in 1 of
2 sampling years; not detected in
other years
Watanabe et
al. 2003

HEXACHLOROCYCLOHEXANE (HCH) 276
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
Louisiana section of
the Mississippi River
White bass
1990–
1994
1.44 ng/g
Average detection in 1 of
3 sampling years; not detected in
other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
White crappie
1990–
1994
1.75 ng/g
Average detection in 1 of
3 sampling years; not detected in
other years
Watanabe et
al. 2003
Southwestern
Michigan
Adult green frogs
1998
0.02 ng/g
0.04 ppb detected in juvenile
frogs
Gilliland et al.
2001
Gulf of California
Clams (Chione californiensis)
Not
reported
(2015 or
earlier)
<0.005–
1.77 ng/g
wet weight
Detection frequency 16.7% in 1 of
3 study areas, not detected in
other areas; adipose tissue
samples; surrounding area has
primarily agricultural activity
Vargas-
Gonzalez et
al. 2016
Lake Apopka, Florida
Largemouth bass (Micropterus
salmoides)
March
2013
ND
Limit of quantification 0.1–
0.5 ng/g wet weight
Dang et al.
2016
United States
Freshwater fish
2020–
2022
ND
Not detected in 253 samples; no
data reported for 2023
b
WQP 2023
c
β-HCH
Upper Steele Bayou,
Mississippi
Fish
1988
ND–
20 ng/g
wet weight
Ford and Hill
1991
Upper Steele Bayou,
Mississippi
Snakes
1988
ND
Ford and Hill
1991
Southwestern
Michigan
Adult green frogs
1998
0.01 ng/g
Not detected in juvenile frogs
Gilliland et al.
2001
Louisiana section of
the Mississippi River
Cobia, long-nose gar, red drum,
red snapper
1990–
1994
ND
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Bigmouth buffalo
1990–
1994
2.25–
11.2 ng/g
Range of average detection in
3 of 3 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Blue catfish
1990–
1994
3.67–
8.33 ng/g
Range of average detection in
3 of 4 sampling years; not
detected in other year
Watanabe et
al. 2003

HEXACHLOROCYCLOHEXANE (HCH) 277
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
Louisiana section of
the Mississippi River
Blue crab
1990–
1994
4.00–
11.0 ng/g
Range of average detections in
2 of 2 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Carp
1990–
1994
5.00–
11.3 ng/g
Range of average detections in
2 of 3 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Channel catfish
1990–
1994
0.333–
7.77 ng/g
Range of average detections in
3 of 4 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Crawfish
1990–
1994
27.5 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Flathead catfish
1990–
1994
0.500 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Freshwater drum
1990–
1994
0.333 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Largemouth bass
1990–
1994
2.00 ng/g
Average detection in 1 of
2 sampling years; not detected in
other year
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
River shrimp
1990–
1994
8.67 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Small-mouth buffalo
1990–
1994
0.25–
2.00 ng/g
Range of average detection in
3 of 4 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Spotted gar
1990–
1994
39.0 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Striped bass
1990–
1994
5.50–
57.9 ng/g
Range of average detection in
2 of 2 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
White bass
1990–
1994
3.11 ng/g
Average detection in 1 of
3 sampling years; not detected in
other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
White crappie
1990–
1994
1.00–
11.0 ng/g
Range of average detection in
2 of 3 sampling years; not
detected in other years
Watanabe et
al. 2003

HEXACHLOROCYCLOHEXANE (HCH) 278
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
Gulf of California
Clams (C. californiensis)
Not
reported
(2015 or
earlier)
ND
Limit of detection 0.01 ng/g wet
weight
Adipose tissue samples;
surrounding area has primarily
agricultural activity
Vargas-
Gonzalez et
al. 2016
Lake Apopka, Florida
Largemouth bass (M. salmoides)
March
2013
ND
Limit of quantification 0.1–
0.5 ng/g wet weight
Dang et al.
2016
United States
Freshwater fish
2020
ND
Not detected in 172 samples
WQP 2023
c
United States
Freshwater fish; Oncorhynchus
kisutch
2022
1.7–
1.8 ng/g
1.9 ng/g
Detected in 11% of 19 samples;
detected only in Oncorhynchus
kisutch (33% of 6 samples); no
data reported for 2021 or 2023
b
WQP 2023
c
γ-HCH
Gulf of Mexico
Oyster
1987
<0.25–
9.06 ng/g
1.74 ng/g
Detection frequency 80%
Sericano et al.
1990
United States
Freshwater fish
1980–
1981
0.02–
0.03 ng/g
Whole body concentrations were
>0.01 ng/g at 1 of 107 monitoring
stations
Schmitt et al.
1985
United States
Freshwater fish
1984
NR–
40 ng/g
<10 ng/g
Schmitt et al.
1990
Southwestern
Michigan
Adult green frogs
1998
0.07 ng/g
Not detected in juvenile frogs
Gilliland et al.
2001
Louisiana section of
the Mississippi River
Blue crab, channel catfish,
cobia, crawfish, flathead catfish,
freshwater drum, long-nose gar,
red drum
1990–
1994
Not detected
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Bigmouth buffalo
1990–
1994
1.00–
1.80 ng/g
Range of average detection in
2 of 3 sampling years; not
detected in other year
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Blue catfish
1990–
1994
2.25–
26.5 ng/g
Range of average detection in
3 of 4 sampling years; not
detected in other year
Watanabe et
al. 2003

HEXACHLOROCYCLOHEXANE (HCH) 279
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
Louisiana section of
the Mississippi River
Carp
1990–
1994
0.714–
5.00 ng/g
Range of average detections in
2 of 3 sampling years; not
detected in other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Largemouth bass
1990–
1994
1.00 ng/g
Average detection in 1 of
2 sampling years; not detected in
other year
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Red snapper
1990–
1994
0.333 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
River shrimp
1990–
1994
1.67 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Small-mouth buffalo
1990–
1994
0.250–
7.00 ng/g
Range of average detection in
4 of 4 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Spotted gar
1990–
1994
857 ng/g
Average detection in 1 of
1 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
Striped bass
1990–
1994
0.500–
1.25 ng/g
Range of average detection in
2 of 2 sampling years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
White bass
1990–
1994
7.56 ng/g
Average detection in 1 of
3 sampling years; not detected in
other years
Watanabe et
al. 2003
Louisiana section of
the Mississippi River
White crappie
1990–
1994
0.750–
1.20 ng/g
Range of average detection in
2 of 3 sampling years; not
detected in other years
Watanabe et
al. 2003
Gulf of California
Clams (C. californiensis)
Not
reported
(2015 or
earlier)
Not detected
Limit of detection 0.005 ng/g wet
weight; adipose tissue samples;
surrounding area has primarily
agricultural activity
Vargas-
Gonzalez et
al. 2016

HEXACHLOROCYCLOHEXANE (HCH) 280
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-11. Organism Monitoring Data for Hexachlorocyclohexanes (HCHs)
Locations
Species
Date(s)
Range
a
Mean
a
Notes
Reference
Lake Apopka, Florida
Largemouth bass (M. salmoides)
March
2013
0.8 ng/g wet
weight
(gastrointestinal
tract)
1.6 ng/g wet
weight (liver)
2.6 ng/g wet
weight (kidney)
0.8 ng/g wet
weight (spleen)
4.8 ng/g wet
weight (brain)
1.8 ng/g wet
weight (gonad)
1.3 ng/g wet
weight (muscle)
117.8 ng/g lipid (gastrointestinal
tract)
29.4 ng/g lipid (liver)
742.6 ng/g lipid (kidney)
102.0 ng/g lipid (spleen)
144.1 ng/g lipid (brain)
77.6 ng/g lipid (gonad)
125.2 ng/g lipid (muscle)
Dang et al.
2016
δ-HCH
Southwestern
Michigan
Adult green frogs
1998
0.03 ng/g
Not detected in juvenile frogs
Gilliland et al.
2001
Gulf of California
Clams (C. californiensis)
Not
reported
(2015 or
earlier)
<0.01–
1.97 ng/g
wet weight
Detection frequency 16.7% in 1 of
3 study areas, not detected in
other areas; adipose tissue
samples; surrounding area has
primarily agricultural activity
Vargas-
Gonzalez et
al. 2016
Lake Apopka, Florida
Largemouth bass (M. salmoides)
March
2013
Not detected
Limit of quantification 0.1–
0.5 ng/g wet weight
Dang et al.
2016
United States
Freshwater fish
2020
ND
Not detected in 191 samples; no
data reported for 2022–2023
b
WQP 2023
c
a
Organism concentration unit conversion: 1 ng/g = 1 ppb.
b
As of June 2023.
c
Data collected by USGS monitoring stations across the United States; mean and ranges do not reflect samples reported as not detected/below detection limit.
Not detected; NR = not reported; USGS = U.S. Geological Survey
HEXACHLOROCYCLOHEXANE (HCH) 281
5. POTENTIAL FOR HUMAN EXPOSURE
residue screening program carried out by the H.E.B. Food Stores of San Antonio between 1989 and 1991
detected γ-HCH in 4 of 429 onion samples (detection limit 0.02 ppm); however, none of the positive
samples exceeded the action level for this commodity (Schattenberg and Hsu 1992). γ-HCH was detected
at levels of ≤10 ppm in 6 out of 5,784 fruit and vegetable commodities analyzed in Canada from 1992 to
1994 (Neidert and Saschenbrecker 1996). α-, β-, and γ-HCH were detected in butter samples from the
United States at mean levels of 0.38, 0.42, and 0.78 ppb, respectively (Kalantzi et al. 2001). HCH
isomers were also detected in butter samples from 20 other countries, with the highest levels being
observed in a single butter sample from India with reported concentrations of 98, 108, and 164 ppb for α-,
β-, and γ-HCH, respectively (Kalantzi et al. 2001).
Based on the most recent pesticide residue monitoring results published by the FDA from 2018, no
γ-HCH residues were detected on food products produced in or imported to the United States (FDA
2020a). The products sampled were broadly encompassing of domestic and imported food and
agricultural commodities, and included fruits, vegetables, grains, beans, nuts, honey, milk, and meat,
amongst many other categories. A recent study, however, detected averages of 0.22 ppb α-HCH and
0.77 ppb γ-HCH in tobacco products (n=20; cigarettes from one pack were pooled for analysis) purchased
in the United States (Quadroni and Bettinetti 2019). It is unclear if these products were domestic or
imported.
Strategies exist to reduce pesticide residues on food products. γ-HCH residues on tomatoes decreased by
23.9% 15 days after application of the pesticide (from 0.1956 to 0.1488 ppm). Processing the tomatoes
(e.g., pureeing, making tomato juice) reduced the residue levels by 100% after the waiting period;
however, washing the tomatoes reduced the residues by up to 55.9% (Bessar et al. 1991). An analysis of
pesticide residues in green coffee and after roasting indicated that technical-grade HCH was found in
green coffee at concentrations ranging from <0.005 to 0.204 ppm. However, storage and roasting reduced
the pesticide residues by 60–67% and up to 98%, respectively, with darker roasting resulting in the
greatest reduction (McCarthy et al. 1992).
5.6 GENERAL POPULATION EXPOSURE
Exposure of the general population to HCH has declined steadily since its use as a pesticide was
discontinued. Human exposure to γ-HCH may result from environmental exposures to contaminated
water or soil, or possible ingestion of small amounts in drinking water. Historically, γ-HCH and its most
persistent metabolite, β-HCH, have been detected in blood, adipose tissue, and breast milk. Based on the
HEXACHLOROCYCLOHEXANE (HCH) 282
5. POTENTIAL FOR HUMAN EXPOSURE
most recent U.S. population survey, γ-HCH is generally no longer detected in blood, but β-HCH still is
(CDC 2019). This is consistent with the decreased general population exposure to γ-HCH as a pesticide.
Medicinal exposure to γ-HCH can occur from prescription scabies and lice treatments. An analysis of
data from 238 families in Missouri between June 1989 and March 1990 indicated that 9.2% of the
families reported using Kwell shampoo (contains γ-HCH) for lice control on children (Davis et al. 1992).
In general, accidental or intentional ingestion of these products would lead to the highest exposures.
Worker exposure constitutes the next highest exposure population, although worker exposure is
decreasing in both the number of workers exposed and the levels of exposure. Lastly, the general
population receives the lowest levels, which occur mainly from ingestion of foods and water with γ-HCH
residues. Living near a waste disposal site contaminated with γ-HCH will also increase the likelihood of
exposure.
Ingestion of food containing pesticide residue, historically a significant route of exposure, is no longer
expected to be a likely route of non-medicinal human exposure to γ-HCH. During studies conducted
between 1982 and 1991, γ-HCH was detected in 4–6% of the foods collected in eight market basket
surveys from different regions of the United States (Gunderson 1988, 1995a, 1995b). The most recent
results of this survey reported no detections (limit of detection 0.4–2.8 ppb) in foods surveyed in 2017 for
α-, β-, γ-, or δ-HCH (FDA 2020b). γ-HCH was also not detected in domestic or imported food products
in the United States by the FDA (FDA 2020a). Foods representative of consumption patterns by eight
infant and adult population groups were prepared for consumption prior to analysis in a revision to FDA's
Total Diet Studies methodology. The estimated mean daily intakes (ng/kg body weight/day) of α-, β-,
and γ-HCH for these groups continuously decreased between all study periods (1982–1984, 1984–1986,
and 1986–1991). From 1986 to 1991 daily intakes ranged from 0.5 to 2.7 ng/kg/day for α-HCH, were all
<1 ng/kg/day for β-HCH, and ranged from 0.6 to 3.2 ng/kg/day for γ-HCH (Gunderson 1988, 1995a,
1995b). An estimated γ-HCH daily dietary intake based on 2003 FDA pesticide residue monitoring data
for fruits and vegetables was determined to be trace only for domestic produce and 0.00754 ng/kg/day for
imported produce (Katz and Winter 2009). While HCH isomers have been previously detected in
freshwater fish, lindane was not detected (<0.25 ng/mL) in the serum of Detroit area anglers who
consumed their catches, in 2013 (Wattigney et al. 2022). Because γ-HCH and its isomers were not
detected in most recently available food monitoring data, current daily intake can be assumed to be
negligible.
A small degree of exposure to γ-HCH from drinking water may be possible. α-, β-, and δ-HCH have
been detected in recent surface water samples, although these may not represent drinking water sources
HEXACHLOROCYCLOHEXANE (HCH) 283
5. POTENTIAL FOR HUMAN EXPOSURE
(WQP 2023). γ-HCH was detected in drinking water samples collected for the 1998 to 2005 EPA 6-year
review of drinking water quality (EPA 2010). Data for HCH were not reported for the most recent
available period, 2006–2011.
Contaminated soils, which have been sampled as recently as 2021, may present another exposure pathway
(WQP 2023). Studies in which soils containing 10 ppm radiolabeled γ-HCH were added to human skin
samples at quantities that exceeded monolayer coverage (5 mg soil/cm
2
skin) demonstrated mean γ-HCH
absorptions of 1.04% from sandy soils and 1.64% from silt soils (Duff and Kissel 1996). However, data
from soil absorption studies can vary due to factors such as the amount of soil added to skin, exposure
time, and possible evaporation of the contaminant.
The results of biomonitoring studies can be used as indicators of human exposures to HCH. The National
Human Adipose Tissue Survey (NHATS) conducted in 1982 showed that β-HCH (the most prevalent
HCH isomer in fatty tissue) was detected in 87% of 46 composite samples at concentrations <19–
570 ng/g (ppb) (EPA 1986). It was detected most often in postmortem samples collected from individuals
from the southern United States. In another survey conducted in 1970–1975, β-HCH was detected in
>90% of the postmortem human adipose tissue samples at an average level of 300 ppb (Kutz et al. 1979).
In a review of the NHATS data available from 1970 to 1983, EPA (1985c) reported that the estimated
1983 national median level of β-HCH was 80 ppb, in comparison to the historic level of 140 ppb. The
median level had decreased over time, but the compound continued to be detected in nearly 100% of the
population surveyed. Median levels were highest in the South census region and tended to increase with
age but had not been found to differ across the sexes or racial groups. A further analysis of the NHATS
data indicated that average β-HCH concentrations in fat had decreased from 0.45 ppm in 1970 to
approximately 0.16 ppm since 1981 (Kutz et al. 1991). In a similar study in Japan, levels of HCHs in the
adipose tissue of Japanese males increased from the late 1940s to 1966, coinciding with an increased
annual production of HCH, and began dropping when HCHs were banned in 1971, with only the only the
more persistent β-HCH isomer detected after 1974 (Loganathan et al. 1993). Recent adipose tissue
concentrations in the United States were not located, but the trend towards lower concentrations may have
continued following the discontinued use of γ-HCH as a pesticide.
A comparison of the levels of α- and β-HCH in the whole blood and biopsy fat of 25 patients showed
median levels of <0.04 ng/g (maximum, <0.04 ng/g [LOD]) and 0.13 ng/g (maximum, 2.60 ng/g) for the
blood and 1.1 ng/g (maximum, 9.6 ng/g) and 18.0 ng/g (maximum, 748.6 ng/g) for the fat tissue,
respectively (Mes 1992). A further comparison of β-HCH levels in breast milk and adipose tissue
HEXACHLOROCYCLOHEXANE (HCH) 284
5. POTENTIAL FOR HUMAN EXPOSURE
samples was made for populations living near the Great Lakes (Canada only) and in other Canadian
regions. Mean β-HCH levels in breast milk (0.6 ng/g, n=70 samples) and adipose tissue (23.4 ng/g,
n=16 samples) were lower near the Great Lakes than in other parts of Canada (0.8 [n=305 samples] and
30.8 ng/g [n=90 samples], respectively) (Mes and Malcolm 1992). In addition, studies indicate that
γ-HCH can also be present in breast milk at a previously reported average level of 0.006 ppm in Alberta,
Canada (Currie et al. 1979). In a study of 50 donors of breast milk in Oahu, Hawaii, Takahashi et al.
(1981) demonstrated HCH in 82% of the samples at a mean level of 81 ppb within a range of 0–480 ppb,
expressed in terms of extractable lipid.
γ-HCH was one of the most frequently detected pesticides in the blood of Virginia residents, although the
number of individuals sampled was not identified (Griffith and Blanke 1975). γ-HCH blood
concentrations were the highest in residents of the middle age group (41–60 years). Some of the
frequency of γ-HCH occurrence in the state was attributed to its common use in commercial vaporizers
and its presence in cigarette smoke (Griffith and Blanke 1975). NHANES analyzed blood and urine
specimens for the presence of HCH isomers. β-HCH was detected in approximately 13.9% of the
U.S. population (12–74 years) in the Northeast, Midwest, and South. The median level for the 91%
quantifiable positive results was 1.7 ppb (Murphy and Harvey 1985).
In a more recent study (1999–2000) of pesticide serum concentrations in pregnant Latina women living in
an agricultural community in California, median serum levels were 36.9 ng/g lipid for β-HCH and
1.1 ng/g lipid for γ-HCH (Bradman et al. 2007). The median serum concentration of β-HCH was 5.9 ng/g
lipid in 48 mothers enrolled in the California Childhood Leukemia Study in 2006–2007 (Whitehead et al.
2015). Maternal and blood cord samples were collected predominantly from Latina women, who were in
their second or third trimester of pregnancy, as part of a study from October 2010 to June 2011 in San
Francisco, California. Sixty-seven percent of maternal blood samples were above the method detection
limit for β-HCH (5 ng/L wet weight or 0.005 ng/g wet weight). The lipid adjusted median cord:maternal
serum ratio was 1.0, suggesting equivalent exposures for the fetus and the mother. (Morello-Frosch et al.
2016). In another study of 10 whole blood samples obtained from a blood donation center in Palo Alto,
California, α-HCH was detected in all samples, β- and δ-HCH were detected in 60% of samples, and
γ-HCH was detected in 80% of samples. Whole blood concentrations were 0.291–0.828, 0.544–1.15,
0.250–0.694, and 0.550–1.26 ng/g for α-, β-, γ-, and δ-HCH, respectively (Hao et al. 2020). Because
HCH is persistent and can be transported long distances, its isomers have been detected in peoples living
near the Arctic Circle. In 2019, β-HCH was detected in 59% of plasma samples (n = 54) from people in
Old Crow, Canada, which is predominantly Vuntut Gwitchin First Nation people (Drysdale et al. 2021).

HEXACHLOROCYCLOHEXANE (HCH) 285
5. POTENTIAL FOR HUMAN EXPOSURE
The geometric mean was below the detection limit (0.01 ng/L or 9.8x10
-6
ng/g) with a 95
th
percentile of
8.3x10
-5
ng/g (0.085 ng/L; 13,000 ng/g lipid adjusted). γ-HCH was not detected (Drysdale et al. 2021).
The Centers for Disease Control and Prevention (CDC) completed its Fourth National Report on Human
Exposure to Environmental Chemicals that was derived from data obtained from NHANES (CDC 2019).
The first report on 27 chemicals was issued in March 2001. This fourth report, released in January 2019,
presents blood and urine levels of environmental chemicals from a sample of people who represent the
noninstitutionalized, civilian U.S. population during 2-year study periods over 1999–2016. Lipid serum
levels of β- and γ-HCH from the most recent available study periods, 2015–2016 and 2011–2012,
respectively, are summarized in Table 5-12. Serum monitoring was not conducted in the following
NHANES study periods.
Table 5-12. Geometric Mean of the Serum Concentration (ng/g) of β-Hexachloro-
cyclohexane (β-HCH) (2015–2016) and γ-Hexachlorocyclohexane (γ-HCH) (2011–
2012) in the U.S. Population
Population group (sex, age)
Geometric mean
Unadjusted standard error
Sample size (pools)
a
β-HCH
Non-Hispanic white
Male, 12–19 years
NA
b
NA
7
Male, 20–39 years
NA
NA
10
Male, 40–59 years
0.012
0.001
10
Male, ≥60 years
0.03
0.004
14
Female, 12–19 years
NA
NA
5
Female, 20–39 years
NA
NA
11
Female, 40–59 years
0.015
0.002
9
Female, ≥60 years
0.095
0.026
13
Non-Hispanic black
Male, 12–19 years
NA
NA
5
Male, 20–39 years
NA
NA
7
Male, 40–59 years
0.013
0.002
7
Male, ≥60 years
0.017
0.001
7
Female, 12–19 years
NA
NA
4
Female, 20–39 years
NA
NA
9
Female, 40–59 years
0.018
0.002
12
Female, ≥60 years
0.095
0.014
7

HEXACHLOROCYCLOHEXANE (HCH) 286
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-12. Geometric Mean of the Serum Concentration (ng/g) of β-Hexachloro-
cyclohexane (β-HCH) (2015–2016) and γ-Hexachlorocyclohexane (γ-HCH) (2011–
2012) in the U.S. Population
Population group (sex, age)
Geometric mean
Unadjusted standard error
Sample size (pools)
a
Mexican American
Male, 12–19 years
NA
NA
6
Male, 20–39 years
0.011
0.002
7
Male, 40-59 years
0.027
0.005
4
Male, ≥60 years
0.058
0.01
5
Female, 12–19 years
NA
NA
5
Female, 20–39 years
0.016
0.003
6
Female, 40–59 years
0.055
0.006
8
Female, ≥60 years
0.16
0.037
7
All Hispanic
Male, 12–19 years
NA
NA
9
Male, 20–39 years
8.86
1.79
11
Male, 40–59 years
17.6
3.9
9
Male, ≥60 years
81.2
13
9
Female, 12–19 years
NA
NA
8
Female, 20–39 years
11.9
2
11
Female, 40–59 years
52.5
5.3
13
Female, ≥60 years
135
22
13
Asian
Male, 12–19 years
0.058
0.015
3
Male, 20–39 years
0.391
c
0.127
5
Male, 40–59 years
0.488
0.114
4
Male, ≥60 years
0.805
0.108
3
Female, 12–19 years
0.056
0.007
3
Female, 20–39 years
0.496
c
0.225
4
Female, 40–59 years
0.166
c
0.078
5
Female, ≥60 years
1.2
0.11
3
γ-HCH
Non-Hispanic white
Male, 12–19 years
NA
NA
6
Male, 20–39 years
NA
NA
12
Male, 40–59 years
NA
NA
12
Male, ≥60 years
NA
NA
12
Female, 12–19 years
NA
NA
5
Female, 20–39 years
NA
NA
13
Female, 40–59 years
NA
NA
11
Female, ≥60 years
NA
NA
14
HEXACHLOROCYCLOHEXANE (HCH) 287
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-12. Geometric Mean of the Serum Concentration (ng/g) of β-Hexachloro-
cyclohexane (β-HCH) (2015–2016) and γ-Hexachlorocyclohexane (γ-HCH) (2011–
2012) in the U.S. Population
Population group (sex, age)
Geometric mean
Unadjusted standard error
Sample size (pools)
a
Non-Hispanic black
Male, 12–19 years
NA
NA
7
Male, 20–39 years
NA
NA
9
Male, 40–59 years
NA
NA
7
Male, ≥60 years
NA
NA
9
Female, 12–19 years
NA
NA
6
Female, 20–39 years
NA
NA
8
Female, 40–59 years
NA
NA
8
Female, ≥60 years
NA
NA
8
Mexican American
Male, 12–19 years
NA
NA
5
Male, 20–39 years
NA
NA
4
Male, 40–59 years
NA
NA
4
Male, ≥60 years
NA
NA
2
Female, 12–19 years
NA
NA
4
Female, 20–39 years
NA
NA
4
Female, 40–59 years
NA
NA
3
Female, ≥60 years
NA
NA
3
All Hispanic
Male, 12–19 years
NA
NA
7
Male, 20–39 years
NA
NA
8
Male, 40–59 years
NA
NA
7
Male, ≥60 years
NA
NA
6
Female, 12–19 years
NA
NA
7
Female, 20–39 years
NA
NA
8
Female, 40–59 years
NA
NA
7
Female, ≥60 years
NA
NA
7
Asian
Male, 12–19 years
NA
NA
3
Male, 20–39 years
NA
NA
6
Male, 40–59 years
NA
NA
6
Male, ≥60 years
NA
NA
4
Female, 12–19 years
NA
NA
4
Female, 20–39 years
NA
NA
6

HEXACHLOROCYCLOHEXANE (HCH) 288
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-12. Geometric Mean of the Serum Concentration (ng/g) of β-Hexachloro-
cyclohexane (β-HCH) (2015–2016) and γ-Hexachlorocyclohexane (γ-HCH) (2011–
2012) in the U.S. Population
Population group (sex, age)
Geometric mean
Unadjusted standard error
Sample size (pools)
a
Female, 40–59 years
NA
NA
6
Female, ≥60 years
NA
NA
3
a
Each pool contained serum from eight people.
b
NA = not available; proportion of results below limit of detection (1.3 ng/g lipid for β-HCH and 0.92 ng/g lipid for
γ-HCH) was too high to provide a valid result.
c
Standard error of the mean is >30%.
a
Source: CDC 2019
Factors such as age, dietary habits, and residence can influence the body burden of γ-HCH in exposed
individuals. In one study, it was shown that women between the ages of 26 and 34 years who lived in a
rural area of India and were nonvegetarians tended to show higher body levels of γ-HCH than other
Indian women who lived in an urban area or who were vegetarians (Saxena et al. 1981a). The higher
levels of γ-HCH in women at an older child-bearing age suggest that a longer life span may cause a
greater accumulation of pesticide in the body. Higher pesticide levels were found in mutton, eggs, and
chicken, which are common in nonvegetarian meals; therefore, there tended to be a higher level of γ-HCH
in the bodies of nonvegetarians. In another study, when corrected for age and BMI, vegans had an almost
statistically significantly lower (p=0.076) mean β-HCH plasma concentration, not adjusted for lipids, than
omnivores. Mean β-HCH plasma concentrations were 6.151 ng/g lipid for vegans and 5.720 ng/g lipid
for omnivores (Arguin et al. 2010). In a study of hair samples, 460 ng/g γ-HCH was detected in samples
from people who worked as pesticide applicators and 40 ng/g γ-HCH was detected in samples from
people who lived close to farms in Atlanta, Georgia. Hair collected from people in Houston, Texas,
representing urban environmental exposure, had 1,500 ng/g γ-HCH detected (Smith-Baker and Saleh
2011). The study authors did not suggest an explanation for the higher levels in the samples from
environmentally exposed persons in Houston, Texas compared with levels in pesticide applicators in
Atlanta, Georgia; however, the sample sizes were very small (eight applicators and eight each
environmentally exposed persons in Atlanta and Houston). In addition, the ages of the volunteers from
whom hair samples were collected were not reported, and hair from older individuals could have higher
accumulation of γ-HCH. Further, there was no information on whether any volunteers had previous
exposure to γ-HCH applied to the scalp for treatment of lice.
HEXACHLOROCYCLOHEXANE (HCH) 289
5. POTENTIAL FOR HUMAN EXPOSURE
A study conducted in Colorado indicated, in general, that no quantitative relationships were demonstrated
between pesticide levels in household dust and pesticide levels in blood. However, γ-HCH levels in
blood sera in a pesticide formulator (16.8 ng/g) and his wife (5 ng/g) were found to be elevated in a
household in which dust levels measured 5.85 ng/g (Starr et al. 1974). It is possible that the γ-HCH found
in the wife's blood and in the household came from the clothes and person of the pesticide formulator.
The Nonoccupational Pesticide Exposure Study (NOPES) conducted by EPA was based on the Total
Exposure Assessment Methodology (TEAM) approach to exposure estimation. NOPES was designed to
provide estimates of nonoccupational exposure to 32 household pesticides in the United States. Samples
were collected at two locations: (1) Jacksonville, Florida, an area representative of high pesticide usage;
and (2) Springfield/Chicopee, Massachusetts, an area of low-to-moderate pesticide usage. Detectable
levels of γ-HCH were found in the personal air samples of 32–70% of the Jacksonville sample population;
the range of mean concentrations in the air samples was 7–22 ng/m
3
. For the Springfield population,
detectable levels of γ-HCH were found in personal air samples collected from 8–10% of the population,
with mean concentrations of 0.7–5 ng/m
3
(EPA 1990).
5.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES
The populations with the most potential for chronic-duration exposure to HCH are workers who work at
facilities that produce, process, or use γ-HCH. Exposure of the general population to γ-HCH tends to be
low because federal regulations limiting its use have taken effect. However, γ-HCH is available in some
prescription medications (e.g., shampoos, lotions), and the possibility of exposure may arise from use of
these products. Individuals living near hazardous waste sites contaminated with HCH may also be
exposed.
Historically, the largest occupational exposures came from people who work with pesticides. A study on
occupational pesticide exposure of commercial seed-treating applicators was conducted in Montana (Grey
et al. 1983). No exposure was detectable on the chest and arm pads, but γ-HCH was detected on the
hands and on the respirator pads. Workers involved with γ-HCH application complained of nasal
irritation if they did not wear a respirator or mask. The α-, β-, γ-, and δ-isomers of HCH have been
detected in the blood serum and adipose tissue of individuals occupationally exposed to HCH in pesticide
formulation. Serum levels of <0.5 ppb–1 ppm α-HCH, <0.9 ppb–0.72 ppm β-HCH, <0.7 ppb–0.17 ppm
γ-HCH, and 0.002–0.16 ppm δ-HCH have been detected in exposed workers (Baumann et al. 1980;
Kashyap 1986; Morgan and Lin 1978; Nigam et al. 1986). Mean adipose tissue levels of 5.8 mg
HEXACHLOROCYCLOHEXANE (HCH) 290
5. POTENTIAL FOR HUMAN EXPOSURE
α-HCH/kg, 45.6 mg β-HCH/kg, and 3.1 mg γ-HCH/kg have also been reported in exposed workers
(Baumann et al. 1980).
A number of case reports (e.g., Bhalla and Thami 2004; Daud et al. 2010; Juan et al. 2004; Paul et al.
2013; Shah et al. 2013; Ramabhatta et al. 2014; Wiles et al. 2015; Yu et al. 2015) have documented toxic
effects in humans overexposed to γ-HCH through excessive dermal application or accidental or
intentional ingestion of products used to treat scabies and head lice; effects observed in these studies are
described in Chapter 2.
HEXACHLOROCYCLOHEXANE (HCH) 291
CHAPTER 6. ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of HCH is available. Where adequate information is not
available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of research
designed to determine the adverse health effects (and techniques for developing methods to determine
such health effects) of HCH.
Data needs are defined as substance-specific informational needs that, if met, would reduce the
uncertainties of human health risk assessment. This definition should not be interpreted to mean that all
data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
6.1 EXISTING INFORMATION ON HEALTH EFFECTS
Studies evaluating the health effects of inhalation, oral, and dermal exposure of humans and animals to
HCH that are discussed in Chapter 2 are summarized in Figures 6-1, 6-2, 6-3, and 6-4. The purpose of
these figures is to illustrate the information concerning the health effects of HCH. The number of human
and animal studies examining each endpoint is indicated regardless of whether an effect was found and
the quality of the study or studies.
6.2 IDENTIFICATION OF DATA NEEDS
Missing information in Figures 6-1, 6-2, 6-3, and 6-4 should not be interpreted as a “data need.” A data
need, as defined in ATSDR’s Decision Guide for Identifying Substance-Specific Data Needs Related to
Toxicological Profiles (ATSDR 1989), is substance-specific information necessary to conduct
comprehensive public health assessments. Generally, ATSDR defines a data gap more broadly as any
substance-specific information missing from the scientific literature.

HEXACHLOROCYCLOHEXANE (HCH) 292
6. ADEQUACY OF THE DATABASE
Figure 6-1. Summary of Existing Health Effects Studies on
α-Hexachlorocyclohexane by Route and Endpoint*
Potential body weight, liver, and cancer effects were the most studied endpoints
The majority of the studies examined oral exposure in animals (versus humans)
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
5
1
3
3
2
4
4
8
1
2
1
16
3
1
Oral Studies
Acute
12%
Intermediate
47%
Chronic
41%
Oral Route Duration
*Includes studies discussed in Chapter 2; the number of studies include those finding no effect. Most
studies examined multiple endpoints. No inhalation or dermal studies in humans or animals were located.
Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of
this figure.

HEXACHLOROCYCLOHEXANE (HCH) 293
6. ADEQUACY OF THE DATABASE
Figure 6-2. Summary of Existing Health Effects Studies on
β-Hexachlorocyclohexane by Route and Endpoint*
Potential developmental, other noncancer, and cancer effects were the most studied endpoints
The majority of the studies examined exposure in humans (versus animals)
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
27
14
20
5
6
2
8
3
2
4
2
2
1
3
4
4
3
8
3
4
3
33
Oral Studies
Acute
15%
Intermediate
23%
Chronic
63%
Oral Route Duration
*Includes studies discussed in Chapter 2; the number of studies include those finding no effect. Most
studies examined multiple endpoints. No inhalation or dermal studies in humans or animals were located.
Human studies of unknown route and/or duration were classified as chronic oral studies for the purpose of
this figure.

HEXACHLOROCYCLOHEXANE (HCH) 294
6. ADEQUACY OF THE DATABASE
Figure 6-3. Summary of Existing Health Effects Studies on γ-Hexachlorocyclohexane by Route and Endpoint*
Potential body weight, liver, and neurological effects were the most studied endpoints
The majority of the studies examined oral exposure in animals (versus humans)
1
1
2
1
2
2
1
2
2
4
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
Inhalation Studies
24
3
7
7
4
4
4
6
1
6
2
34
28
33
11
4
16
40
6
2
6
4
22
11
33
Oral Studies
3
1
1
3
1
1
2
3
7
Dermal Studies
Acute
43%
Intermediate
29%
Chronic
29%
Inhalation Route Duration
Acute
23%
Intermediate
35%
Chronic
42%
Oral Route Duration
Acute
83%
Intermediate
17%
Chroni
0%
Dermal Route Duration
*Includes studies discussed in Chapter 2; the number of studies include those finding no effect. Most studies
examined multiple endpoints. Human studies of unknown route and/or duration were classified as chronic oral
studies for the purpose of this figure.

HEXACHLOROCYCLOHEXANE (HCH) 295
6. ADEQUACY OF THE DATABASE
Figure 6-4. Summary of Existing Health Effects Studies on
δ-Hexachlorocyclohexane and Unspecified
Hexachlorocyclohexanes by Route and Endpoint*
Potential hepatic and cancer effects were the most studied endpoints
The majority of the studies examined exposure in animals (versus humans)
Cancer
Other Noncancer
Developmental
Reproductive
Neurological
Immunological
Endocrine
Ocular
Dermal
Renal
Hepatic
Musculoskeletal
Hematological
Gastrointestinal
Cardiovascular
Respiratory
Body weight
Death
1
3
2
2
1
1
1
3
3
33
Oral Studies
1
1
1
Dermal Studies
Intermediate
20%
Chronic
80%
Oral Route Duration
Intermediate
100%
Dermal Route Duration
*Includes studies discussed in Chapter 2; the number of studies include those finding no effect.
Most studies examined multiple endpoints. No inhalation or dermal studies in humans or
inhalation studies in animals were located. Human studies of unknown route and/or duration
were classified as chronic oral studies for the purpose of this figure.
HEXACHLOROCYCLOHEXANE (HCH) 296
6. ADEQUACY OF THE DATABASE
Acute-Duration MRLs. The inhalation database is inadequate to derive acute-duration inhalation
MRLs for any HCH isomer. The oral database is adequate to derive acute-duration oral MRLs for β- and
γ-HCH, but not for α- or δ-HCH. Acute oral studies providing data on effects of α- and δ-HCH at low
doses are needed.
Intermediate-Duration MRLs. The inhalation database is inadequate to derive intermediate-duration
inhalation MRLs for any HCH isomer. The oral database is adequate to derive intermediate-duration oral
MRLs for α-, β-, and γ-HCH, but not for δ-HCH. Intermediate oral studies providing data on effects of
δ-HCH at low doses are needed.
Chronic-Duration MRLs. The inhalation database is inadequate to derive chronic-duration inhalation
MRLs for any HCH isomer. The oral database is adequate to derive a chronic-duration oral MRL for
α-HCH, but not for β-, γ-, or δ-HCH. Chronic-duration oral studies providing data on effects of β-, γ-,
and δ-HCH at low doses are needed.
Health Effects.
Hepatic. Available animal studies provide abundant evidence for hepatic effects after oral
exposure to α- and β-HCH for intermediate and chronic durations, and to γ-HCH for all exposure
durations. Very limited data are available for liver effects in animals exposed chronically to
δ-HCH by oral administration. Additional studies examining sensitive liver endpoints after acute-
duration oral exposure to α- and β-HCH, all durations of oral exposure to δ-HCH, and inhalation
exposure of all durations to all HCH isomers would complete the database for this health effect.
Neurotoxicity. Studies examining sensitive neurological and neurobehavioral effects in animals
exposed to α-, β-, and δ-HCH by oral and inhalation exposure are needed, as studies of γ-HCH
have shown neurotoxicity after inhalation, oral, and dermal exposure of animals and case-reports
have demonstrated severe neurological effects in exposed humans. For γ-HCH, specialized
neurotoxicity studies of inhalation exposure (all durations) are needed.
Developmental. There are no data on the developmental effects of α- or δ-HCH and very
limited data on the developmental effects of β-HCH. One epidemiological study in children
under 4 years of age suggested that β-HCH in serum may be associated with deficits in cochlear
function, but the findings varied by age at blood sampling; further assessment of the potential
HEXACHLOROCYCLOHEXANE (HCH) 297
6. ADEQUACY OF THE DATABASE
ototoxicity of β-HCH in humans or animals is needed to enable conclusions regarding this health
effect. Data in animals exposed via oral administration of γ-HCH demonstrate a wide variety of
serious effects on the developing organism, including effects on birth outcomes, reproductive
tract development, and the development of the central nervous system and heart. Therefore, the
lack of information on potential developmental toxicity of the other isomers represents a
significant data gap. In addition, there are no studies of these endpoints in animals exposed to
any HCH isomer by inhalation; availability of such information is needed to provide adequate
data for derivation of inhalation MRLs.
Immunotoxicity. There are no data on the effects of α- or δ-HCH on the immune system of
animals exposed by any route, and very limited data on the effects of β-HCH after oral exposure.
Data in animals exposed via oral administration of γ-HCH demonstrate immune suppression in a
variety of species after acute- and intermediate-durations. Specialized studies examining the
functioning of the immune system in animals exposed orally to α-, β-, and δ-HCH are needed, as
are studies of immunotoxicity in animals exposed by inhalation to the HCH isomers.
For the key health outcomes, especially those shown above, data on the mechanisms by which HCH
isomers induce toxicity are limited. Additional mechanistic studies may improve the understanding of the
human relevance of toxic effects observed in animals.
Epidemiology and Human Dosimetry Studies. In the United States, γ-HCH and technical HCH
are no longer used for agricultural purposes, and HCH is not produced in the United States. Currently
authorized uses are limited to prescription shampoos or lotions containing 1% γ-HCH for treatment of lice
and scabies. As a result of the limited current exposures to HCH isomers, additional follow-up of
occupational cohorts established previously may be the most useful approach to obtaining additional
human data. Other epidemiological studies have used blood or tissue levels of HCH isomers in the
general population to evaluate past exposure, an approach that is viable for the more persistent β-HCH
isomer, but not for the isomers with shorter half-lives.
Biomarkers of Exposure and Effect. Methods exist for the analysis of HCH isomers in blood
(normalized by lipid content) and hair and for HCH metabolites in urine. Serum measurements of γ-HCH
represent short-term exposure because γ-HCH is metabolized and excreted rapidly. Due to its high lipid
solubility and persistence, β-HCH levels represent longer-term exposures. However, reported blood
levels of HCH have not been quantitatively correlated with ambient HCH levels or past exposure.
HEXACHLOROCYCLOHEXANE (HCH) 298
6. ADEQUACY OF THE DATABASE
Methods that measure the levels of HCH metabolites in urine are not specific enough to detect exposure
to HCH alone. More information could be provided by studies designed to correlate biomarkers of
exposure with exposure levels. No biomarkers of effect, specific for HCH isomers, have been identified
in the literature. Several studies have demonstrated increases in lipid peroxidation and depletion of
antioxidants in the central nervous system, liver, male reproductive tract, and maternal or fetal tissues in
animals exposed to γ-HCH; however, these are nonspecific effects induced by a wide range of
compounds. Additional studies designed to assess mechanisms of action and/or adverse outcome
pathways may serve to identify specific biomarkers of effect for health outcomes of concern for HCH
isomers (e.g., liver, neurological, developmental, and immune system effects).
Absorption, Distribution, Metabolism, and Excretion. Information is available to evaluate the
toxicokinetics of HCH isomers following oral and dermal exposure in animals and humans. Studies
evaluating toxicokinetic properties following inhalation exposure would be helpful. Limited information
suggests differences in the metabolism of the HCH isomers. Additional data on metabolism of the α-, β-,
and δ-HCH isomers would be beneficial, especially if such information was linked to differences in
specific health outcomes. In vitro studies using rat liver microsomes have demonstrated the formation of
a reactive epoxide metabolite; however, investigations have not been conducted to examine the epoxide
formation in vivo or its role in inducing mutagenic and carcinogenic effects. Further information on the
possible role of epoxide formation in carcinogenesis in vivo, as well as its rate of formation under various
conditions, would be useful.
Comparative Toxicokinetics. The development and validation of additional PBPK models that
compare predictions against observations in humans could provide valuable information in extrapolating
animal toxicity data to humans.
Children’s Susceptibility. Data needs relating to both prenatal and childhood exposures, and
developmental effects are discussed in detail in the Developmental Toxicity subsection above. Limited
data are available on the toxicokinetics or health effects of α- and β-HCH isomers on exposed children.
Further, additional animal studies evaluating potential early life susceptibility to neurotoxicity and/or
cancer after exposure to γ-HCH would be useful.
Physical and Chemical Properties. Sufficient information is available on the physical and chemical
properties of γ-HCH and the other HCH isomers (see Chapter 4) to permit an assessment of the
environmental fate of these compounds. No additional studies are warranted at this time.
HEXACHLOROCYCLOHEXANE (HCH) 299
6. ADEQUACY OF THE DATABASE
Production, Import/Export, Use, Release, and Disposal. Production methods for HCH are well
described in the literature (IARC 1979). γ-HCH is used as an insecticide and as a therapeutic scabicide
and pediculicide for treatment of ectoparasites in humans and animals (Budavari et al. 1989). The
production and use of γ-HCH as a pesticide has been restricted in the United States, and the use of γ-HCH
was voluntarily canceled in 2006 (EPA 2006b). Recent data suggest that the uses and import/export
volumes of γ-HCH are decreasing (EPA 2012, 2016; Hauzenberger et al. 2002). Release of γ-HCH to
environmental media has been primarily from its use as a pesticide. Wastes containing γ-HCH must be
contained, incinerated, and disposed of in landfills (EPA 1975). Carbon absorption or flocculation are
useful treatment methods for the removal of HCH from aqueous effluent streams, except when methanol
is also contained in the effluents (NLM 2021). Disposal methods are currently subject to revision under
EPA guidance.
Environmental Fate. HCH released to the environment partitions to the atmosphere, soils, and
sediments (Atkins and Eggleton 1971; Lewis and Lee 1976; Melancon et al. 1986; Saleh et al. 1982;
Stanley et al. 1971). HCH is transported in the atmosphere, surface water, and groundwater (Mackay and
Leinonen 1975; Nordmeyer et al. 1992; Stanley et al. 1971). HCH is transformed via biodegradation in
soils and surface waters (Govind et al. 1991; Kar and Singh 1979b; Kennedy et al. 1990; Macholz and
Kujawa 1985; Sharom et al. 1980; Tu 1976). Wet and dry deposition are significant removal processes
for HCH in the atmosphere (Atkins and Eggleton 1971; Hamada et al. 1981; Wiberg et al. 2001).
Additional information on the transport, transformation, and persistence of the individual isomers in soils
and groundwater, particularly at hazardous waste sites, are needed to identify the most important routes of
human exposure to HCH. There is information regarding the half-lives for γ-HCH in water (3–30, 30–
300, and >300 days for river, lake, and groundwater, respectively) (Zoeteman et al. 1980). Reported half-
lives determined in groundwater of a contaminated site were 223, 62–287, and 120–632 days for α-, β-,
and δ-HCH isomers, respectively (Bashir et al. 2015). Hydrolysis occurs slowly under most
environmental conditions, but the rate is much more rapid under alkaline conditions. At 25°C, hydrolysis
half-lives of 92, 648, and 771 hours were observed for γ-HCH at pH 9.3, 7.8, and 7.3, respectively (Saleh
et al. 1982). The alkaline hydrolysis (pH 9.78) half-life of α-HCH was calculated at 1,083 hours (Zhang
et al. 2014). The degradation of HCH in the atmosphere occurs through the reaction with
photochemically generated hydroxyl radicals, and half-lives of γ- and α-HCH are around 100 days, but
can be much longer based upon environmental conditions (Brubaker and Hites 1998).
HEXACHLOROCYCLOHEXANE (HCH) 300
6. ADEQUACY OF THE DATABASE
Bioavailability from Environmental Media. Evidence of absorption following inhalation and
dermal exposure is available for workers involved in the formulation of pesticide products containing
HCH isomers and in the use of γ-HCH (Baumann et al. 1980; Grey et al. 1983). Dietary intake is not a
major route of exposure for the general population (FDA 2020a, 2020b). Additional information on the
absorption of γ-HCH, following ingestion of or contact with media containing residues of the compound,
would be helpful. As mentioned in Section 6.3.1, Duff and Kissel (1996) showed that bioavailability of
γ-HCH via dermal exposure depended upon levels of soil loading. Dermal absorption ranged from
0.45 to 2.35%. For populations living in the vicinity of hazardous waste sites, additional information on
absorption following dermal contact with, or ingestion of, contaminated soil is needed, given the expected
strong sorption of the compound to soil particulates. Because of the potential of HCH to contaminate air,
drinking water, and soil, further information on the bioavailability of the HCH isomers from these
environmental media are needed for assessing possible health concerns for humans.
Food Chain Bioaccumulation. γ-HCH in surface waters and soils is taken up and bioconcentrated by
terrestrial and aquatic organisms (Just et al. 1990; Matsumura and Benezet 1973; Ramamoorthy 1985;
Schimmel et al. 1977; Verma and Pillai 1991; Viswanathan et al. 1988). Uptake from soils and
bioconcentration by plants and terrestrial organisms appears to be limited (Chen et al. 2013; Šmídová et
al. 2015; Verma and Pillai 1991; Wild and Jones 1992). Plant uptake from air may be greater (Yang et al.
2007). Limited information suggests that the compound is not biomagnified in terrestrial food chains
because of its metabolism by terrestrial organisms (Schmitt et al. 1985). Trophic level transfer of γ-HCH
has been observed (Bemy et al. 2003). Bioconcentration values in zebra fish for α- and β-HCH have been
reported (Butte et al. 1991). Among the HCH isomers, β-HCH accumulates the most in the food chain
(Szokolay et al. 1977). Additional information on the potential bioaccumulation of α-, β-, and δ-HCH
isomers in terrestrial and aquatic food chains is needed.
Exposure Levels in Environmental Media. γ-HCH has been detected in air, surface water and
groundwater, sediment, soil, and food. A gradual decrease of α- and γ-HCH air has been seen across the
decades (Atlas and Giam 1988; Cortes and Hites 2000; WQP 2021), and there is evidence of decreases of
α- and β-HCH in surface water and groundwater although the data have a large range (WQP 2021).
Trends for soil, reflecting varying land uses, are not as clear for the isomers. Although the use of γ-HCH
as a pesticide was voluntarily canceled in 2006 (EPA 2006b), it is uncertain whether new environmental
measurements will show considerably lower levels of HCH since there are remaining impacts from
importing and processing HCH, and evidence of persistency of the isomers. For example, a study of a
pesticide reformulating and packaging facility reported groundwater contamination at the site (Chartrand
HEXACHLOROCYCLOHEXANE (HCH) 301
6. ADEQUACY OF THE DATABASE
et al. 2015). Therefore, additional information on the levels of γ-, α-, β-, and δ-HCH isomers would be
beneficial to determine current potential human exposure to the chemicals from environmental media,
particularly near hazardous waste sites.
Exposure Levels in Humans. HCH can be detected in the blood (Baumann et al. 1980; Bradman et
al. 2007; Griffith and Blanke 1975; Hao et al. 2020; Murphy and Harvey 1985; Whitehead et al. 2015),
urine (Murphy and Harvey 1985), adipose tissue (Baumann et al. 1980; EPA 1986), breast milk
(Takahashi et al. 1981), hair (Smith-Baker and Saleh 2011), and semen (Stachel et al. 1989) of exposed
individuals. Most of the data on the body burden of HCH in adipose tissue and breast milk are prior to
the 2006 voluntary cancellation of γ-HCH for agricultural use. Additional information after this time
point would be helpful to assess current population body burdens. Additionally, most of the data on the
body burden of HCH are from adipose tissue and blood serum analyses conducted postmortem or on
occupationally exposed individuals. The disadvantage of using postmortem blood is that the HCH
concentration may change after death. The occupational studies often do not report environmental levels;
therefore, it is not possible to correlate body HCH levels with environmental levels. The results of the
NHATS conducted in 1982 showed that β-HCH, the most prevalent isomer in fatty tissue, was detected
most often in postmortem samples collected from individuals from the southern United States. Samples
of human milk that were collected over the years in certain populations and used to monitor other
contaminants (e.g., polychlorinated biphenyls) could be tested for HCHs content. Additional information
is needed on exposure to γ-, α-, β-, and δ-HCH isomers in populations living in the vicinity of hazardous
waste sites.
This information is necessary for assessing the need to conduct health studies on these populations.
Exposures of Children. Prenatal exposure of children to HCH has been demonstrated; it is well
documented that placental transfer of HCH occurs, and HCH levels have been measured in placenta and
cord blood in humans (Morello-Frosch et al. 2016; Nair et al. 1996; Saxena et al. 1981b) and in amniotic
fluid and fetal tissues in mice (Srivastava and Raizada 1993). Infants have previously also been exposed
via ingestion of breast milk and cow’s milk. Exposure may also occur via ingestion of water containing
HCH and possibly through incidental ingestion of household dust; exposure is less likely from food and
animal products. It has been demonstrated that household dust can be an important source of
environmental HCH (Starr et al. 1974). This occurs especially if the parents work in facilities that
process or use HCH and can bring home residues of HCH via their work clothes, skin, hair, tools, or other
objects removed from the workplace. A take-home exposure study on pesticide applicators might be
HEXACHLOROCYCLOHEXANE (HCH) 302
6. ADEQUACY OF THE DATABASE
useful if such occupational exposure settings occur. Limited studies conducted on exposure of infants and
children to γ-HCH from application of 1% γ-HCH lotion as scabicide indicated dermal absorption
occurred (Ginsburg et al. 1977). Adipose tissue is a major storage depot for HCH. Although data from a
national human adipose tissue survey exist (EPA 1986), no quantitative data are currently available on the
body burden of HCH in children. These studies are needed because unique exposure pathways for
children exist, and children may be different from adults in their weight-adjusted intake of HCH because
of their higher surface area to volume ratio and higher ingestion rate of household dust.
6.3 ONGOING STUDIES
No ongoing studies were identified in the National Institutes of Health (NIH) RePORTER (2023)
database, which tracks projects funded by NIH.

HEXACHLOROCYCLOHEXANE (HCH) 303
CHAPTER 7. REGULATIONS AND GUIDELINES
Pertinent international and national regulations, advisories, and guidelines regarding HCH in air, water,
and other media are summarized in Table 7-1. This table is not an exhaustive list, and current regulations
should be verified by the appropriate regulatory agency.
ATSDR develops MRLs, which are substance-specific guidelines intended to serve as screening levels by
ATSDR health assessors and other responders to identify contaminants and potential health effects that
may be of concern at hazardous waste sites. See Section 1.3 and Appendix A for detailed information on
the MRLs for HCH.
Table 7-1. Regulations and Guidelines Applicable to Hexachlorocyclohexane
(HCH)
Agency
Description
Information
Reference
Air
EPA
RfC
Not evaluated
IRIS 1987a, IRIS
1987b, IRIS
1987c, IRIS
1987d, IRIS
1987f
WHO
Air quality guidelines
No data
WHO 2010
Water & Food
EPA
Drinking water standards and health
advisories
EPA 2018a
γ-HCH
1-Day health advisory (10-kg child)
1 mg/L
10-Day health advisory (10-kg child)
1 mg/L
DWEL
0.2 mg/L
National primary drinking water regulations
EPA 2009
γ-HCH
MCL and MCLG
0.0002 mg/L
RfD
β-HCH
0.00006 mg/kg/day
EPA 2006c
γ-HCH
3x10
-4
mg/kg/day
IRIS 1987c
WHO
Drinking water quality guidelines
WHO 2022
γ-HCH
Guideline value
0.002 mg/L
ADI
0–0.005 mg/kg body weight
FDA
Substances added to food
a
Not listed
FDA 2023
Allowable level in bottled water
FDA 2017
γ-HCH
0.0002 mg/L

HEXACHLOROCYCLOHEXANE (HCH) 304
7. REGULATIONS AND GUIDELINES
Table 7-1. Regulations and Guidelines Applicable to Hexachlorocyclohexane
(HCH)
Agency
Description
Information
Reference
Cancer
HHS
Carcinogenicity classification
NTP 2021
γ-HCH, technical HCH and other HCH
isomers
Reasonably anticipated to be
human carcinogens
EPA
Carcinogenicity classification
α-HCH
Group B2
b
IRIS 1987a
β-HCH
Group C
c
IRIS 1987b
γ-HCH
Suggestive evidence of
carcinogenicity, but not
sufficient to assess human
carcinogenic potential
EPA 2002
δ-HCH
Group D
d
IRIS 1987d
Technical HCH
Group B2
b
IRIS 1987f
Inhalation unit risk
α-HCH
1.8x10
-3
per µg/m
3
IRIS 1987a
β-HCH
5.3x10
-4
per µg/m
3
IRIS 1987b
Technical HCH
5.1x10
-4
per µg/m
3
IRIS 1987f
Oral slope factor
α-HCH
6.3 per mg/kg/day
IRIS 1987a
β-HCH
1.8 per mg/kg/day
IRIS 1987b
Technical HCH
1.8 per mg/kg/day
IRIS 1987f
IARC
Carcinogenicity classification
γ-HCH
Group 1
e
IARC 2018
HCH
Group 2B
f
IARC 1987
Occupational
OSHA
PEL (8-hour TWA) for general industry,
shipyards, and construction
OSHA 2021a,
OSHA 2021b,
OSHA 2021c
γ-HCH
0.5 mg/m
3
g
NIOSH
REL (up to 10-hour TWA)
NIOSH 2019
γ-HCH
0.5 mg/m
3
g
IDLH
NIOSH 1994
γ-HCH
50 mg/m
3
Emergency Criteria
EPA
AEGLs-air
No data
EPA 2018b
HEXACHLOROCYCLOHEXANE (HCH) 305
7. REGULATIONS AND GUIDELINES
Table 7-1. Regulations and Guidelines Applicable to Hexachlorocyclohexane
(HCH)
Agency
Description
Information
Reference
DOE
PACs-air
DOE 2016
γ-HCH
TEEL-1
h
9.1 mg/m
3
TEEL-2
h
100 mg/m
3
TEEL-3
h
1,000 mg/m
3
a
The Substances Added to Food inventory replaces EAFUS and contains the following types of ingredients: food and
color additives listed in FDA regulations, flavoring substances evaluated by FEMA or JECFA, GRAS substances
listed in FDA regulations, substances approved for specific uses in food prior to September 6, 1958, substances that
are listed in FDA regulations as prohibited from use in food, delisted color additives, and some substances "no
longer FEMA GRAS."
b
Group B2: probable human carcinogen.
c
Group C: possible human carcinogen.
d
Group D: not classifiable as to human carcinogenicity.
e
Group 1: carcinogenic to humans.
f
Group 2B: possibly carcinogenic to humans.
g
Skin notation.
h
Definitions of PAC terminology are available from DOE (2022).
ADI = acceptable daily intake; AEGL = acute exposure guideline levels; DOE = Department of Energy;
DWEL = drinking water equivalent level; EAFUS = Everything Added to Food in the United States;
EPA = Environmental Protection Agency; FAO = Food and Agriculture Organization; FDA = Food and Drug
Administration; FEMA = Flavor and Extract Manufacturers Association of the United States; GRAS = generally
recognized as safe; HHS = Department of Health and Human Services; IARC = International Agency for Research
on Cancer; IDLH = immediately dangerous to life or health; IRIS = Integrated Risk Information System;
JECFA = Joint FAO/WHO Expert Committee on Food Additives; MCL = maximum contaminant level;
MCLG = maximum contaminant level goal; NIOSH = National Institute for Occupational Safety and Health;
NTP = National Toxicology Program; OSHA = Occupational Safety and Health Administration; PAC = protective
action criteria; PEL = permissible exposure limit; REL = recommended exposure limit; RfC = inhalation reference
concentration; RfD = oral reference dose; TEEL = temporary emergency exposure limit; TWA = time-weighted
average; WHO = World Health Organization
HEXACHLOROCYCLOHEXANE (HCH) 306
CHAPTER 8. REFERENCES
Abd El-Moneam NM, Shreadah MA, El-Assar SA, et al. 2017. Protective role of antioxidants capacity
of Hyrtios aff. Erectus sponge extract against mixture of persistent organic pollutants (POPs)-
induced hepatic toxicity in mice liver: biomarkers and ultrastructural study. Environ Sci Pollut Res
Int 24(27):22061-22072. https://doi.org/10.1007/s11356-017-9805-8.
Abolhassani M, Asadikaram G, Paydar P, et al. 2019. Organochlorine and organophosphorous pesticides
may induce colorectal cancer; A case-control study. Ecotoxicol Environ Saf 178:168-177.
https://doi.org/10.1016/j.ecoenv.2019.04.030.
Abou Ghayda R, Sergeyev O, Burns JS, et al. 2020. Peripubertal serum concentrations of organochlorine
pesticides and semen parameters in Russian young men. Environ Int 144:106085.
https://doi.org/10.1016/j.envint.2020.106085.
Adamski JC, Pugh AL. 1996. Occurrence of pesticides in ground water of the Ozark Plateaus Province.
Water Res Bull 31:97-105.
Agrahari A, Singh A, Srivastava A, et al. 2019. Overexpression of cerebral cytochrome P450s in
prenatally exposed offspring modify the toxicity of lindane in rechallenged offspring. Toxicol Appl
Pharmacol 371:20-37. https://doi.org/10.1016/j.taap.2019.03.022.
Ahamed M, Anand M, Kumar A, et al. 2006. Childhood aplastic anaemia in Lucknow, India: incidence,
organochlorines in the blood and review of case reports following exposure to pesticides. Clin
Biochem 39(7):762-766. https://doi.org/10.1016/j.clinbiochem.2006.03.021.
Ahdaya SM, Monroe RJ, Guthrie FE. 1981. Absorption and distribution of intubated insecticides in
fasted mice. Pestic Biochem Physiol 16:38-46.
Ahmed FE, Hart RW, Lewis NJ. 1977. Pesticide induced DNA damage and its repair in cultured human
cells. Mutat Res 42:161-174.
Ahmed RS, Suke SG, Seth V, et al. 2008. Protective effects of dietary ginger (Zingiber officinales
Rosc.) on lindane-induced oxidative stress in rats. Phytother Res 22(7):902-906.
https://doi.org/10.1002/ptr.2412.
Akhlaq M, Gupta AK, Yunus M. 2006. Effect of lindane pretreatment on the onset of acetaminophen
induced hepatic damage in the rat. Chem Environ Res 15(1-2):93-102.
Akkina J, Reif J, Keefe T, et al. 2004. Age at natural menopause and exposure to organochlorine
pesticides in Hispanic women. J Toxicol Environ Health A 67(18):1407-1422.
https://doi.org/10.1080/15287390490483845.
Aks SE, Krantz A, Hryhorczuk DO. 1995. Acute accidental lindane ingestion in toddlers. Ann Emerg
Med 26(5):647-651.
Akyeampong E, Bend JR, Luginaah I, et al. 2022. Urinary pesticide residual levels and acute respiratory
infections in children under 5 years of age: Findings from the Offinso North farm health study.
Environ Health Insights 16:11786302221094418. https://doi.org/10.1177/11786302221094418.
Alavanja MC, Hofmann JN, Lynch CF, et al. 2014. Non-Hodgkin lymphoma risk and insecticide,
fungicide and fumigant use in the agricultural health study. PLoS One 9(10):e109332.
https://doi.org/10.1371/journal.pone.0109332.
Albertson TE, Joy RM, Stark LG. 1985. Facilitation of kindling in adult rats following neonatal
exposure to lindane. Dev Brain Res 17:263-266.
Albro PW, Thomas R. 1974. Intestinal absorption of hexachlorobenzene and hexachlorocyclohexane
isomers in rats. Bull Environ Contam Toxicol 12:289-294.
Al-Hussaini TK, Abdelaleem AA, Elnashar I, et al. 2018. The effect of follicullar fluid pesticides and
polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological
and clinical outcome. Eur J Obstet Gynecol Reprod Biol 220:39-43.
https://doi.org/10.1016/j.ejogrb.2017.11.003.
Ali SS, Shakoori AR. 1998. Studies on the toxicity of lindane in albino rat: Histopathological effects in
liver. Punjab Univ J Zool 13:149-166.
HEXACHLOROCYCLOHEXANE (HCH) 307
8. REFERENCES
Ali U, Bajwa A, Iqbal Chaudhry MJ, et al. 2016. Significance of black carbon in the sediment-water
partitioning of organochlorine pesticides (OCPs) in the Indus River, Pakistan. Ecotoxicol Environ
Saf 126:177-185. https://doi.org/10.1016/j.ecoenv.2015.12.024.
Allen RH, Mage DT, Gondy G, et al. 2006. Investigation of job-related pesticide exposure in the third
national health and nutrition examination survey. Arch Environ Occup Health 61(2):75-86.
https://doi.org/10.3200/AEOH.61.2.75-86.
Allsup T, Walsh D. 1982. Gas chromatographic analysis of chlorophenylmercapturic acid lindane
metabolites. J Chromatogr 236:421-428.
Alvarado-Hernandez DL, Montero-Montoya R, Serrano-Garcia L, et al. 2013. Assessment of exposure
to organochlorine pesticides and levels of DNA damage in mother-infant pairs of an agrarian
community. Environ Mol Mutagen 54(2):99-111. https://doi.org/10.1002/em.21753.
Alvarez-Pedrerol M, Ribas-Fitó N, Torrent M, et al. 2008a. Thyroid disruption at birth due to prenatal
exposure to beta-hexachlorocyclohexane. Environ Int 34(6):737-740.
https://doi.org/10.1016/j.envint.2007.12.001.
Alvarez-Pedrerol M, Ribas-Fitó N, Torrent M, et al. 2008b. Effects of PCBs, p,p'-DDT, p,p'-DDE, HCB
and beta-HCH on thyroid function in preschool children. Occup Environ Med 65(7):452-457.
https://doi.org/10.1136/oem.2007.032763.
Alvarez-Pedrerol M, Guxens M, Ibarluzea J, et al. 2009. Organochlorine compounds, iodine intake, and
thyroid hormone levels during pregnancy. Environ Sci Technol 43(20):7909-7915.
https://doi.org/10.1021/es9007273.
Amyes SJ. 1990. Lindane: Combined oncogenicity and toxicity study by dietary administration to
Wistar rats for 104 weeks. Suffolk, England: Life Science Research Limited. LSR Report No.
90/CIL002/0839.
Anand M, Taneja A. 2020. Organochlorine pesticides residue in placenta and their influence on
anthropometric measures of infants. Environ Res 182:109106.
https://doi.org/10.1016/j.envres.2019.109106.
Anand M, Gupta GSD, Gopal K, et al. 1991. Influence of dietary protein deficiency on EEG
neurotransmitters and neurobehavior after chronic exposure to HCH. Toxicol Environ Chem 34:1-
11. https://doi.org/10.1080/02772249109357770.
Anand M, Meera P, Kumar R, et al. 1995. Possible role of calcium in the cardiovascular effects of
prolonged administration of gamma-HCH (lindane) in rats. J Appl Toxicol 15(4):245-248.
https://doi.org/10.1002/jat.2550150403.
Anand M, Singh L, Agarwal P, et al. 2019. Pesticides exposure through environment and risk of pre-
term birth: a study from Agra city. Drug Chem Toxicol 42(5):471-477.
https://doi.org/10.1080/01480545.2017.1413107.
Andersen ME, Krishnan K. 1994. Relating in vitro to in vivo exposures with physiologically based
tissue dosimetry and tissue response models. In: Salem H, ed. Animal test alternatives:
Refinement, reduction, replacement. New York, NY: Marcel Dekker, Inc., 9-25.
Andrews JE, Gray LE. 1990. The effects of lindane and linuron on calcium metabolism, bone
morphometry and the kidney in rats. Toxicology 60:99-107. https://doi.org/10.1016/0300-
483X(90)90165-D.
Angerer J, Heinrich R, Laudehr H. 1981. Occupational exposure to hexachlorocyclohexane. V. Gas
chromatographic determination of monohydroxychlorobenzenes (chlorophenols) in urine. Int Arch
Occup Environ Health 48:319-324.
Angerer J, Maass R, Heinrich R. 1983. Occupational exposure to hexachlorocyclohexane. VI.
Metabolism of g-hexachlorocyclohexane in man. Int Arch Occup Environ Health 52:59-67.
Angsubhakorn S, Bhamarapravati N, Romruen K, et al. 1981. Further study of α-benzene hexachloride
inhibition of aflatoxin B1 hepatocarcinogenesis in rats. Br J Cancer 43:881-883.
Anilakumar KR, Saritha V, Khanum F, et al. 2009. Ameliorative effect of ajwain extract on
hexachlorocyclohexane-induced lipid peroxidation in rat liver. Food Chem Toxicol 47(2):279-282.
https://doi.org/10.1016/j.fct.2008.09.061.
HEXACHLOROCYCLOHEXANE (HCH) 308
8. REFERENCES
Appenzeller BMR, Hardy EM, Grova N, et al. 2017. Hair analysis for the biomonitoring of pesticide
exposure: comparison with blood and urine in a rat model. Arch Toxicol 91(8):2813-2825.
https://doi.org/10.1007/s00204-016-1910-9.
Arguin H, Sanchez M, Bray GA, et al. 2010. Impact of adopting a vegan diet or an olestra
supplementation on plasma organochlorine concentrations: results from two pilot studies. Br J Nutr
103(10):1433-1441. https://doi.org/10.1017/S000711450999331X.
Arisi ACM, Simizu K, Kogake M, et al. 1994. Brain and liver lipid peroxidation levels following acute
and short-term lindane administration in the rat. Toxicology Letters 74:61-68.
https://doi.org/10.1016/0378-4274(94)90074-4.
Aronson KJ, Wilson JW, Hamel M, et al. 2010. Plasma organochlorine levels and prostate cancer risk. J
Expo Sci Environ Epidemiol 20(5):434-445. https://doi.org/10.1038/jes.2009.33.
Arrebola JP, Pumarega J, Gasull M, et al. 2013. Adipose tissue concentrations of persistent organic
pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ Res 122:31-37.
https://doi.org/10.1016/j.envres.2012.12.001.
Arrebola JP, Ocaña-Riola R, Arrebola-Moreno AL, et al. 2014. Associations of accumulated exposure to
persistent organic pollutants with serum lipids and obesity in an adult cohort from Southern Spain.
Environ Pollut 195:9-15. https://doi.org/10.1016/j.envpol.2014.08.003.
Arrebola JP, Belhassen H, Artacho-Cordon F, et al. 2015a. Risk of female breast cancer and serum
concentrations of organochlorine pesticides and polychlorinated biphenyls: a case-control study in
Tunisia. Sci Total Environ 520:106-113. https://doi.org/10.1016/j.scitotenv.2015.03.045.
Arrebola JP, Fernandez MF, Martin-Olmedo P, et al. 2015b. Historical exposure to persistent organic
pollutants and risk of incident hypertension. Environ Res 138:217-223.
https://doi.org/10.1016/j.envres.2015.02.018.
Arrebola JP, Ramos JJ, Bartolomé M, et al. 2019. Associations of multiple exposures to persistent toxic
substances with the risk of hyperuricemia and subclinical uric acid levels in BIOAMBIENT.ES
study. Environ Int 123:512-521. https://doi.org/10.1016/j.envint.2018.12.030.
Atkins DHF, Eggleton AEJ. 1971. Studies of atmospheric washout and deposition of γ-HHC, dieldrin,
and p,p-DDT using radiolabelled pesticides. In: Nuclear techniques in environmental pollution:
Proceedings of a symposium on use of nuclear techniques in the measurement and control of
environmental pollution held by the International Atomic Energy Agency in Salzburg, 26-30 October
1970. Vienna: International Atomic Energy Agency, 521-533.
Atlas E, Giam CS. 1988. Ambient concentrations and precipitation scavenging of atmospheric organic
pollutants. Water Air Soil Pollut 38:19-36.
ATSDR. 1989. Decision guide for identifying substance-specific data needs related to toxicological
profiles; Notice. Agency for Toxic Substances and Disease Registry. Federal Register
54(174):37618-37634.
ATSDR. 2018. Draft guidance for the preparation of toxicological profiles. Agency for Toxic
Substances and Disease Registry.
https://www.atsdr.cdc.gov/toxprofiles/guidance/profile_development_guidance.pdf. September 29,
2022.
ATSDR. 2022. Hexachlorocyclohexanes. Full SPL data. Substance priority list (SPL) resource page.
Agency for Toxic Substances and Disease Registry.
https://www.atsdr.cdc.gov/spl/resources/index.html. July 6, 2023.
Attia AM, Richardson BA, Rodriguez C, et al. 1991. Lindane may enhance nocturnal pineal N-
acetyltransferase activity via β-adrenergic receptors. Brain Res 554(1-2):253-256.
https://doi.org/10.1016/0006-8993(91)90197-4.
Attia AM, El-Banna SG, Nomeir FR, et al. 2011. Lindane-induced biochemical perturbations in rat
serum and attenuation by omega-3 and Nigella sativa seed oil. Indian J Biochem Biophys 48(3):184-
190.
HEXACHLOROCYCLOHEXANE (HCH) 309
8. REFERENCES
Aulakh RS, Bedi JS, Gill JP, et al. 2007. Occurrence of DDT and HCH insecticide residues in human
biopsy adipose tissues in Punjab, India. Bull Environ Contam Toxicol 78(5):330-334.
https://doi.org/10.1007/s00128-007-9187-6.
Baker MT, Nelson RM, Van DR. 1985. The formation of chlorobenzene and benzene by the reductive
metabolism of lindane in rat liver microsomes. Arch Biochem Biophys 236:506-514.
Band PR, Abanto Z, Bert J, et al. 2011. Prostate cancer risk and exposure to pesticides in British
Columbia farmers. Prostate 71(2):168-183. https://doi.org/10.1002/pros.21232.
Banerjee BD, Koner BC, Ray A, et al. 1996. Influence of subchronic exposure to lindane on humoral
immunity in mice. Indian J Exp Biol 34(11):1109-1113.
Barnes DG, Dourson M. 1988. Reference dose (RfD): Description and use in health risk assessments.
Regul Toxicol Pharmacol 8:471-486.
Barros SB, Simizu K, Junqueira VB. 1991. Liver lipid peroxidation-related parameters after short-term
administration of hexachlorocyclohexane isomers to rats. Toxicology Letters 56:137-144.
Barry KH, Koutros S, Berndt SI, et al. 2011. Genetic variation in base excision repair pathway genes,
pesticide exposure, and prostate cancer risk. Environ Health Perspect 119(12):1726-1732.
https://doi.org/10.1289/ehp.1103454.
Bashir S, Hitzfeld KL, Gehre M, et al. 2015. Evaluating degradation of hexachlorcyclohexane (HCH)
isomers within a contaminated aquifer using compound-specific stable carbon isotope analysis
(CSIA). Water Res 71:187-196. https://doi.org/10.1016/j.watres.2014.12.033.
Bassig BA, Shu XO, Sjodin A, et al. 2020. Prediagnostic blood levels of organochlorines and risk of
non-Hodgkin lymphoma in three prospective cohorts in China and Singapore. Int J Cancer
146(3):839-849. https://doi.org/10.1002/ijc.32350.
Baumann K, Angerer J, Heinrich R, et al. 1980. Occupational exposure to hexachlorocyclohexane. I.
Body burden of HCH-isomers. Int Arch Occup Health 47:119-127.
Beard AP, Rawlings NC. 1998. Reproductive effects in mink (Mustela vison) exposed to the pesticides
lindane, carbofuran and pentachlorophenol in a multigenerational study. J Reprod Fertil 113:93-104.
https://doi.org/10.1530/jrf.0.1130095.
Beard AP, Rawlings NC. 1999. Thyroid function and effects on reproduction in ewes exposed to the
organochlorine pesticides lindane or pentachlorophenol (PCP) from conception. J Toxicol Environ
Health A 58:509-530.
Beard AP, McRae AC, Rawlings NC. 1997. Reproductive efficiency in mink (Mustela vison) treated
with the pesticides lindane, carbofuran and pentachlorophenol. J Reprod Fertil 111:21-28.
https://doi.org/10.1530/jrf.0.1110021.
Beard AP, Bartlewski PM, Chandolia RK, et al. 1999a. Reproductive and endocrine function in rams
exposed to the organochlorine pesticides lindane and pentachlorophenol from conception. J Reprod
Fertil 115:303-314.
Beard AP, Bartleweski PM, Rawlings NC. 1999b. Endocrine and reproductive function in ewes exposed
to the organochlorine pesticides lindane or pentachlorophenol. Journal of Toxicology and
Environmental Health 56(1):23-46. https://doi.org/10.1080/009841099158213.
Bedi JS, Gill JP, Aulakh RS, et al. 2013. Pesticide residues in human breast milk: risk assessment for
infants from Punjab, India. Sci Total Environ 463-464:720-726.
https://doi.org/10.1016/j.scitotenv.2013.06.066.
Bemy PJ, Veniat A, Mazallon M. 2003. Bioaccumulation of lead, cadmium, and lindane in zebra
mussels (Dreissena polymorpha) and associated risk for bioconcentration in tufted duck (Aythia
fuligula). Bull Environ Contam Toxicol 71(1):90-97. https://doi.org/10.1007/s00128-003-0135-9.
Benjamin N, Kushwah A, Sharma RK, et al. 2006. Histopathological changes in liver, kidney and
muscles of pesticides exposed malnourished and diabetic rats. Indian J Exp Biol 44(3):228-232.
Berg GL. 1988. Lindane. In: Farm chemicals handbook. Willoughby, OH: Meister Publishing Co.,
Berg V, Charles D, Bergdahl IA, et al. 2021. Pre- and post-diagnostic blood profiles of chlorinated
persistent organic pollutants and metabolic markers in type 2 diabetes mellitus cases and controls; a
pilot study. Environ Res 195:110846. https://doi.org/10.1016/j.envres.2021.110846.
HEXACHLOROCYCLOHEXANE (HCH) 310
8. REFERENCES
Berny P, Lachaux O, Buronfosse T, et al. 2002. Zebra mussels (Dreissena polymorpha) as indicators of
freshwater contamination with lindane. Environ Res 90(2):142-151.
https://doi.org/10.1006/enrs.2002.4371.
Berry DH, Brewster MA, Watson R, et al. 1987. Untoward effects associated with lindane abuse [letter].
Am J Dis Child 141:125-126.
Bessar BAA, Korany K, Szabo AS. 1991. Effect of home preparative procedures and technological
processes on lindane residues in tomato. Acta Aliment 20:25-30.
Bevenue A, Hylin JW, Kawano Y, et al. 1972. Pesticides in water: Organochlorine pesticide residues in
water, sediment, algae and fish: Hawaii 1970-1971. Pestic Monit J 6:56-72.
Bhalla M, Thami GP. 2004. Reversible neurotoxicity after an overdose of topical lindane in an infant.
Pediatr Dermatol 21(5):597-599. https://doi.org/10.1111/j.0736-8046.2004.21515.x.
Bhatt DK, Bano M. 2009. Modulation of tricarboxylic acid cycle dehydrogenases during
hepatocarcinogenesis induced by hexachlorocyclohexane in mice. Exp Toxicol Pathol 61(4):325-
332. https://doi.org/10.1016/j.etp.2008.09.004.
Bhatt DK, Nagda G. 2012. Modulation of acid phosphatase and lactic dehydrogenase in
hexachlorocyclohexane-induced hepatocarcinogenesis in mice. J Biochem Mol Toxicol 26(11):439-
444. https://doi.org/10.1002/jbt.21441.
Biberhofer J, Stevens RJJ. 1987. Organochlorine contaminants in ambient waters of Lake Ontario.
Environment Canada, Inland Waters/Lands Director Scientific Series No. 159.
Boffa MJ, Brough PA, Ead RD. 1995. Lindane neurotoxicity. Br J Dermatol 133(6):1013.
Boll M, Weber LW, Stampfl A. 1995. The effect of γ-hexachlorocyclohexane (lindane) on the activities
of liver lipogenic enzymes and on serum lipids in rats. Z Naturforsch C J Biosci 50(1-2):135-142.
https://doi.org/10.1515/znc-1995-1-220.
Bosch AL. 1987a. Dermal absorption of 14C-lindane in male rats. Madison, WI: Hazelton Laboratories
America, Inc. HLA Study No. 6188-103.
Bosch AL. 1987b. Dermal absorption of 14C-lindane in male rabbits. Madison, WI: Hazelton
Laboratories America, Inc. HLA Study No. 6188-104.
Bradman AS, Schwartz JM, Fenster L, et al. 2007. Factors predicting organochlorine pesticide levels in
pregnant Latina women living in a United States agricultural area. J Expo Sci Environ Epidemiol
17(4):388-399. https://doi.org/10.1038/sj.jes.7500525.
Brannen KC, Devaud LL, Liu J. 1998. Prenatal exposure to neurotoxicants dieldrin or lindane alters tert-
butylbicyclophosphorothionate binding to GABA(A) receptors in fetal rat brainstem. Dev Neurosci
20:34-41.
Brassow HL, Baumann K, Lehnert G. 1981. Occupational exposure to hexachlorocyclohexane. II.
Health conditions of chronically exposed workers. Int Arch Occup Environ Health 48:81-87.
Braun JM, Kalkbrenner AE, Just AC, et al. 2014. Gestational exposure to endocrine-disrupting
chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children:
the HOME study. Environ Health Perspect 122(5):513-520. https://doi.org/10.1289/ehp.1307261.
Brauner EV, Sorensen M, Gaudreau E, et al. 2012. A prospective study of organochlorines in adipose
tissue and risk of non-Hodgkin lymphoma. Environ Health Perspect 120(1):105-111.
https://doi.org/10.1289/ehp.1103573.
Bravo N, Grimalt JO, Chashchin M, et al. 2019. Drivers of maternal accumulation of organohalogen
pollutants in Arctic areas (Chukotka, Russia) and 4,4'-DDT effects on the newborns. Environ Int
124:541-552. https://doi.org/10.1016/j.envint.2019.01.049.
Breton P, Bouvet S, Delamanche I, et al. 2005. Electrocorticogram disturbances detected in rats exposed
from early stages of life to residual doses of lindane. Pesticide Biochemistry and Physiology
81(2):97-104. https://doi.org/10.1016/j.pestbp.2004.10.001.
Brubaker WW, Hites RA. 1998. Gas-phase oxidation products of biphenyl and polychlorinated
biphenyls. Environ Sci Technol 32:3913-3918.
HEXACHLOROCYCLOHEXANE (HCH) 311
8. REFERENCES
Buck Louis GM, Chen Z, Peterson CM, et al. 2012. Persistent lipophilic environmental chemicals and
endometriosis: the ENDO Study. Environ Health Perspect 120(6):811-816.
https://doi.org/10.1289/ehp.1104432.
Budavari S, O'Neil MJ, Smith A, et al. 1989. Lindane. In: The Merck index. Rahway, NJ: Merck &
Co., Inc, 866-867.
Burns JS, Williams PL, Korrick SA, et al. 2014. Association between chlorinated pesticides in the serum
of prepubertal Russian boys and longitudinal biomarkers of metabolic function. Am J Epidemiol
180(9):909-919. https://doi.org/10.1093/aje/kwu212.
Butte W, Fox K, Zauke GP. 1991. Kinetics of bioaccumulation and clearance of isomeric
hexachlorocyclohexanes. Sci Total Environ 109-110:377-382.
Callan AC, Hinwood AL, Heyworth J, et al. 2016. Sex specific influence on the relationship between
maternal exposures to persistent chemicals and birth outcomes. International Journal of Hygiene and
Environmental Health 219(8):734-741. https://doi.org/10.1016/j.ijheh.2016.09.018.
Cantor KP, Strickland PT, Brock JW, et al. 2003. Risk of non-Hodgkin's lymphoma and prediagnostic
serum organochlorines: β-hexachlorocyclohexane, chlordane/heptachlor-related compounds,
dieldrin, and hexachlorobenzene. Environ Health Perspect 111(2):179-183.
https://doi.org/10.1289/ehp.4347.
Capt A, Luzy AP, Esdaile D, et al. 2007. Comparison of the human skin grafted onto nude mouse model
with in vivo and in vitro models in the prediction of percutaneous penetration of three lipophilic
pesticides. Regul Toxicol Pharmacol 47(3):274-287. https://doi.org/10.1016/j.yrtph.2006.11.008.
Caquet T, Thybaud E, Lebras S, et al. 1992. Fate and biological effects of lindane and deltamethrin in
fresh-water mesocosms. Aquat Toxicol 23:261-278.
CDC. 2005. Unintentional topic lindane ingestions - United States, 1998-2003. Centers for Disease
Control and Prevention. MMWR Rep 54(21):533-535.
CDC. 2019. Fourth national report on human exposure to environmental chemicals, updated tables.
January 2019. Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/.
December 11, 2019.
Cerón JJ, Panizo CG, Montes A. 1995. Toxicological effects in rabbits induced by endosulfan, lindane,
and methylparathion representing agricultural byproducts contamination. Bull Environ Contam
Toxicol 54:258-265.
Chadwick RW, Freal JJ. 1972a. The identification of five unreported lindane metabolites recovered from
rat urine. Bull Environ Contam Toxicol 7:137-146.
Chadwick RW, Freal JJ. 1972b. Comparative acceleration of lindane metabolism to chlorophenols by
pretreatment of rats with lindane or with DDT and lindane. Food Cosmet Toxicol 10:789-795.
Chadwick R, Peoples A, Cranmer M. 1972. The effect of ascorbic acid deficiency and protein quality on
stimulation of hepatic microsomal enzymes in guinea pigs. Toxicol Appl Pharmacol 22:308-309.
Chadwick RW, Copeland MF, Chadwick C. 1978a. Enhanced pesticide metabolism: A previously
unreported effect of dietary fibre in mammals. Food Cosmet Toxicol 16:217-225.
Chadwick RW, Faeder EJ, King LC, et al. 1978b. Effect of acute and chronic Cd exposure on lindane
metabolism. Ecotoxicol Environ Saf 2:301-316.
Chadwick RW, Freal JJ, Sovocool GW, et al. 1978c. The identification of three previously unreported
lindane metabolites from mammals. Chemosphere 8:633-640.
Chadwick RW, Copeland MF, Mole ML, et al. 1981. Comparative effect of pretreatment with
phenobarbital, Aroclor 1254, and β-naphthoflavone on the metabolism of lindane. Pestic Biochem
Physiol 15:120-136.
Chadwick RW, Copeland MF, Wolff GL, et al. 1985. Effects of age and obesity on the metabolism of
lindane by black a/a, yellow A
vy
/a, and pseudoagouti A
vy
/a phenotypes of (YS x VY) F
1
hybrid mice.
J Toxicol Environ Health 16:771-796.
Chadwick RW, Cooper RL, Chang J, et al. 1988. Possible antiestrogenic activity of lindane in female
rats. J Biochem Toxicol 3:147-158. https://doi.org/10.1002/jbt.2570030303.
HEXACHLOROCYCLOHEXANE (HCH) 312
8. REFERENCES
Chand B, Ramachandran M. 1980. Effect of dietary hexachlorocyclohexane on certain biochemical
changes in albino rat. Indian J Exp Biol 18:735-736.
Charles D, Berg V, Nost TH, et al. 2022. Longitudinal changes in concentrations of persistent organic
pollutants (1986-2016) and their associations with type 2 diabetes mellitus. Environ Res 204(Pt
B):112129. https://doi.org/10.1016/j.envres.2021.112129.
Chartrand M, Passeport E, Rose C, et al. 2015. Compound specific isotope analysis of
hexachlorocyclohexane isomers: a method for source fingerprinting and field investigation of in situ
biodegradation. Rapid Commun Mass Spectrom 29(6):505-514. https://doi.org/10.1002/rcm.7146.
Chen ZM, Zabik MJ, Leavitt RA. 1984. Comparative study of thin film photodegradative rates for 36
pesticides. Ind Eng Chem Prod Res Dev 23:5-11.
Chen Z, Zhao Y, Guo T, et al. 2013. Accumulation and phytoavailability of hexachlorocyclohexane
isomers and cadmium in Allium sativum L. under the stress of hexachlorocyclohexane and cadmium.
Bull Environ Contam Toxicol 90(2):182-187. https://doi.org/10.1007/s00128-012-0882-6.
Chen MW, Santos HM, Que DE, et al. 2018. Association between organochlorine pesticide levels in
breast milk and their effects on female reproduction in a Taiwanese population. Int J Environ Res
Public Health 15(5):931. https://doi.org/10.3390/ijerph15050931.
Cheslack-Postava K, Rantakokko P, Kiviranta H, et al. 2022. Maternal serum persistent organic
pollutant exposure and offspring diagnosed ADHD in a national birth cohort. Environ Res 212(Pt
A):113145. https://doi.org/10.1016/j.envres.2022.113145.
Chiou CT, McGroddy SE, Kile DE. 1998. Partition characteristics of polycyclic aromatic hydrocarbons
on soils and sediments. Environ Sci Technol 32:264-269.
Cifone MA. 1990. Lindane (technical): In the in vitro rat primary hepatocyte unscheduled DNA
synthesis assay. Kensington, MD: Hazelton Laboratories America, Inc. HLA Study No. 12024-0-
447.
Clark DE, Smalley HE, Crookshank HR, et al. 1974. Residues in food and feed: Chlorinated
hydrocarbon insecticide residues in feed and carcasses of feedlot cattle, Texas–1972. Pestic Monit J
8:180-183.
Clayton G, Clayton F, eds. 1981. Hexachlorocyclohexane. In: Patty's industrial hygiene and toxicology.
Vol. IIB. 3rd ed. New York, NY: John Wiley & Sons, 3740-3749.
Clewell HJ. 1995. The application of physiologically based pharmacokinetic modeling in human health
risk assessment of hazardous substances. Toxicol Lett 79(1-3):207-217.
https://doi.org/10.1016/0378-4274(95)03372-r.
Clewell HJ, Andersen ME. 1985. Risk assessment extrapolations and physiological modeling. Toxicol
Ind Health 1(4):111-131.
Cocco P, Brennan P, Ibba A, et al. 2008. Plasma polychlorobiphenyl and organochlorine pesticide level
and risk of major lymphoma subtypes. Occup Environ Med 65(2):132-140.
https://doi.org/10.1136/oem.2007.033548.
Cole RH, Frederick RE, Healy RP, et al. 1984. Preliminary findings of the priority pollutant monitoring
project of the nationwide urban runoff program. J Water Pollut Control Fed 56:898-908.
Conley BE. 1952. Health hazards of electric vaporizing devices for insecticides. JAMA 149:367-369.
Cornacoff JB, Lauer LD, House RV, et al. 1988. Evaluation of the immunotoxicity of β-
hexachlorocyclohexane (β-HCH). Fundam Appl Toxicol 11(2):293-299.
https://doi.org/10.1016/0272-0590(88)90154-6.
Cortes DR, Hites RA. 2000. Detection of statistically significant trends in atmospheric concentrations of
semivolatile compounds. Environ Sci Technol 34:2826-2829.
Crockett AB, Wiersma GB, Tai H, et al. 1974. Pesticides in soil: Pesticide residue levels in soils and
crops, FY-70 - National Soils Monitoring Program (II). Pestic Monit J 8:69-97.
Cupul-Uicab LA, Klebanoff MA, Brock JW, et al. 2013. Prenatal exposure to persistent organochlorines
and childhood obesity in the U.S. collaborative perinatal project. Environ Health Perspect
121(9):1103-1109. https://doi.org/10.1289/ehp.1205901.
HEXACHLOROCYCLOHEXANE (HCH) 313
8. REFERENCES
Curren MS, Davis K, Liang CL, et al. 2014. Comparing plasma concentrations of persistent organic
pollutants and metals in primiparous women from northern and southern Canada. Sci Total Environ
479-480:306-318. https://doi.org/10.1016/j.scitotenv.2014.01.017.
Currie RA, Kadis VW, Breitkreitz WE, et al. 1979. Pesticide residues in human milk, Alberta, Canada -
1966-1970, 1977-1978. Pestic Monit J 13:52-55.
Czeglédi-Jankó G, Avar P. 1970. Occupational exposure to lindane: Clinical and laboratory findings. Br
J Ind Med 27:283-286.
Dallaire R, Dewailly E, Pereg D, et al. 2009. Thyroid function and plasma concentrations of
polyhalogenated compounds in Inuit adults. Environ Health Perspect 117(9):1380-1386.
https://doi.org/10.1289/ehp.0900633.
Dalsenter PR, Faqi AS, Webb J, et al. 1996. Reproductive toxicity and tissue concentrations of lindane
in adult male rats. Hum Exp Toxicol 15(5):406-410. https://doi.org/10.1177/096032719601500508.
Dalsenter PR, Faqi AS, Chahoud I. 1997a. Serum testosterone and sexual behavior in rats after prenatal
exposure to lindane. Bull Environ Contam Toxicol 59(3):360-366.
https://doi.org/10.1007/s001289900486.
Dalsenter PR, Faqi AS, Webb J, et al. 1997b. Reproductive toxicity and toxicokinetics of lindane in the
male offspring of rats exposed during lactation. Hum Exp Toxicol 16(3):146-153.
https://doi.org/10.1177/096032719701600303.
Dang VD, Kroll KJ, Supowit SD, et al. 2016. Tissue distribution of organochlorine pesticides in
largemouth bass (Micropterus salmoides) from laboratory exposure and a contaminated lake.
Environ Pollut 216:877-883. https://doi.org/10.1016/j.envpol.2016.06.061.
Daniels SI, Chambers JC, Sanchez SS, et al. 2018. Elevated levels of organochlorine pesticides in South
Asian immigrants are associated with an increased risk of diabetes. J Endocr Soc 2(8):832-841.
https://doi.org/10.1210/js.2017-00480.
Danopoulos E, Melissinos K, Katsas G. 1953. Serious poisoning by hexachlorocyclohexane. Arch Ind
Hyg 8:582-587.
Daud Y, Rehman D, Farooq U. 2010. Lindane toxicity in a 7 year old boy. J Ayub Med Coll
Abbottabad 22(4):223.
Davies JE, Dedhia H, Morgade C, et al. 1983. Lindane poisonings. Arch Dermatol 119:142-144.
Davis JR, Brownson RC, Garcia R. 1992. Family pesticide use in the home, garden, orchard, and yard.
Arch Environ Contam Toxicol 22:260-266.
De Roos AJ, Schinasi LH, Miligi L, et al. 2021. Occupational insecticide exposure and risk of non-
Hodgkin lymphoma: A pooled case-control study from the InterLymph Consortium. Int J Cancer
149(10):1768-1786. https://doi.org/10.1002/ijc.33740.
Debost-Legrand A, Warembourg C, Massart C, et al. 2016. Prenatal exposure to persistent organic
pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res
146:207-217. https://doi.org/10.1016/j.envres.2016.01.005.
DeJongh J, Blaauboer BJ. 1997. Simulation of lindane kinetics in rats. Toxicology 122:1-9.
Desalegn AA, Iszatt N, Stigum H, et al. 2021. A case-cohort study of perinatal exposure to potential
endocrine disrupters and the risk of cryptorchidism in the Norwegian HUMIS study. Environ Int
157:106815. https://doi.org/10.1016/j.envint.2021.106815.
Desi I. 1974. Neurotoxicological effect of small quantities of lindane. Animal studies. Int Arch
Arbeitsmed 33(2):153-162. https://doi.org/10.1007/BF00538999.
Desi I, Varga L, Farkas I. 1978. Studies on the immunosuppressive effect of organochlorine and
organophosphoric pesticides in subacute experiments. J Hyg Epidemiol Microbiol Immunol
22(1):115-122.
Dewan A, Gupta SK, Jani JP, et al. 1980. Effect of lindane on antibody response to typhoid vaccine in
weanling rats. J Environ Sci Health B 15(4):395-402. https://doi.org/10.1080/03601238009372191.
Dewan P, Jain V, Gupta P, et al. 2013. Organochlorine pesticide residues in maternal blood, cord blood,
placenta, and breastmilk and their relation to birth size. Chemosphere 90(5):1704-1710.
https://doi.org/10.1016/j.chemosphere.2012.09.083.
HEXACHLOROCYCLOHEXANE (HCH) 314
8. REFERENCES
Deziel NC, Warren JL, Huang H, et al. 2021. Exposure to polychlorinated biphenyls and organochlorine
pesticides and thyroid cancer in Connecticut women. Environ Res 192:110333.
https://doi.org/10.1016/j.envres.2020.110333.
Di Consiglio E, De Angelis G, Traina ME, et al. 2009. Effect of lindane on CYP-mediated steroid
hormone metabolism in male mice following in utero exposure. J Appl Toxicol 29(8):648-655.
https://doi.org/10.1002/jat.1452.
Dick IP, Blain PG, Williams FM. 1997a. The percutaneous absorption and skin distribution of lindane in
man. I. In vivo studies. Hum Exp Toxicol 16:645-651.
Dick IP, Blain PG, Williams FM. 1997b. The percutaneous absorption and skin distribution of lindane in
man. II. In vitro studies. Hum Exp Toxicol 16:652-657.
Dietrich DR, Swenberg JA. 1990. Lindane induces nephropathy and renal accumulation of α2µ- globulin
in male but not in female Fischer 344 rats or male NBR rats. Toxicology Letters 53:179-181.
Dietrich DR, Swenberg JA. 1991. NCI-Black-Reiter (NBR) male rats fail to develop renal disease
following exposure to agents that induce α-2µ-globulin (α
2µ
) nephropathy. Fundam Appl Toxicol
16:749-762.
Dikshith TSS, Chandra P, Datta KK. 1973. Effect of lindane on the skin of albino rats. Experientia
29(6):684-685. https://doi.org/10.1007/BF01944772.
Dikshith TSS, Datta KK, Kushwah HS, et al. 1978. Histopathological and biochemical changes in
guinea pigs after repeated dermal exposure to benzene hexachloride. Toxicology 10:55-66.
https://doi.org/10.1016/0300-483X(78)90055-0.
Dikshith TSS, Carrera G, Raizada RB, et al. 1989a. Interaction of hexachlorocyclohexane (HCH) and
chloropropham (CIPC) in male rats. Toxicology Letters 45:281-288. https://doi.org/10.1016/0378-
4274(89)90019-2.
Dikshith TSS, Raizada RB, Srivastava MK, et al. 1989b. Dermal toxicity of hexachlorocyclohexane
(HCH) in rabbit. Indian J Exp Biol 27(3):252-257.
Dikshith TS, Srivastava MK, Raizada RB. 1990. Fetotoxicity of hexachlorocyclohexane (HCH) in mice:
Morphological, biochemical and residue evaluations. Vet Hum Toxicol 32(6):524-527.
Dikshith TSS, Raizada RB, Srivastava MK. 1991a. Long-term dietary study and development of no-
observed-effect level (NOEL) of technical hexachlorocyclohexane to rats. J Toxicol Environ Health
34:495-507. https://doi.org/10.1080/15287399109531585.
Dikshith TS, Srivastava MK, Raizada RB. 1991b. Response of young rats to repeated oral
administration of technical hexachlorocyclohexane. Vet Hum Toxicol 33(3):235-237.
Dikshith TS, Raizada RB, Singh V, et al. 1991c. Repeated dermal toxicity of technical HCH and methyl
parathion (50EC) to female rats (Rattus norvigicus). Indian J Exp Biol 29(2):149-155.
Dimitriadou L, Malarvannan G, Covaci A, et al. 2016. Levels and profiles of brominated and chlorinated
contaminants in human breast milk from Thessaloniki, Greece. Sci Total Environ 539:350-358.
https://doi.org/10.1016/j.scitotenv.2015.08.137.
Doan TQ, Berntsen HF, Verhaegen S, et al. 2019. A mixture of persistent organic pollutants relevant for
human exposure inhibits the transactivation activity of the aryl hydrocarbon receptor in vitro.
Environ Pollut 254(Pt B):113098. https://doi.org/10.1016/j.envpol.2019.113098.
DOE. 2016. Lindane; (gamma-Benzenehexachloride); includes the isomers (319-84-6, 319-85-7, 608-
73-1). PAC Database. U.S. Department of Energy. https://pacteels.pnnl.gov/. July 16, 2023.
DOE. 2022. DOE-HDBK-1046-2016 (reaffirmed 2022), temporary emergency exposure limits for
chemicals: methods and practice. U.S. Department of Energy.
https://www.standards.doe.gov/standards-documents/1000/1046-Bhdbk-2016-reaff-
2022/@@images/file. July 16, 2023.
Dorfler U, Schneider P, Scheunert I. 1991a. Volatilization rates of pesticides from soil and plant surfaces
under controlled conditions. Toxicol Environ Chem 31-32:87-95.
Dorfler U, Adler-Koehler R, Schneider P, et al. 1991b. A laboratory model system for determining the
volatility of pesticides from soil and plant surfaces. Chemosphere 23:485-496.
HEXACHLOROCYCLOHEXANE (HCH) 315
8. REFERENCES
Drysdale M, Ratelle M, Skinner K, et al. 2021. Human biomonitoring results of contaminant and
nutrient biomarkers in Old Crow, Yukon, Canada. Sci Total Environ 760:143339.
https://doi.org/10.1016/j.scitotenv.2020.143339.
Duff RM, Kissel JC. 1996. Effect of soil loading on dermal absorption efficiency from contaminated
soil. J Toxicol Environ Health 48:98-106.
Dzwonkowska A, Hubner H. 1986. Induction of chromosomal aberrations in the Syrian hamster by
insecticides tested in vivo. Arch Toxicol 58:152-156.
Egeler P, Meller RM, Knacker T, et al. 1997. Bioaccumulation of lindane and hexachlorobenzene by
tubificid sludgeworms (oligochaeta) under standardized laboratory conditions. Chemosphere
35(4):835- 852.
Eichler D, Heupt W, Paul W. 1983. Comparative study on the distribution of α- and g-
hexachlorocyclohexane in the rat with particular reference to the problem of isomerization.
Xenobiotica 13:639-647.
Eisenreich SJ, Looney BB, Thornton JD. 1981. Airborne organic contaminants in the Great Lakes
ecosystem. Environ Sci Technol 15:30-38.
Eitzer BD, Chevalier A. 1999. Landscape care pesticide residues in residential drinking water wells.
Bull Environ Contam Toxicol 62:420-427.
El-Masri HA, Mumtaz MM, Yushak ML. 2004. Application of physiologically-based pharmacokinetic
modeling to investigate the toxicological interaction between chlorpyrifos and parathion in the rat.
Environ Toxicol Pharmacol 16(1-2):57-71. https://doi.org/10.1016/j.etap.2003.10.002.
Elserougy S, Beshir S, Saad-Hussein A, et al. 2013. Organochlorine pesticide residues in biological
compartments of healthy mothers. Toxicol Ind Health 29(5):441-448.
https://doi.org/10.1177/0748233712436645.
E
ngst R, Macholz RH, Kujawa H. 1979. [Metabolism of lindane in microbial organisms, warm
- bl
ooded
animals and humans]. Gig Sanit 10:64-65.
Engst R, Macholz R, Kujawa M, et al. 1976. The metabolism of lindane and its metabolites gamma-
2,3,4,5,6-pentachlorocyclohexene, pentachlorobenzene, and pentachlorophenol in rats and the
pathways of lindane metabolism. J Environ Sci Health B 11:95-117.
Ennaceur S. 2017. Study of the genotoxic and cytotoxic effects of the α-, β-, and γ-
Hexachlorocyclohexane isomers in human lymphocyte cells using the cytokinesis-block
micronucleus assay. Drug Chem Toxicol 40(1):85-89.
https://doi.org/10.1080/01480545.2016.1175008.
EPA. 1974. Pesticide in the Illinois waters of Lake Michigan. Washington, DC: U.S. Environmental
Protection Agency. EPA660374002.
EPA. 1975. Guidelines for the disposal of small quantities of unused pesticides. Washington, DC: U.S.
Environmental Protection Agency. EPA670276057.
https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=300051QF.txt. May 5, 2021.
EPA. 1979. Water-related environmental fate of 129 priority pollutants. Volume I: Introduction and
technical background, metals and inorganics, pesticides and PCBs. Washington, DC: U.S.
Environmental Protection Agency. EPA440479029a.
EPA. 1980. Manual of analytical methods for the analysis of pesticides in humans and environmental
samples. Research Triangle Park, NC: U.S. Environmental Protection Agency. EPA600880038.
https://nepis.epa.gov/Exe/ZyPDF.cgi/20007QPD.PDF?Dockey=20007QPD.PDF. May 5, 2021.
EPA. 1982a. Aquatic fate process data for organic priority pollutants. Washington, DC: U.S.
Environmental Protection Agency. EPA440481014.
EPA. 1982b. Retention and transformation of selected pesticides and phosphorous in soil-water systems:
A critical review. Athens, GA: U.S. Environmental Protection Agency. EPA600382060.
EPA. 1985a. Drinking water criteria document for lindane. Cincinnati, OH: U.S. Environmental
Protection Agency. EPA600X841821.
EPA. 1985b. Guidance for the reregistration of pesticide products containing lindane as the active
ingredient. Washington, DC: U.S. Environmental Protection Agency. EPARS85027.
HEXACHLOROCYCLOHEXANE (HCH) 316
8. REFERENCES
EPA. 1985c. Baseline estimates and time trends for beta-benzene hexachloride, hexachlorobenzene, and
polychlorinated biphenyls in human adipose tissue: 1970-1983. Washington, DC: U.S.
Environmental Protection Agency. EPA560585025.
EPA. 1986. Broad scan analysis of the FY82 national human adipose tissue survey specimens. Volume
1 - Executive summary. Washington, DC: U.S. Environmental Protection Agency.
EPA. 1988a. Data evaluation report: EPA Id No.: 52904-C. Lindane. Review of a 13-week dermal
toxicity study (with interim kill and recovery period) in the rat; MRID 408217-01. U.S.
Environmental Protection Agency. Record No. 231696.
https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-009001_18-May-
89_123.pdf. May 4, 2021.
EPA. 1988b. Recommendations for and documentation of biological values for use in risk assessment.
Washington, DC: U.S. Environmental Protection Agency. EPA600687008.
https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=34855. May 5, 2021.
EPA. 1989a. List of hazardous substances and reportable quantities. U.S. Environmental Protection
Agency. Code of Federal Regulations. 40 CFR 302.4.
EPA. 1989b. Hydrolysis rate constants for enhancing property-reactivity relationships. Athens, GA:
U.S. Environmental Protection Agency. EPA600389063. PB89220479.
EPA. 1990. Nonoccupational pesticide exposure study (NOPES): Final report. Research Triangle Park,
NC: U.S. Environmental Protection Agency. EPA600390003.
EPA. 1991a. Data evaluation report: Lindane. Reproductive performance study in rats treated
continuously through two successive generations; MRID 422461-01. U.S. Environmental Protection
Agency. MRID42246101.
EPA. 1991b. Αlpha-2μ-globulin: Association with chemically-induced renal toxicity and neoplasia in
the male rat. Final draft. Washington, DC: U.S. Environmental Protection Agency.
EPA625391019F.
EPA. 1993. Notice of receipt of requests for amendments to delete uses in certain pesticide registrations.
U.S. Environmental Protection Agency. Federal Register 58(220):60630-60631.
EPA. 1995. Method 508: Determination of chlorinated pesticides in water by gas chromatography with
an electron capture device. Cincinnati, OH: U.S. Environmental Protection Agency.
https://www.o2si.com/docs/epa-method-508.pdf. May 5, 2021.
EPA. 1999a. Data evaluation report: Lindane (gamma HCH). Study type: Acute oral (gavage)
neurotoxicity - rat (81-8); MRID 44769201. U.S. Environmental Protection Agency.
EPA. 1999b. Data evaluation report: Lindane (gamma HCH). Study type: Subchronic oral neurotoxicity
- rat (82-7); MRID 44781101. U.S. Environmental Protection Agency.
EPA. 1999c. Data evaluation report: Lindane. Developmental neurotoxicity - rat [870.6300; (83-6)];
MRID 45073501. U.S. Environmental Protection Agency.
EPA. 1999d. Compendium of methods for the determination of toxic organic compounds in ambient air.
Second edition. Washington, DC: U.S. Environmental Protection Agency. EPA625R96010b.
https://www.epa.gov/sites/production/files/2019-11/documents/tocomp99.pdf. April 2, 2021.
EPA. 2000a. Data evaluation report: Lindane. Oncogenicity feeding - mouse [OPPTS 870.4200 (83-2)];
MRID No. 45291402. U.S. Environmental Protection Agency.
EPA. 2000b. Method 1656, Revision A: Organo-halide pesticides in wastewater, soil, sludge, sediment,
and tissue by GC/HSD. U.S. Environmental Protection Agency. EPA821R00017.
https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1002CPW.txt. May 5, 2021.
EPA. 2001. Cancer assessment document. Evaluation of the carcinogenic potential of lindane. Final
report. U.S. Environmental Protection Agency.
https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/628259. May 11, 2021.
EPA. 2002. Registration eligibility decision for lindane. Case 315. U.S. Environmental Protection
Agency.
EPA. 2006a. Lindane; Notice of receipt of requests to voluntarily cancel lindane pesticide registrations.
U.S. Environmental Protection Agency. Federal Register 71:49445.
HEXACHLOROCYCLOHEXANE (HCH) 317
8. REFERENCES
EPA. 2006b. Lindane; cancellation order. U.S. Environmental Protection Agency. Federal Register
71:74905.
EPA. 2006c. Assessment of lindane and other hexachlorocyclohexane isomers. Washington, DC: U.S.
Environmental Protection Agency. https://www.regulations.gov/document/EPA-HQ-OPP-2006-
0034-0002. March 24, 2021.
EPA. 2007. Method 1699: Pesticides in water, soil, sediment, biosolids, and tissue by HRGC/HRMS
Washington, DC: U.S. Environmental Protection Agency. EPA821R08001.
https://www.epa.gov/sites/production/files/2015-10/documents/method_1699_2007.pdf. May 5,
2021.
EPA. 2009. National primary drinking water regulations. U.S. Environmental Protection Agency.
EPA816F090004. https://www.epa.gov/sites/production/files/2016-
06/documents/npwdr_complete_table.pdf. September 7, 2017.
EPA. 2010. Six-year review 2 contaminant occurrence data (1998-2005). U.S. Environmental
Protection Agency. https://www.epa.gov/dwsixyearreview/six-year-review-2-contaminant-
occurrence-data-1998-2005. May 5, 2021.
EPA. 2012. 2012 Chemical data reporting results. U.S. Environmental Protection Agency. February 20,
2019. https://www.epa.gov/chemical-data-reporting/access-cdr-data. May 5, 2021.
EPA. 2014. Applicability of treatment standards. U.S. Environmental Protection Agency. Code of
Federal Regulations. 40 CFR 268.40. https://www.ecfr.gov/cgi-bin/text-
idx?node=pt40.29.268&rgn=div5. May 7, 2021.
EPA. 2016. 2016 Chemical data reporting results. U.S. Environmental Protection Agency.
https://www.epa.gov/chemical-data-reporting/access-cdr-data. May 5, 2021.
EPA. 2018a. 2018 Edition of the drinking water standards and health advisories. Washington, DC: U.S.
Environmental Protection Agency. EPA822S12001.
https://www.epa.gov/system/files/documents/2022-01/dwtable2018.pdf. July 16, 2023.
EPA. 2018b. Acute Exposure Guideline Levels (AEGLs) values. U.S. Environmental Protection
Agency. https://www.epa.gov/sites/production/files/2018-
08/documents/compiled_aegls_update_27jul2018.pdf. April 12, 2020.
EPA. 2020a. Initial list of hazardous air pollutants with modifications. U.S. Environmental Protection
Agency. https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications. May 5, 2021.
EPA. 2020b. 2017 National emissions inventory (NEI) data. U.S. Environmental Protection Agency.
https://www.epa.gov/air-emissions-inventories/2017-national-emissions-inventory-nei-data. March
30, 2021.
EPA. 2020c. List of lists. Consolidated list of chemicals subject to the Emergency Planning and
Community Right To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation
and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act. U.S. Environmental
Protection Agency. EPA550B20001. https://www.epa.gov/sites/production/files/2015-
03/documents/list_of_lists.pdf. May 12, 2021.
EPA. 2021. Air toxics data. Ambient monitoring archive. U.S. Environmental Protection Agency.
https://www3.epa.gov/ttnamti1/toxdat.html#data. April 2, 2021.
EPA. 2022a. 2020 CDR data. U.S. Environmental Protection Agency. https://www.epa.gov/chemical-
data-reporting/access-cdr-data#2020. October 26, 2022.
EPA. 2022b. Toxic chemical release inventory reporting forms and instructions: Revised 2021 version.
U.S. Environmental Protection Agency. EPA740B22002.
https://ordspub.epa.gov/ords/guideme_ext/guideme_ext/guideme/file/ry_2021_rfi.pdf. August 22,
2023.
Etim OE, Farombi EO, Usoh IF, et al. 2006. The protective effect of aloe vera juice on lindane induced
hepatotoxicity and genotoxicity. Pak J Pharm Sci 19(4):337-340.
Everett CJ, Matheson EM. 2010. Biomarkers of pesticide exposure and diabetes in the 1999-2004
national health and nutrition examination survey. Environ Int 36(4):398-401.
https://doi.org/10.1016/j.envint.2010.02.010.
HEXACHLOROCYCLOHEXANE (HCH) 318
8. REFERENCES
Everett CJ, Thompson OM. 2015. Association of DDT and heptachlor epoxide in human blood with
diabetic nephropathy. Rev Environ Health 30(2):93-97. https://doi.org/10.1515/reveh-2015-0003.
Fabisiková A, Drobná B, Conka K, et al. 2012. The effect of prenatal and postnatal exposure to PCBs
and some pesticides on mental and psychomotor development of infants at the age of 10 month.
Organohalogen Compounds 74:948-951. https://dioxin20xx.org/wp-
content/uploads/pdfs/2012/1241.pdf. August 4, 2023.
Fagan J. 1981. Henoch-Schonlein purpura and (γ)-benzene hexachloride [letter]. Pediatrics 67:310-311.
Fang J, Liu H, Zhao H, et al. 2019a. Association of prenatal exposure to organochlorine pesticides and
birth size. Sci Total Environ 654:678-683. https://doi.org/10.1016/j.scitotenv.2018.10.384.
Fang J, Liu H, Zhao H, et al. 2019b. Association of in utero hexachlorocyclohexane exposure with
gestational age. Ecotoxicol Environ Saf 174:263-269. https://doi.org/10.1016/j.ecoenv.2019.02.089.
Farm Chemicals Handbook. 1993. Lindane. In: Pesticide dictionary. Willoughby, OH: Meister
Publishing Company, C204.
Fatih Fidan A, Hakk Cigerci I, Baysu-Sozbilir N, et al. 2008. The effects of the dose-dependent γ-
hexachlorocyclohexane (lindane) on blood and tissue antioxidant defense systems, lipid peroxidation
and histopathological changes in rats. J Anim Vet Adv 7(11):1480-1488.
Fazzalari FA. 1978. Compilation of odor and taste threshold values data (Committee E-18).
Philadelphia, PA: American Society for Testing and Materials. ASTM Data Series DS 48A.
https://doi.org/10.1520/DS48A-EB.
FDA. 2015. Lindane shampoo and lindane lotion. U.S. Food and Drug Administration.
https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/lindane-
shampoo-and-lindane-lotion. May 5, 2021.
FDA. 2017. Subpart B - Requirements for specific standardized beverages. Bottled water. U.S. Food
and Drug Administration. Code of Federal Regulations. 21 CFR 165.110.
https://www.gpo.gov/fdsys/pkg/CFR-2017-title21-vol2/pdf/CFR-2017-title21-vol2-sec165-110.pdf.
September 7, 2017.
FDA. 2020a. Pesticide residue monitoring report and data for FY 2018. U.S. Food and Drug
Administration. https://www.fda.gov/food/pesticides/pesticide-residue-monitoring-report-and-data-
fy-2018. May 5, 2021.
FDA. 2020b. Analytical results of the total diet study. U.S. Food and Drug Administration.
https://www.fda.gov/food/total-diet-study/analytical-results-total-diet-study. May 5, 2021.
FDA. 2023. Substances added to food. U.S. Food and Drug Administration.
https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FoodSubstances. July 16, 2023.
Feldmann RJ, Maibach HI. 1974. Percutaneous penetration of some pesticides and herbicides in man.
Toxicol Appl Pharmacol 28:126-132.
Fendinger NJ, Adams DD, Glotfelty DE. 1992. The role of gas ebullition in the transport of organic
contaminants from sediments. Sci Total Environ 112:189-201.
Fenster L, Eskenazi B, Anderson M, et al. 2006. Association of in utero organochlorine pesticide
exposure and fetal growth and length of gestation in an agricultural population. Environ Health
Perspect 114(4):597-602. https://doi.org/10.1289/ehp.8423.
Fernandez MF, Olmos B, Granada A, et al. 2007. Human exposure to endocrine-disrupting chemicals
and prenatal risk factors for cryptorchidism and hypospadias: a nested case-control study. Environ
Health Perspect 115 Suppl 1:8-14. https://doi.org/10.1289/ehp.9351.
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, et al. 1992. Persistence of some pesticides in the
aquatic environment. Bull Environ Contam Toxicol 48:747-755.
Fischer TF. 1994. Lindane toxicity in a 24-year-old woman. Ann Emerg Med 24(5):972-974.
Fitzhugh OG, Nelson AA. 1947. The comparative chronic toxicities of fumaric, tartaric, oxalic, and
maleic acids. J Am Pharm Assoc Am Pharm Assoc 36(7):217-219.
https://doi.org/10.1002/jps.3030360708.
Fitzhugh OG, Nelson AA, Frawley JP. 1950. The chronic toxicities of technical benzene hexachloride
and its alpha, beta and gamma isomers. J Pharmacol Exp Ther 100(1):59-66.
HEXACHLOROCYCLOHEXANE (HCH) 319
8. REFERENCES
Fitzloff JF, Pan JC. 1984. Epoxidation of the lindane metabolite, β-PCCH, by human- and rat-liver
microsomes. Xenobiotica 14:599-604.
Fitzloff JF, Portig J, Stein K. 1982. Lindane metabolism by human and rat liver microsomes.
Xenobiotica 12:197-202.
Fonseca RG, Resende LAL, Silva MD, et al. 1993. Chronic motor neuron disease possibly related to
intoxication with organochlorine insecticides. Acta Neurol Scand 88:56-58.
Ford WM, Hill EP. 1991. Organochlorine pesticides in soil sediments and aquatic animals in the Upper
Steele Bayou watershed of Mississippi (USA). Arch Environ Contam Toxicol 20:160-167.
Forrester MB, Sievert JS, Stanley SK. 2004. Epidemiology of lindane exposures for pediculosis reported
to Poison Centers in Texas, 1998-2002. J Toxicol Clin Toxicol 42(1):55-60.
https://doi.org/10.1081/clt-120028745.
Frank R, Braun HE, Stonefield KI, et al. 1990. Organochlorine and organophosphorus residues in the fat
of domestic farm animal species, Ontario, Canada 1986–1988. Food Addit Contam 7:629-636.
Franz TJ, Lehman PA, Franz SF, et al. 1996. Comparative percutaneous absorption of lindane and
permethrin. Archives of Dermatology 132(8):901-905.
Freire C, Lopez-Espinosa MJ, Fernandez M, et al. 2011. Prenatal exposure to organochlorine pesticides
and TSH status in newborns from Southern Spain. Sci Total Environ 409(18):3281-3287.
https://doi.org/10.1016/j.scitotenv.2011.05.037.
Freire C, Koifman RJ, Sarcinelli P, et al. 2012. Long term exposure to organochlorine pesticides and
thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environ Res 117:68-
74. https://doi.org/10.1016/j.envres.2012.06.009.
Freire C, Koifman RJ, Sarcinelli PN, et al. 2013. Long-term exposure to organochlorine pesticides and
thyroid status in adults in a heavily contaminated area in Brazil. Environ Res 127:7-15.
https://doi.org/10.1016/j.envres.2013.09.001.
Freire C, Koifman RJ, Sarcinelli PN, et al. 2014. Association between serum levels of organochlorine
pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int J Hyg
Environ Health 217(2-3):370-378. https://doi.org/10.1016/j.ijheh.2013.07.012.
Freire C, Koifman RJ, Koifman S. 2015. Hematological and hepatic alterations in Brazilian population
heavily exposed to organochlorine pesticides. J Toxicol Environ Health A 78(8):534-548.
https://doi.org/10.1080/15287394.2014.999396.
Friberg L, Martensson J. 1953. Case of panmyelophthisis after exposure to chlorophenothane and
benzene hexachloride. AMA Arch Ind Hyg 8:166-169.
Fukata H, Omori M, Osada H, et al. 2005. Necessity to measure PCBs and organochlorine pesticide
concentrations in human umbilical cords for fetal exposure assessment. Environ Health Perspect
113(3):297-303. https://doi.org/10.1289/ehp.7330.
Fytianos K, Vasilkiotis G, Weil L, et al. 1985. Preliminary study of organochlorine compounds in milk
products, human milk, and vegetables. Bull Environ Contam Toxicol 34:504-508.
Gaines T. 1960. The acute toxicity of pesticides to rats. Toxicol Appl Pharmacol 2:88-99.
https://doi.org/10.1016/0041-008x(60)90074-0.
Garcia-Villarino M, Signes-Pastor AJ, Riano-Galan I, et al. 2022. Serum concentrations of persistent
organic pollutants mixture during pregnancy and anogenital distance in 8-year-old children from the
INMA-Asturias cohort. Environ Res 213:113607. https://doi.org/10.1016/j.envres.2022.113607.
Gartrell MJ, Craun JC, Podrebarac DS, et al. 1986a. Pesticides, selected elements, and other chemicals
in infant and toddler total diet samples, October 1980-March 1982. J AOAC 69:123-145.
Gartrell MJ, Craun JC, Podrebarac DS, et al. 1986b. Pesticides, selected elements, and other chemicals
in adult total diet samples, October 1980-March 1982. J AOAC 69:146-161.
Gasull M, Pumarega J, Tellez-Plaza M, et al. 2012. Blood concentrations of persistent organic pollutants
and prediabetes and diabetes in the general population of Catalonia. Environ Sci Technol
46(14):7799-7810. https://doi.org/10.1021/es300712g.
HEXACHLOROCYCLOHEXANE (HCH) 320
8. REFERENCES
Gasull M, Castell C, Pallarès N, et al. 2018. Blood concentrations of persistent organic pollutants and
unhealthy metabolic phenotypes in normal-weight, overweight, and obese individuals. Am J
Epidemiol 187(3):494-506. https://doi.org/10.1093/aje/kwx267.
Gautam AK, Gandhi DN, Jani JP, et al. 1989. Histological and pharmacological changes in vas deferens
of rats exposed to hexachlorocyclohexane. Res Commun Chem Pathol Pharmacol 63(3):463-466.
Génard-Walton M, Warembourg C, Duros S, et al. 2023. Serum persistent organic pollutants and
diminished ovarian reserve: a single-exposure and mixture exposure approach from a French case-
control study. Hum Reprod 38(4):701-715. https://doi.org/10.1093/humrep/dead028.
Genualdi SA, Hageman KJ, Ackerman LK, et al. 2011. Sources and fate of chiral organochlorine
pesticides in western U.S. National Park ecosystems. Environ Toxicol Chem 30(7):1533-1538.
https://doi.org/10.1002/etc.538.
Genuis SJ, Lane K, Birkholz D. 2016. Human elimination of organochlorine pesticides: Blood, urine,
and sweat study. Biomed Res Int 2016:1624643. https://doi.org/10.1155/2016/1624643.
Gewin HM. 1939. Benzene hexachloride and aplastic anemia. JAMA 14:296-297.
Geyer H, Scheunert I, Bruggemann R, et al. 1997. Half-lives and bioconcentration of lindane (g-HCH)
in different fish species and relationship with their lipid content. Chemosphere 35(1-2):343-351.
Ghosh R, Siddarth M, Singh N, et al. 2017. Organochlorine pesticide level in patients with chronic
kidney disease of unknown etiology and its association with renal function. Environ Health Prev
Med 22(1):49. https://doi.org/10.1186/s12199-017-0660-5.
Gilbert ME. 1995. Repeated exposure to lindane leads to behavioral sensitization and facilitates
electrical kindling. Neurotoxicol Teratol 17:131-141. https://doi.org/10.1016/0892-0362(94)00064-
K.
Gilbert ME, Mack CM. 1995. Seizure thresholds in kindled animals are reduced by the pesticides
lindane and endosulfan. Neurotoxicol Teratol 17:143-150. https://doi.org/10.1016/0892-
0362(94)00065-L.
Gilliland CD, Summer CL, Silliland MG, et al. 2001. Organochlorine insecticides, polychlorinated
biphenyls, and metals in water, sediment, and green frogs from southwestern Michigan.
Chemosphere 44:327-339.
Ginsburg CM, Lowry W, Reisch JS. 1977. Absorption of lindane (gamma benzene hexachloride) in
infants and children. J Pediatrics 91:998-1000.
Gladen BC, Shkiryak-Nyzhnyk ZA, Chyslovska N, et al. 2003. Persistent organochlorine compounds
and birth weight. Annals of Epidemiology 13(3):151-157. https://doi.org/10.1016/S1047-
2797(02)00268-5.
Goel A, McConnell LL, Torrents A, et al. 2010. Environmental factors affecting the levels of legacy
pesticides in the airshed of Delaware and Chesapeake Bays, USA. Environ Toxicol Chem
29(9):1893-1906. https://doi.org/10.1002/etc.243.
Goldner WS, Sandler DP, Yu F, et al. 2013. Hypothyroidism and pesticide use among male private
pesticide applicators in the agricultural health study. J Occup Environ Med 55(10):1171-1178.
https://doi.org/10.1097/JOM.0b013e31829b290b.
Gopal K, Anand M, Khanna RN, et al. 1992. Some neurotoxicological consequences of
hexachlorocyclohexane (HCH) stress in rats fed on protein deficient diet. Toxicol Environ Chem
36:57-63. https://doi.org/10.1080/02772249209357827.
Gopalaswamy UV, Aiyar AS. 1984. Biotransformation of lindane in the rat. Bull Environ Contam
Toxicol 32:148-156.
Govind R, Flaherty PA, Dobbs RA. 1991. Fate and effects of semivolatile organic pollutants during
anaerobic digestion of sludge. Water Res 25:547-556.
Grabarczyk M, Kopec-Szlezak J, Szczepanska I, et al. 1990. The effect of gamma-
hexachlorocyclohexane (lindane) on blood cells, kidney and liver tissues in rabbits. Haematologia
23:171-179.
Grey WE, Marthre DE, Rogers SJ. 1983. Potential exposure of commercial seed-treating applicators to
the pesticides carboxin-thiram and lindane. Bull Environ Contam Toxicol 31:244-250.
HEXACHLOROCYCLOHEXANE (HCH) 321
8. REFERENCES
Griffith FD, Blanke RV. 1975. Pesticides in people: Blood organochlorine pesticide levels in Virginia
residents. Pestic Monit J 8:219-224.
Gunderson EL. 1988. FDA total diet study, April 1982–April 1984: Dietary intakes of pesticides,
selected elements, and other chemicals. J AOAC 71:1200-1209.
Gunderson EL. 1995a. Dietary intakes of pesticides, selected elements, and other chemicals: FDA total
diet study, June 1984-April 1986. J AOAC Int 78(4):910-921.
Gunderson EL. 1995b. FDA total diet study, July 1986-April 1991, dietary intakes of pesticides, selected
elements, and other chemicals. J AOAC Int 78(6):1353-1363.
Guo H, Jin Y, Cheng Y, et al. 2014. Prenatal exposure to organochlorine pesticides and infant birth
weight in China. Chemosphere 110:1-7. https://doi.org/10.1016/j.chemosphere.2014.02.017.
Gupta A, Agarwal R, Shukla GS. 1999. Functional impairment of blood–brain barrier following
pesticide exposure during early development in rats. Hum Exp Toxicol 18(3):174-179.
Gupta C, Tripathi DN, Vikram A, et al. 2011. Quercetin inhibits diethylnitrosamine-induced hepatic
preneoplastic lesions in rats. Nutr Cancer 63(2):234-241.
https://doi.org/10.1080/01635581.2011.523806.
Haider K. 1979. Degradation and metabolization of lindane and other hexachlorocyclohexane isomers
by anaerobic and aerobic soil microorganisms. Z Naturforsch C Biosci 34(11):1066-1069.
https://doi.org/10.1515/znc-1979-1138.
Hall RC, Hall RC. 1999. Long-term psychological and neurological complications of lindane poisoning.
Psychosomatics 40(6):513-517.
Hamada M, Kawano E, Kawamura S, et al. 1981. Radiation- and photo-induced degradation of five
isomers of 1,2,3,4,5,6-hexachlorocyclohexane. Agric Biol Chem 45:659-665.
Han X, Meng L, Li Y, et al. 2019. Associations between exposure to persistent organic pollutants and
thyroid function in a case-control study of East China. Environ Sci Technol 53(16):9866-9875.
https://doi.org/10.1021/acs.est.9b02810.
Han X, Zhang F, Meng L, et al. 2020. Exposure to organochlorine pesticides and the risk of type 2
diabetes in the population of East China. Ecotoxicol Environ Saf 190:110125.
https://doi.org/10.1016/j.ecoenv.2019.110125.
Hanada M, Yutani C, Miyaji T. 1973. Induction of hepatoma in mice by benzene hexachloride. Gann
64(5):511-513. https://doi.org/10.20772/cancersci1959.64.5_511.
Hanig JP, Yoder PD, Krop S. 1976. Convulsions in weanling rabbits after a single topical application of
1% lindane. Toxicol Appl Pharmacol 38:463-469. https://doi.org/10.1016/0041-008X(76)90177-0.
Hansch C, Leo A. 1995. Hexachlorocyclohexane. In: Substituent constants for correlation analysis in
chemistry and biology. New York, NY: John Wiley and Sons, 202.
Hao W, Kingston HM, Dillard A, et al. 2020. Quantification of persistent organic pollutants in human
whole blood samples using stir bar sorptive extraction coupled with GC/MS/MS and isotope dilution
mass spectrometry. Microchemical Journal 153:104279.
https://doi.org/10.1016/j.microc.2019.104279.
Hargrave BT, Vass WP, Erickson PE, et al. 1988. Atmospheric transport of organochlorines to the
Arctic Ocean. Tellus 40B:480-493.
Harman‐Fetcho JA, McConnell LL, Baker JE. 1999. Agricultural pesticides in the Patuxent River, a
tributary of the Chesapeake Bay. Journal of Environmental Quality 28(3):928-938.
https://doi.org/10.2134/jeq1999.00472425002800030025x.
Harner T, Wideman JL, Jantunen LM, et al. 1999. Residues of organochlorine pesticides in Alabama
soils. Environ Pollut 106(3):323-332. https://doi.org/10.1016/s0269-7491(99)00110-4.
Harris CJ, Williford EA, Kemberling SR, et al. 1969. Pesticide intoxications in Arizona. Ariz Med
26:872-876.
Hassoun EA, Stohs SJ. 1996a. Comparative teratological studies on TCDD, endrin, and lindane in
C57BL/6J and DBA/2J mice. Comp Biochem Physiol 113C(3):393-398.
https://doi.org/10.1016/0742-8413(96)00011-4.
HEXACHLOROCYCLOHEXANE (HCH) 322
8. REFERENCES
Hassoun EA, Stohs SJ. 1996b. TCDD, endrin, and lindane induced oxidative stress in fetal and placental
tissues of C57BL/6J and DBA/2J mice. Comp Biochem Physiol 115C(1):11-18.
Hassoun EA, Bagchi D, Stohs SJ. 1996. TCDD, endrin, and lindane induced increases in lipid
metabolites in maternal sera and amniotic fluids of pregnant C57BL/6J and DBA/2J mice. Res
Commun Mol Pathol Pharmacol 94(2):157-169.
Hauzenberger I, Perthen-Palmisano B, Hermann M. 2002. Lindane. Report presented at the third
meeting of the POPs Expert Group in Geneva, Switzerland in June 2002.
Hayes WJ. 1982. Benzene hexachloride and lindane. In: Pesticides studied in man. Baltimore, MD:
Williams and Wilkins, 211-228.
Heiberg OM, Wright HN. 1955. Benzene hexachloride poisoning. Arch Ind Health 11:457-458.
Heinisch E, Jonas K, Klein S. 1993. HCH isomers in soil and vegetation from the surroundings of an
industrial landfill of the former GDR, 1971-1989. Sci Total Environ (Suppl Part 1):151-159.
Herbst M, Weisse I, Koellmer H. 1975. A contribution to the question of the possible
hepatocarcinogenic effects of lindane. Toxicology 4(1):91-96. https://doi.org/10.1016/0300-
483x(75)90025-6.
Hernik A, Struciński P, Buckley BT, et al. 2016. Relationship between paired cord blood and milk POPs
levels as a tool for assessing perinatal exposure, a pilot study. Human and Ecological Risk
Assessment 22(7):1456-1468. https://doi.org/10.1080/10807039.2016.1185688.
Herrero-Mercado M, Waliszewski SM, Caba M, et al. 2010. Organochlorine pesticide levels in umbilical
cord blood of newborn in Veracruz, Mexico. Bull Environ Contam Toxicol 85(4):367-371.
https://doi.org/10.1007/s00128-010-0108-8.
Herrero-Mercado M, Waliszewski SM, Caba M, et al. 2011. Organochlorine pesticide gradient levels
among maternal adipose tissue, maternal blood serum and umbilical blood serum. Bull Environ
Contam Toxicol 86(3):289-293. https://doi.org/10.1007/s00128-011-0204-4.
Hfaiedh N, Murat J-C, Elfeki A. 2011. Protective effects of garlic (Allium sativum) extract upon
lindane-induced oxidative stress and related damages in testes and brain of male rats. Pesticide
Biochemistry and Physiology 100(2):187-192. https://doi.org/10.1016/j.pestbp.2011.03.009.
Hfaiedh N, Murat JC, Elfeki A. 2012. A combination of ascorbic acid and α-tocopherol or a combination
of Mg and Zn are both able to reduce the adverse effects of lindane-poisoning on rat brain and liver.
J Trace Elem Med Biol 26(4):273-278. https://doi.org/10.1016/j.jtemb.2012.04.002.
Hiskia A, Mylonas A, Tsipi D, et al. 1997. Photocatalytic degradation of lindane in aqueous solution.
Pestic Sci 50:171-174.
Hitachi M, Yamada K, Takayama S. 1975. Brief communication: Cytologic changes induced in rat liver
cells by short-term exposure to chemical substances. J Natl Cancer Inst 54(5):1245.
Hjermitslev MH, Long M, Wielsoe M, et al. 2020. Persistent organic pollutants in Greenlandic pregnant
women and indices of foetal growth: The ACCEPT study. Sci Total Environ 698:134118.
https://doi.org/10.1016/j.scitotenv.2019.134118.
Hoff RM, Muir DCG, Grift NP. 1992a. Annual cycle of polychlorinated biphenyls and organohalogen
pesticides in air in southern Ontario. 1. Air concentration data. Environ Sci Technol 26:266-275.
Hoff RM, Muir DCG, Grift NP. 1992b. Annual cycle of polychlorinated biphenyls and organohalogen
pesticides in air in southern Ontario. 2. Atmospheric transport and sources. Environ Sci Technol
26:276-283.
Hollifield HC. 1979. Rapid nephelometric estimate of water solubility of highly insoluble organic
chemicals of environmental interest. Bull Environ Contam Toxicol 23:579-586.
Hong HL, Boorman GA. 1993. Residual myelotoxicity of lindane in mice. Fundam Appl Toxicol
21(4):500-507. https://doi.org/10.1006/faat.1993.1126.
Howsam M, Grimalt JO, Guino E, et al. 2004. Organochlorine exposure and colorectal cancer risk.
Environ Health Perspect 112(15):1460-1466. https://doi.org/10.1289/ehp.7143.
Hoyer A, Grandjean P, Jorgensen T, et al. 1998. Organochlorine exposure and risk of breast cancer.
Lancet 352:1816-1820.
HEXACHLOROCYCLOHEXANE (HCH) 323
8. REFERENCES
Hulth L, Hoglund L, Bergman A, et al. 1978. Convulsive properties of lindane, lindane metabolites, and
the lindane isomer a-hexachlorocyclohexane: Effects on the convulsive threshold for
pentylenetetrazol and the brain content of g-aminobutyric acid (GABA) in the mouse. Toxicol Appl
Pharmacol 46:101-108.
Humphreys EH, Janssen S, Heil A, et al. 2008. Outcomes of the California ban on pharmaceutical
lindane: clinical and ecologic impacts. Environ Health Perspect 116(3):297-302.
https://doi.org/10.1289/ehp.10668.
IARC. 1979. Hexachlorocyclohexane. In: IARC monographs on the evaluation of the carcinogenic risk
of chemicals to humans: Some halogenated hydrocarbons. Vol. 20. Geneva, Switzerland:
International Agency for Research on Cancer, 195-241.
IARC. 1987. Hexachlorocyclohexanes. IARC Monographs on the evaluation of carcinogenic risks to
humans. Supplement 7. Overall evaluations of carcinogenicity: An updating of IARC monographs
volumes 1–42. Lyon, France: International Agency for Research on Cancer. 220-222.
https://publications.iarc.fr/139. March 23, 2021.
IARC. 2018. DDT, lindane, and 2,4-D. Volume 113. IARC Monographs on the evaluation of
carcinogenic risks to humans. Lyon, France: International Agency for Research on Cancer.
https://publications.iarc.fr/550. March 23, 2021.
Ibarluzea JM, Fernandez MF, Santa-Marina L, et al. 2004. Breast cancer risk and the combined effect of
environmental estrogens. Cancer Causes Control 15(6):591-600.
https://doi.org/10.1023/B:CACO.0000036167.51236.86.
IRIS. 1987a. alpha-Hexachlorocyclohexane (alpha-HCH). CASRN 319-84-6. Integrated Risk
Information System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0162_summary.pdf. March 22,
2021.
IRIS. 1987b. beta-Hexachlorocyclohexane (beta-HCH). CASRN 319-85-7. Integrated Risk Information
System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0244_summary.pdf. March 22,
2021.
IRIS. 1987c. gamma-Hexachlorocyclohexane (gamma-HCH). CASRN 58-89-9. Integrated Risk
Information System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0065_summary.pdf. March 22,
2021.
IRIS. 1987d. delta-Hexachlorocyclohexane (delta-HCH). CASRN 319-86-8. Integrated Risk
Information System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0163_summary.pdf. March 22,
2021.
IRIS. 1987e. epsilon-Hexachlorocyclohexane (ε-HCH). CASRN 6108-10-7. Integrated Risk
Information System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0164_summary.pdf. March 22,
2021.
IRIS. 1987f. Technical hexachlorocyclohexane (t-HCH). CASRN 608-73-1. Integrated Risk
Information System. Chemical assessment summary. U.S. Environmental Protection Agency.
https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0165_summary.pdf. March 22,
2021.
Ishidate MJ, Odashima S. 1977. Chromosome tests with 134 compounds on Chinese hamster cells in
vitro - a screening for chemical carcinogens. Mutat Res 49:337-354.
Ito N, Nagasaki H, Arai M, et al. 1973. Histologic and ultrastructural studies on the
hepatocarcinogenicity of benzene hexachloride in mice. J Natl Cancer Inst 51:817-826.
https://doi.org/10.1093/jnci/51.3.817.
Ito N, Nagasaki H, Aoe H, et al. 1975. Development of hepatocellular carcinomas in rats treated with
benzene hexachloride. J Natl Cancer Inst 54(3):801-805.
HEXACHLOROCYCLOHEXANE (HCH) 324
8. REFERENCES
Ito N, Hananouchi M, Sugihara S, et al. 1976. Reversibility and irreversibility of liver tumors in mice
induced by the α isomer of 1,2,3,4,5,6-hexachlorocyclohexane. Cancer Res 36(7 pt 1):2227-2234.
Itoh H, Iwasaki M, Kasuga Y, et al. 2014. Association between serum organochlorines and global
methylation level of leukocyte DNA among Japanese women: a cross-sectional study. Sci Total
Environ 490:603-609. https://doi.org/10.1016/j.scitotenv.2014.05.035.
Iverson F, Ryan JJ, Lizotte R, et al. 1984. In vivo and in vitro binding of α- and g-hexachloro-
cyclohexane to mouse liver macromolecules. Toxicology Letters 20:331-335.
Jacobsen BN, Nyholm N, Pedersen BM, et al. 1991. Microbial degradation of pentachlorophenol and
lindane in laboratory-scale activated sludge reactors. Water Sci Technol 23:349-356.
Jain P, Kapoor A, Rubeshkumar P, et al. 2022. Sudden deaths due to accidental leakage of Lindane from
a storage tank in a village, Sitapur, Uttar Pradesh, India, 2020: A field epidemiological investigation.
Environ Epidemiol 6(3):e213. https://doi.org/10.1097/ee9.0000000000000213.
Jantunen LM, Bidleman TF, Harner T, et al. 2000. Toxaphene, chlordane, and other organochlorine
pesticides in Alabama air. Environmental Science & Technology 34(24):5097-5105.
https://doi.org/10.1021/es001197y.
Jeddy Z, Kordas K, Allen K, et al. 2018. Prenatal exposure to organochlorine pesticides and early
childhood communication development in British girls. Neurotoxicology 69:121-129.
https://doi.org/10.1016/j.neuro.2018.10.003.
Jenssen D, Ramel C. 1980. The micronucleus test as part of a short-term mutagenicity test program for
the prediction of carcinogenicity evaluated by 143 agents tested. Mutat Res 75:191-202.
Johri A, Yadav S, Dhawan A, et al. 2007. Overexpression of cerebral and hepatic cytochrome P450s
alters behavioral activity of rat offspring following prenatal exposure to lindane. Toxicol Appl
Pharmacol 225(3):278-292. https://doi.org/10.1016/j.taap.2007.08.006.
Johri A, Yadav S, Dhawan A, et al. 2008. Responsiveness of cerebral and hepatic cytochrome P450s in
rat offspring prenatally exposed to lindane. Toxicol Appl Pharmacol 231(1):10-16.
https://doi.org/10.1016/j.taap.2008.03.019.
Jonnalagadda PR, Jahan P, Venkatasubramanian S, et al. 2012. Genotoxicity in agricultural farmers from
Guntur district of South India-A case study. Hum Exp Toxicol 31(7):741-747.
https://doi.org/10.1177/0960327111408151.
Joseph P, Shivanandappa T, Krishnakumari MK. 1992a. Influence of vitamin A on
hexachlorocyclohexane (HCH) toxicity in the rat. J Nutr Biochem 3:408-414.
https://doi.org/10.1016/0955-2863(92)90015-B.
Joseph P, Shivanandappa T, Narasimhamurthy K, et al. 1992b. Effect of vitamin A on
hexachlorocyclohexane (HCH) toxicity in the rat. Gen Pharmacol 23:1159-1164.
Joseph P, Viswanatha S, Krishnakumari MK. 1992c. Role of vitamin A in the haematotoxicity of
hexachlorocyclohexane (HCH) in the rat. J Environ Sci Health B 27(3):269-280.
https://doi.org/10.1080/03601239209372779.
Joy RM, Stark LG, Albertson TE. 1982. Proconvulsant effects of lindane: Enhancement of amygdaloid
kindling in the rat. Neurobehav Toxicol Teratol 4(3):347-354.
Juan WH, Yang LC, Hong HS. 2004. Acute generalized exanthematous pustulosis induced by topical
lindane. Dermatology 209(3):239-240. https://doi.org/10.1159/000079899.
Jung D, Becher H, Edler L, et al. 1997. Elimination of β-hexachlorocyclohexane in occupationally
exposed persons. J Toxicol Environ Health 51:23-34.
Junque E, Garcia S, Martinez MA, et al. 2020. Changes of organochlorine compound concentrations in
maternal serum during pregnancy and comparison to serum cord blood composition. Environ Res
182:108994. https://doi.org/10.1016/j.envres.2019.108994.
Just AC, Hawker DW, Connell DW. 1990. Partitioning of lindane between sediment, water, and the
crustacean Metapenaeus macleayi. Aust J Marine Freshwater Res 41:389-397.
Kachuri L, Beane Freeman LE, Spinelli JJ, et al. 2020. Insecticide use and risk of non-Hodgkin
lymphoma subtypes: A subset meta-analysis of the North American Pooled Project. Int J Cancer
147(12):3370-3383. https://doi.org/10.1002/ijc.33164.
HEXACHLOROCYCLOHEXANE (HCH) 325
8. REFERENCES
Kalantzi OI, Alcock RE, Johnston PA, et al. 2001. The global distribution of PCBs and organochlorine
pesticides in butter. Environ Sci Technol 35:1013-1018.
Kalantzi OI, Hewitt R, Ford KJ, et al. 2004. Low dose induction of micronuclei by lindane.
Carcinogenesis 25(4):613-622. https://doi.org/10.1093/carcin/bgh048.
Kalsch W, Knacker T, Robertz M, et al. 1998. Partitioning and mineralization of [
14
C]lindane in a
laboratory sediment-water system. Environmental Toxicology and Chemistry 17(4):662-669.
https://doi.org/10.1002/etc.5620170420.
Kamal El-Dein EM, Aness LM, Elsayed Aly SM. 2016. Effects of α-lipoic acid on γ-radiation and
lindane-induced heart toxicity in rats. Pakistan Journal of Zoology 48(5):1523-1529.
Kanja LW, Skaare JU, Ojwang SBO, et al. 1992. A comparison of organochlorine pesticide-residues in
maternal adipose-tissue, maternal blood, cord blood, and human-milk from mother infant pairs.
Arch Environ Contam Toxicol 22:21-24.
Kannan K, Battula S, Loganathan BG, et al. 2003. Trace organic contaminants, including toxaphene and
trifluralin, in cotton field soils from Georgia and South Carolina, USA. Arch Environ Contam
Toxicol 45(1):30-36. https://doi.org/10.1007/s00244-002-0267-7.
Kao CC, Que DE, Bongo SJ, et al. 2019. Residue levels of organochlorine pesticides in breast milk and
its associations with cord blood thyroid hormones and the offspring's neurodevelopment. Int J
Environ Res Public Health 16(8):1438. https://doi.org/10.3390/ijerph16081438.
Kar S, Singh PK. 1979a. Mutagenicity of pesticides carbofuran and hexachlorocyclohexane to blue-
green alga Nostoc muscorum. Microbios Lett 12:79-82.
Kar S, Singh PK. 1979b. Detoxification of pesticides carbofuran and hexachlorocyclohexane by blue-
green algae Nostoc muscorum and Wollea bharadwajae. Microbios Lett 10:111-114.
Karnik AB, Thakore KN, Nigam SR, et al. 1981. Studies on glucose-6-phosphatase, fructose-1,2-
diphosphatase activity, glycogen distribution and endoplasmic reticulum changes during
hexachlorocyclohexane induced hepato-carcinogenesis in pure inbred Swiss mice. Neoplasm
28:575-584.
Kashyap SK. 1986. Health surveillance and biological monitoring of pesticide formulators in India.
Toxicology Letters 33:107-114.
Kashyap SK, Nigam SK, Gupta RC, et al. 1979. Carcinogenicity of hexachlorocyclohexane (BHC) in
pure inbred Swiss mice. J Environ Sci Health B 14(3):305-318.
https://doi.org/10.1080/03601237909372130.
Katsumata K, Katsumata K. 2003. Norwegian scabies in an elderly patient who died after treatment with
γBHC. Intern Med 42(4):367-369. https://doi.org/10.2169/internalmedicine.42.367.
Katz JM, Winter CK. 2009. Comparison of pesticide exposure from consumption of domestic and
imported fruits and vegetables. Food Chem Toxicol 47(2):335-338.
https://doi.org/10.1016/j.fct.2008.11.024.
Kaur N, Starling AP, Calafat AM, et al. 2020. Longitudinal association of biomarkers of pesticide
exposure with cardiovascular disease risk factors in youth with diabetes. Environ Res 181:108916.
https://doi.org/10.1016/j.envres.2019.108916.
Keerthinarayana S, Bandyopadhyay M. 1998. Assessment of equilibrium time and effect of co-solutes in
lindane sorption. J Environ Sci Health 33(2):179-209. https://doi.org/10.1080/03601239809373138.
Keith LH, Garrison AW, Allen FR, et al. 1976. Identification and analysis of organic pollutants in
drinking water from 13 U.S. cities. In: Keith LH, ed. Identification and analysis of organic
pollutants in water. Ann Arbor, MI: Ann Arbor Science Publishers Inc, 329-373.
Kennedy DW, Aust SD, Bumpus JA. 1990. Comparative biodegradation of alkyl halide insecticides by
the white rot fungus, Phanerochaete chrysosporium (BKM-F-1767). Appl Environ Microbiol
56:2347- 2353.
Khanjani N, Sim MR. 2006. Reproductive outcomes of maternal contamination with cyclodiene
insecticides, hexachlorobenzene and β-benzene hexachloride. Sci Total Environ 368(2-3):557-564.
https://doi.org/10.1016/j.scitotenv.2006.03.029.
HEXACHLOROCYCLOHEXANE (HCH) 326
8. REFERENCES
Khanna RN, Das M, Anand M. 2002. Influence of phenobarbital and carbon tetrachloride on the
modulation of tissue retention profile of hexachlorocyclohexane in rats. Biomed Environ Sci
15(2):119-129.
Khanna RN, Anband M, Gopal K, et al. 1988. Effect of repeated exposure to lindane and cadmium on
lindane metabolism in rats. Toxicology Letters 42:177-182.
Khanna RN, Gupta R, Gupta GSD, et al. 1990. Effects of the level of dietary protein on the toxicity of
hexachlorocyclohexane in rats. Toxicol Environ Chem 25:91-103.
Khare S, Rizvi A, Shukla O, et al. 1977. Epidemic outbreak of neuro-ocular manifestations due to
chronic BHC poisoning. J Assoc Physicians India 25:215-222.
Khera KS, Whalen C, Trivett G, et al. 1979. Teratogenicity studies on pesticidal formulations of
dimethoate, diuron, and lindane in rats. Bull Environ Contam Toxicol 22(4-5):522-529.
https://doi.org/10.1007/BF02026981.
Khurana R, Mahipal S, Chauhan R. 1999. Effect of pesticides on delayed type hypersensitivity reaction
in sheep. Indian Journal of Animal Sciences 69(11):0367-8318.
Kim S, Park J, Kim HJ, et al. 2013. Association between several persistent organic pollutants and
thyroid hormone levels in serum among the pregnant women of Korea. Environ Int 59:442-448.
https://doi.org/10.1016/j.envint.2013.07.009.
Kim KS, Lee YM, Kim SG, et al. 2014. Associations of organochlorine pesticides and polychlorinated
biphenyls in visceral vs. subcutaneous adipose tissue with type 2 diabetes and insulin resistance.
Chemosphere 94:151-157. https://doi.org/10.1016/j.chemosphere.2013.09.066.
Kim KS, Lee YM, Lee HW, et al. 2015. Associations between organochlorine pesticides and cognition
in U.S. elders: National Health and Nutrition Examination Survey 1999-2002. Environ Int 75:87-92.
https://doi.org/10.1016/j.envint.2014.11.003.
Kiraly J, Szentesi I, Ruzicska M, et al. 1979. Chromosome studies in workers producing
organophosphate insecticides. Arch Environ Contam Toxicol 8:309-319.
Kirk ER, Othmer DF. 1985. Benzene hexachloride. In: Concise encyclopedia of chemical technology.
New York, NY: John Wiley & Sons, 269-270.
Klonne DR, Kintigh WJ. 1988. Lindane technical: Fourteen-week dust aerosol inhalation study on mice.
Export, PA: Bushy Run Research Center. BRRC #51-524. MetPath #14014.
Knap AH, Binkley KS. 1991. Chlorinated organic compounds in the troposphere over the western North
Atlantic Ocean measured by aircraft. Atmos Environ 25:1507-1516.
Kokroko J, Kogut K, Harley K, et al. 2020. Prenatal β-hexachlorocyclohexane (β-HCH) exposure and 7-
year child IQ in the CHAMACOS birth cohort. Neurotox Res 37(3):553-563.
https://doi.org/10.1007/s12640-020-00160-w.
Koner BC, Banerjee BD, Ray A. 1998. Organochlorine pesticide-induced oxidative stress and immune
suppression in rats. Indian J Exp Biol 36(4):395-398.
Kopec-Szlezak J, Goralczyk K, Wozniak J, et al. 1989. Changes in serum and internal organs during
increased accumulation of gamma-hexachlorocyclohexane in adipose tissue of rabbits. Mater Med
Pol 21:286-291.
Kornvig S, Wielsoe M, Long M, et al. 2021. Prenatal exposure to persistent organic pollutants and
metals and problematic child behavior at 3-5 years of age: a Greenlandic cohort study. Sci Rep
11(1):22182. https://doi.org/10.1038/s41598-021-01580-0.
Koutros S, Andreotti G, Berndt SI, et al. 2011. Xenobiotic-metabolizing gene variants, pesticide use, and
the risk of prostate cancer. Pharmacogenet Genomics 21(10):615-623.
https://doi.org/10.1097/FPC.0b013e3283493a57.
Kováčik J, Antoš V, Micalizzi G, et al. 2018. Accumulation and toxicity of organochlorines in green
microalgae. J Hazard Mater 347:168-175. https://doi.org/10.1016/j.jhazmat.2017.12.056.
Kramer MS, Hutchison TA, Rudnick SA, et al. 1980. Operational criteria for adverse drug reactions in
evaluating suspected toxicity of a popular scabicide. Clin Pharmacol Ther 27:149-155.
HEXACHLOROCYCLOHEXANE (HCH) 327
8. REFERENCES
Krishnan K, Andersen ME, Clewell HJ, et al. 1994. Physiologically based pharmacokinetic modeling of
chemical mixtures. In: Yang RSH, ed. Toxicology of chemical mixtures: Case studies,
mechanisms, and novel approaches. San Diego, CA: Academic Press, 399-437.
Kujawa M, Engst R, Macholz R. 1977. On the metabolism of lindane. In: Zaidi SH, ed. Environmental
pollution and human health: Proceedings of the international symposium on industrial toxicology,
November 4-7, 1975. Lucknow: Industrial Toxicology Research Centre, 661-672.
Kumar D, Khan PK, Sinha SP. 1995. Cytogenetic toxicity and no-effect limit dose of pesticides. Food
Chem Toxicol 33:309-314.
Kumar V, Yadav CS, Singh S, et al. 2010. CYP 1A1 polymorphism and organochlorine pesticides levels
in the etiology of prostate cancer. Chemosphere 81(4):464-468.
https://doi.org/10.1016/j.chemosphere.2010.07.067.
Kuntz KW, Warry ND. 1983. Chlorinated organic contaminants in water and suspended sediments of
the lower Niagara River. J Great Lakes Res 9:241-248.
Kurihara N, Tanaka K, Nakajima M. 1979. Mercapturic acid formation from lindane in rats. Pestic
Biochem Physiol 10:137-150.
Kurihara N, Uchida M, Fujita T, et al. 1973. Studies on BHC isomers and related compounds. V. Some
physicochemical properties of BHC isomers. Pesticide Biochemistry and Physiology 2(4):383-390.
https://doi.org/10.1016/0048-3575(73)90050-3.
Kutz FW, Strassman SC, Spearling JF. 1979. Survey of selected organochlorine pesticides in the general
population of the United States: Fiscal years 1970-1975. Ann NY Acad Sci 320:60-68.
Kutz FW, Wood PH, Bottimore DP. 1991. Organochlorine pesticides and polychlorinated biphenyls in
human adipose tissue. Rev Environ Contam Toxicol 120:1-82.
La Sala G, Farini D, De Felici M. 2009. Proapoptotic effects of lindane on mouse primordial germ cells.
Toxicol Sci 108(2):445-451. https://doi.org/10.1093/toxsci/kfp027.
Lahiri P, Chakravarty J, Sircar S. 1990. Residue accumulation in mice chronically fed lindane (g-HCH).
Proc Indian Natl Sci Acad Part B Biol Sci 56:277-280.
Lakkad BC, Nigam SK, Karnik AB, et al. 1982. Dominant-lethal study of technical-grade
hexachlorocyclohexane in Swiss mice. Mutat Res 101:315-320.
Lam T, Williams PL, Lee MM, et al. 2014. Prepubertal organochlorine pesticide concentrations and age
of pubertal onset among Russian boys. Environ Int 73:135-142.
https://doi.org/10.1016/j.envint.2014.06.020.
Lam T, Williams PL, Lee MM, et al. 2015. Prepubertal serum concentrations of organochlorine
pesticides and age at sexual maturity in Russian boys. Environ Health Perspect 123(11):1216-1221.
https://doi.org/10.1289/ehp.1409022.
Landgren O, Kyle RA, Hoppin JA, et al. 2009. Pesticide exposure and risk of monoclonal gammopathy
of undetermined significance in the Agricultural Health Study. Blood 113(25):6386-6391.
https://doi.org/10.1182/blood-2009-02-203471.
Lange M, Nitzche K, Zesch A. 1981. Percutaneous absorption of lindane by healthy volunteers and
scabies patients: Dependency of penetration kinetics in serum upon frequency of application, time,
and mode of washing. Arch Dermatol Res 271:387-399.
Lapertot ME, Pulgarin C. 2006. Biodegradability assessment of several priority hazardous substances:
choice, application and relevance regarding toxicity and bacterial activity. Chemosphere 65(4):682-
690. https://doi.org/10.1016/j.chemosphere.2006.01.046.
Laug EP, Nelson AA, Fitzhugh OG, et al. 1950. Liver cell alteration and DDT storage in the fat of the
rat induced by dietary levels of 1 to 50 p.p.m. DDT. Journal of Pharmacology and Experimental
Therapeutics 98(3):268-273.
Lauritzen HB, Larose TL, Oien T, et al. 2018. Prenatal exposure to persistent organic pollutants and
child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health 17(1):9.
https://doi.org/10.1186/s12940-017-0338-x.
HEXACHLOROCYCLOHEXANE (HCH) 328
8. REFERENCES
Laws SC, Carey SA, Hart DW, et al. 1994. Lindane does not alter the estrogen receptor or the estrogen-
dependent induction of progesterone receptors in sexually immature or ovariectomized adult rats.
Toxicology 92:127-142.
Law SA, Bidleman TF, Martin MJ, et al. 2004. Evidence of enantioselective degradation of α-
hexachlorocyclohexane in groundwater. Environ Sci Technol 38(6):1633-1638.
https://doi.org/10.1021/es030508c.
Lebov JF, Engel LS, Richardson D, et al. 2015. Pesticide exposure and end-stage renal disease risk
among wives of pesticide applicators in the Agricultural Health Study. Environ Res 143(Pt A):198-
210. https://doi.org/10.1016/j.envres.2015.10.002.
Lee B, Groth P. 1977. Scabies: Transcutaneous poisoning during treatment [letter]. Pediatrics 59:643.
Lee DH, Jacobs DR, Porta M. 2007. Association of serum concentrations of persistent organic pollutants
with the prevalence of learning disability and attention deficit disorder. J Epidemiol Community
Health 61(7):591-596. https://doi.org/10.1136/jech.2006.054700.
Lee HA, Park SH, Hong YS, et al. 2016. The effect of exposure to persistent organic pollutants on
metabolic health among Korean children during a 1-year follow-up. Int J Environ Res Public Health
13(3):270. https://doi.org/10.3390/ijerph13030270.
Lee YM, Ha CM, Kim SA, et al. 2017. Low-dose persistent organic pollutants impair insulin secretory
function of pancreatic β-cells: Human and in vitro evidence. Diabetes 66(10):2669-2680.
https://doi.org/10.2337/db17-0188.
Lee YM, Kim SA, Choi GS, et al. 2018a. Association of colorectal polyps and cancer with low-dose
persistent organic pollutants: A case-control study. PLoS One 13(12):e0208546.
https://doi.org/10.1371/journal.pone.0208546.
Lee JY, Lee KM, Lee DH, et al. 2018b. Association of low-dose exposure to persistent organic
pollutants with E-cadherin promoter methylation in healthy Koreans. Biomarkers 23(3):293-298.
https://doi.org/10.1080/1354750X.2017.1417482.
Leeder JS, Kearns GL. 1997. Pharmacogenetics in pediatrics: Implications for practice. Ped Clin North
America 44:55-77.
Lenters V, Iszatt N, Forns J, et al. 2019. Early-life exposure to persistent organic pollutants (OCPs,
PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a
Norwegian birth cohort. Environ Int 125:33-42. https://doi.org/10.1016/j.envint.2019.01.020.
Lerro CC, Jones RR, Langseth H, et al. 2018. A nested case-control study of polychlorinated biphenyls,
organochlorine pesticides, and thyroid cancer in the Janus Serum Bank cohort. Environ Res
165:125-132. https://doi.org/10.1016/j.envres.2018.04.012.
Lerro CC, Beane Freeman LE, DellaValle CT, et al. 2021. Pesticide exposure and incident thyroid
cancer among male pesticide applicators in agricultural health study. Environ Int 146:106187.
https://doi.org/10.1016/j.envint.2020.106187.
Lewis RG, Lee RE. 1976. Air pollution from pesticides: Sources, occurence [sic], and dispersion. In:
Lee RE, ed. Air pollution from pesticides and agricultural processes. Cleveland, OH: CRC Press,
5-50.
Li J, Jiang S, Chang Y, et al. 2013. Association among serum organochlorine pesticide residues,
glutathione S-transferase M1 genetic polymorphism and female breast cancer. Adv Breast Cancer
Res 2:19-23. https://doi.org/10.4236/abcr.2013.22005.
Li C, Cheng Y, Tang Q, et al. 2014. The association between prenatal exposure to organochlorine
pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ Res 129:47-51.
https://doi.org/10.1016/j.envres.2013.12.009.
Li S, Wang X, Yang L, et al. 2016. Interaction between β-hexachlorocyclohexane and ADIPOQ
genotypes contributes to the risk of type 2 diabetes mellitus in East Chinese adults. Sci Rep 6:37769.
https://doi.org/10.1038/srep37769.
Lide DR. 1991. Hexachlorocyclohexane. In: CRC handbook of chemistry and physics: A ready-
reference book of chemical and physical data. Boston, MA: CRC Press, 35-195.
Lifshitz M, Gavrilov V. 2002. Acute lindane poisoning in a child. Isr Med Assoc J 4(9):731-732.
HEXACHLOROCYCLOHEXANE (HCH) 329
8. REFERENCES
Lim JE, Nam C, Yang J, et al. 2017. Serum persistent organic pollutants (POPs) and prostate cancer
risk: A case-cohort study. Int J Hyg Environ Health 220(5):849-856.
https://doi.org/10.1016/j.ijheh.2017.03.014.
Lindenau A, Fischer B, Seiler P, et al. 1994. Effects of persistent chlorinated hydrocarbons on
reproductive tissues in female rabbits. Hum Reprod 9(5):772-780.
https://doi.org/10.1093/oxfordjournals.humrep.a138595.
Liu PT, Morgan DP. 1986. Comparative toxicity and biotransformation of lindane in C57BL/6 and
DBA/2 mice. Life Sci 39:1237-1244. https://doi.org/10.1016/0024-3205(86)90184-0.
Llorens J, Tusell JM, Sunol C, et al. 1989. Effects of lindane on spontaneous behavior of rats analyzed
by multivariate statistics. Neurotoxicol Teratol 11:145-151. https://doi.org/10.1016/0892-
0362(89)90053-6.
Llorens J, Tusell JM, Sunol C, et al. 1990. On the effects of lindane on the plus-maze model of anxiety.
Nerutoxicol Teratol 12:643-647. https://doi.org/10.1016/0892-0362(90)90078-Q.
Loganathan BG, Tanabe S, Hidaka Y, et al. 1993. Temporal trends of persistent organochlorine residues
in human adipose tissue from Japan, 1928-1985. Environ Pollut 81:31-39.
Loge JP. 1965. Aplastic anemia following exposure to benzene hexachloride (lindane). JAMA 193:104-
108.
Lopez-Carrillo L, Lopez-Cervantes M, Torres-Sanchez L, et al. 2002. Serum levels of beta-
hexachlorocyclohexane, hexachlorobenzene and polychlorinated biphenyls and breast cancer in
Mexican women. Eur J Cancer 11(2):129-135.
Lopez-Espinosa MJ, Granada A, Carreno J, et al. 2007. Organochlorine pesticides in placentas from
southern Spain and some related factors. Placenta 28(7):631-638.
https://doi.org/10.1016/j.placenta.2006.09.009.
Lopez-Espinosa MJ, Vizcaino E, Murcia M, et al. 2010. Prenatal exposure to organochlorine compounds
and neonatal thyroid stimulating hormone levels. J Expo Sci Environ Epidemiol 20(7):579-588.
https://doi.org/10.1038/jes.2009.47.
Lopez-Espinosa MJ, Murcia M, Iniguez C, et al. 2011. Prenatal exposure to organochlorine compounds
and birth size. Pediatrics 128(1):e127-e134. https://doi.org/10.1542/peds.2010-1951.
Luebeck EG, Graslkraupp B, Timmermanntrosiener I, et al. 1995. Growth kinetics of enzyme-altered
liver foci in rats treated with phenobarbital or α-hexachlorocyclohexane. Toxicology and Applied
Pharmacology 130(2):304-315. https://doi.org/10.1006/taap.1995.1035.
Macholz RM, Kujawa M. 1985. Recent state of lindane metabolism: Part III. Res Rev 94:119-149.
Macholz RM, Knoll R, Lewerenz HJ, et al. 1982a. Metabolism of alpha-hexachlorocyclohexane: Free
metabolites in urine and organs of rats. Xenobiotica 12(4):277-231.
Macholz RM, Knoll R, Lewerenz HJ, et al. 1982b. Biodegradation of beta-hexachlorocyclohexane: Free
metabolites in rat urine and organs. Arch Toxicol 50:85-88.
Mackay D, Leinonen PJ. 1975. Rate of evaporation of low-solubility contaminants from water bodies to
atmosphere. Environ Sci Technol 9:1178-1180.
Madrigal JM, Sargis RM, Persky V, et al. 2021. Multiple organochlorine pesticide exposures and
measures of sex steroid hormones in adult males: Cross-sectional findings from the 1999-2004
National Health and Nutrition Examination Survey. Int J Hyg Environ Health 231:113609.
https://doi.org/10.1016/j.ijheh.2020.113609.
Magliano DJ, Ranciere F, Slama R, et al. 2021. Exposure to persistent organic pollutants and the risk of
type 2 diabetes: a case-cohort study. Diabetes Metab 47(5):101234.
https://doi.org/10.1016/j.diabet.2021.101234.
Malaiyandi M, Muzika K, Benoit FM. 1982. Isomerization of γ-hexachlorocyclohexane to its α-isomer
by ultra-violet light irradiation. J Environ Sci Health A 17:299-311.
Maranghi F, Rescia M, Macrì C, et al. 2007. Lindane may modulate the female reproductive
development through the interaction with ER-β: an in vivo-in vitro approach. Chem Biol Interact
169(1):1-14. https://doi.org/10.1016/j.cbi.2007.04.008.
HEXACHLOROCYCLOHEXANE (HCH) 330
8. REFERENCES
Marks KJ, Howards PP, Smarr MM, et al. 2021. Prenatal exposure to mixtures of persistent endocrine
disrupting chemicals and early menarche in a population-based cohort of British girls. Environ
Pollut 276:116705. https://doi.org/10.1016/j.envpol.2021.116705.
Marsalek J, Schroeter H. 1988. Annual loadings of toxic contaminants in urban runoff from the
Canadian Great Lakes basin. Water Pollut Res J Can 23:360-378.
Martinez AO, Martinez-Conde E. 1995. The neurotoxic effects of lindane at acute and subchronic
dosages. Ecotoxicol Environ Saf 30:101-105. https://doi.org/10.1006/eesa.1995.1011.
Martinez E, de Vera N, Artigas F. 1991. Differential response of rat brain polyamines to convulsant
agents. Life Sci 48:77-84. https://doi.org/10.1016/0024-3205(91)90427-D.
Marvin C, Painter S, Williams D, et al. 2004. Spatial and temporal trends in surface water and sediment
contamination in the Laurentian Great Lakes. Environ Pollut 129(1):131-144.
https://doi.org/10.1016/j.envpol.2003.09.029.
Mathur AK, Narang S, Gupta BN, et al. 1992. Effect of dermal exposure to LAS detergent and HCH
pesticide in guinea pigs: Biochemical and histopathologic changes in liver and kidney. J Toxicol
Cutaneous Ocul Toxicol 11(1):3-13. https://doi.org/10.3109/15569529209042589.
Mathur AK, Narang S, Gupta BN, et al. 1993. Interaction of linear alkylbenzene sulfonate and
hexachlorocyclohexane in guinea pigs after dermal application. J Toxicol Cutaneous Ocul Toxicol
12(1):25-34. https://doi.org/10.3109/15569529309057656.
Matsumura F, Benezet HJ. 1973. Studies on the bioaccumulation and microbial degradation of 2,3,7,8-
tetrachlorodibenzo-p-dioxin. Environ Health Perspect 5:253-258.
Matsuoka LY. 1981. Convulsions following application of gamma-benzene hexachloride [letter]. J Am
Acad Dermatol 5:98-99.
Matsuura I, Saitoh T, Tani E, et al. 2005. Evaluation of a two-generation reproduction toxicity study
adding endpoints to detect endocrine disrupting activity using lindane. J Toxicol Sci 30(Special
Issue):S135-S161. https://doi.org/10.2131/jts.30.s135.
Mattioli F, Robbiano L, Adamo D, et al. 1996. Genotoxic effects of α-hexachlorocyclohexane in primary
cultures of rodent and human hepatocytes. Mutagenesis 11(1):79-83.
McCarthy JP, Adinolfi J, McMullin SL, et al. 1992. NCA survey of pesticide residues in brewed coffees.
Colloq Sci Int Cafe 14:175-181.
McCready D, Aronson KJ, Chu W, et al. 2004. Breast tissue organochlorine levels and metabolic
genotypes in relation to breast cancer risk Canada. Cancer Causes Control 15(4):399-418.
https://doi.org/10.1023/B:CACO.0000027505.32564.c2.
McGlynn KA, Quraishi SM, Graubard BI, et al. 2008. Persistent organochlorine pesticides and risk of
testicular germ cell tumors. J Natl Cancer Inst 100(9):663-671. https://doi.org/10.1093/jnci/djn101.
McNamara BP, Krop S. 1948. Observations on the pharmacology of the isomers of
hexachlorocyclohexane. J Pharmacol Exp Ther 92:140-146.
McQueen EG, Brosnan C, Ferry DG. 1968. Poisoning from a rose spray containing lindane and
malathion. N Z Med J 67:533-537.
McTernan WF, Pereira JA. 1991. Biotransformation of lindane and 2,4-D in batch enrichment cultures.
Water Res 25:1417-1423.
Medehouenou TCM, Ayotte P, Carmichael PH, et al. 2019. Exposure to polychlorinated biphenyls and
organochlorine pesticides and risk of dementia, Alzheimer's disease and cognitive decline in an older
population: a prospective analysis from the Canadian Study of Health and Aging. Environ Health
18(1):57. https://doi.org/10.1186/s12940-019-0494-2.
Mediratta PK, Tanwar K, Reeta KH, et al. 2008. Attenuation of the effect of lindane on immune
responses and oxidative stress by Ocimum sanctum seed oil (OSSO) in rats. Indian J Physiol
Pharmacol 52(2):171-177.
Meera P, Rao PR, Shanker R, et al. 1992. Immunomodulatory effects of γ-HCH (lindane) in mice.
Immunopharmacol Immunotoxicol 14(1-2):261-282. https://doi.org/10.3109/08923979209009224.
Melancon SM, Pollard JE, Hern SC. 1986. Evaluation of SESOIL, PRZM and PESTAN in a laboratory
column leaching experiment. Environ Toxicol Chem 5:865-878.
HEXACHLOROCYCLOHEXANE (HCH) 331
8. REFERENCES
Mendeloff AI, Smith DE. 1955. Exposure to insecticides, bone marrow failure, gastrointestinal bleeding,
and uncontrollable infections. Am J Med 9:274-284.
Mendez MA, Garcia-Esteban R, Guxens M, et al. 2011. Prenatal organochlorine compound exposure,
rapid weight gain, and overweight in infancy. Environ Health Perspect 119(2):272-278.
https://doi.org/10.1289/ehp.1002169.
Meng G, Feng Y, Nie Z, et al. 2016. Internal exposure levels of typical POPs and their associations with
childhood asthma in Shanghai, China. Environ Res 146:125-135.
https://doi.org/10.1016/j.envres.2015.12.026.
Mes J. 1992. Organochlorine residues in human blood and biopsy fat and their relationship. Bull
Environ Contam Toxicol 48:815-820.
Mes J, Malcolm S. 1992. Comparison of chlorinated hydrocarbon residues in human populations from
the Great Lakes and other regions of Canada. Chemosphere 25:417-424.
Miao Y, Rong M, Li M, et al. 2021. Serum concentrations of organochlorine pesticides, biomarkers of
oxidative stress, and risk of breast cancer. Environ Pollut 286:117386.
https://doi.org/10.1016/j.envpol.2021.117386.
Miao Y, Zeng JY, Rong M, et al. 2022. Organochlorine pesticide exposures, metabolic enzyme genetic
polymorphisms and semen quality parameters among men attending an infertility clinic.
Chemosphere 303(Pt 1):135010. https://doi.org/10.1016/j.chemosphere.2022.135010.
Michalakis M, Tzatzarakis M, Alegakis A, et al. 2012. Pesticides levels (DDTs, HCHs and DAPs) in
blood and hair samples of children diagnosed with hypospadias. Toxicology Letters 211:S187.
https://doi.org/10.1016/j.toxlet.2012.03.673.
Mill T. 1999. Predicting photoreaction rates in surface waters. Chemosphere 38:1379-1390.
Min JY, Cho JS, Lee KJ, et al. 2011. Potential role for organochlorine pesticides in the prevalence of
peripheral arterial diseases in obese persons: results from the National Health and Nutrition
Examination Survey 1999-2004. Atherosclerosis 218(1):200-206.
https://doi.org/10.1016/j.atherosclerosis.2011.04.044.
Minh NH, Someya M, Minh TB, et al. 2004. Persistent organochlorine residues in human breast milk
from Hanoi and Hochiminh City, Vietnam: contamination, accumulation kinetics and risk
assessment for infants. Environ Pollut 129(3):431-441.
https://doi.org/10.1016/j.envpol.2003.11.012.
Mladenović D, Hrncić D, Vucević D, et al. 2007. Ethanol suppressed seizures in lindane-treated rats.
Electroencephalographic and behavioral studies. J Physiol Pharmacol 58(4):641-656.
Mørck TA, Erdmann SE, Long M, et al. 2014. PCB concentrations and dioxin-like activity in blood
samples from Danish school children and their mothers living in urban and rural areas. Basic Clin
Pharmacol Toxicol 115(1):134-144. https://doi.org/10.1111/bcpt.12214.
Morello-Frosch R, Cushing LJ, Jesdale BM, et al. 2016. Environmental chemicals in an urban
population of pregnant women and their newborns from San Francisco. Environ Sci Technol
50(22):12464-12472. https://doi.org/10.1021/acs.est.6b03492.
Morgan DP, Lin LI. 1978. Blood organochlorine pesticide concentrations, clinical hematology, and
biochemistry in workers occupationally exposed to pesticides. Arch Environ Contam Toxicol 7:423-
447.
Morgan DP, Roberts RJ, Walter AW, et al. 1980. Anemia associated with exposure to lindane. Arch
Environ Health 35:307-310.
Morgan MK, Wilson NK, Chuang JC. 2014. Exposures of 129 preschool children to organochlorines,
organophosphates, pyrethroids, and acid herbicides at their homes and daycares in North Carolina.
Int J Environ Res Public Health 11(4):3743-3764. https://doi.org/10.3390/ijerph110403743.
Moriya M, Ohta T, Watanabe K, et al. 1983. Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat Res 116:185-216.
Mortazavi N, Asadikaram G, Ebadzadeh MR, et al. 2019. Organochlorine and organophosphorus
pesticides and bladder cancer: A case-control study. J Cell Biochem 120(9):14847-14859.
https://doi.org/10.1002/jcb.28746.
HEXACHLOROCYCLOHEXANE (HCH) 332
8. REFERENCES
Mougin C, Pericaud C, Malosse C, et al. 1996. Biotransformation of the insecticide lindane by the white
rot basidiomycete Phanerochaete chrysosporium. Pestic Sci 47:51-59.
Mougin C, Pericaud C, Dubroca J, et al. 1997. Enhanced mineralization of lindane in soils supplemented
with the white rot basidiomycete Phanerochaete chrysosporium. Soil Biol Biochem 29(9):1321-
1324.
Mudawal A, Srivastava A, Singh A, et al. 2018. Proteomic approaches to investigate age related
vulnerability to lindane induced neurodegenerative effects in rats. Food Chem Toxicol 115:499-510.
https://doi.org/10.1016/j.fct.2018.03.049.
Muller D, Klepel H, Macholz RM, et al. 1981. Electroneurophysiological studies on neurotoxic effects
of hexachlorocyclohexane isomers and gamma-pentachlorocyclohexene. Bull Environ Contam
Toxicol 27(5):704-706. https://doi.org/10.1007/BF01611085.
Mumtaz MM, Ray M, Crowell SR, et al. 2012a. Translational research to develop a human PBPK
models tool kit-volatile organic compounds (VOCs). J Toxicol Environ Health A 75(1):6-24.
https://doi.org/10.1080/15287394.2012.625546.
Mumtaz M, Fisher J, Blount B, et al. 2012b. Application of physiologically based pharmacokinetic
models in chemical risk assessment. J Toxicol 2012:904603. https://doi.org/10.1155/2012/904603.
Munir KM, Soman CS, Bhide SV. 1983. Hexachlorocyclohexane-induced tumorigenicity in mice under
different experimental conditions. Tumori 69:383-386.
https://doi.org/10.1177/030089168306900503.
Munk ZM, Nantel A. 1977. Acute lindane poisoning with development of muscle necrosis. Can Med
Assoc J 117:1050-1054.
Murli H. 1990. Lindane (technical): In an in vitro cytogenetic assay measuring chromosomal aberration
frequencies in Chinese Hamster Ovary (CHO) cells with multiple harvests under conditions of
metabolic activation. Kensington, MD: Hazelton Laboratories America, Inc. HLA Study No.
12024-0-437C.
Murphy R, Harvey C. 1985. Residues and metabolites of selected persistent halogenated hydrocarbons
in blood specimens from a general population survey. Environ Health Perspect 60:115-120.
Mustafa MD, Banerjee BD, Ahmed RS, et al. 2013. Gene-environment interaction in preterm delivery
with special reference to organochlorine pesticides. Mol Hum Reprod 19(1):35-42.
https://doi.org/10.1093/molehr/gas039.
Mustieles V, Fernandez MF, Martin-Olmedo P, et al. 2017. Human adipose tissue levels of persistent
organic pollutants and metabolic syndrome components: Combining a cross-sectional with a 10-year
longitudinal study using a multi-pollutant approach. Environ Int 104:48-57.
https://doi.org/10.1016/j.envint.2017.04.002.
Naeher LP, Barr DB, Rithmire N, et al. 2009. Pesticide exposure resulting from treatment of lice
infestation in school-aged children in Georgia. Environ Int 35(2):358-362.
https://doi.org/10.1016/j.envint.2008.09.001.
Nagaraja TN, Desiraju T. 1994. Brain regional variations in the levels of biogenic amines, glutamate,
GABA and glutamate decarboxylase activity in developing and adult rats exposed chronically to
hexachlorocyclohexane. Biogenic Amines 10:141-149.
Nagasaki H, Kawabata H, Miyata K, et al. 1975. Effect of various factors on induction of liver tumors in
animals by the α-isomer of benzene hexachloride. Gann 66(2):185-191.
https://doi.org/10.20772/cancersci1959.66.2_185.
Nagda G, Bhatt DK. 2011. Alleviation of lindane induced toxicity in testis of Swiss mice (Mus
musculus) by combined treatment with vitamin C, vitamin E and α-lipoic acid. Indian J Exp Biol
49(3):191-199.
Nagy Z, Mile I, Antoni F. 1975. The mutagenic effect of pesticides on Escherichia coli WP2 try. Acta
Microbiol Acad Sci Hung 22(3):309-314.
Nair A, Mandapati R, Dureja P, et al. 1996. DDT and HCH load in mothers and their infants in Delhi,
India. Bull Environ Contam Toxicol 56:58-64.
HEXACHLOROCYCLOHEXANE (HCH) 333
8. REFERENCES
Namulanda G, Maisonet M, Taylor E, et al. 2016. In utero exposure to organochlorine pesticides and
early menarche in the Avon Longitudinal Study of parents and children. Environ Int 94:467-472.
https://doi.org/10.1016/j.envint.2016.06.001.
Nantel A, Ayotte L, Benedatti JL, et al. 1977. A group of adults acutely poisoned by food contaminated
with lindane. Acta Pharmacol Toxicol 41(Suppl 2):250.
NAS/NRC. 2006. Human biomonitoring for environmental chemicals. Washington, DC: The National
Academies Press, National Research Council. https://doi.org/10.17226/11700.
NCI. 1977. Bioassay of lindane for possible carcinogenicity. Bethesda, MD: National Cancer Institute.
DHEW publication no. (NIH) 77-814.
https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr014.pdf. August 4, 2023.
Neidert E, Saschenbrecker PW. 1996. Occurrence of pesticide residues in selected agricultural food
commodities available in Canada. J AOAC Int 79:549-566.
Neururer H, Womastek R. 1991. [Pesticides in the air]. Bodenkultur 42:57-70. (German)
Nigam SK, Lakkad BC, Karnick AB, et al. 1979. Effect of hexachlorocyclohexane feeding on testicular
tissue of pure inbred Swiss mice. Bull Environ Contam Toxicol 23(4-5):431-437.
https://doi.org/10.1007/BF01769983.
Nigam SK, Karnik AB, Majumder SK, et al. 1986. Serum hexachlorocyclohexane residues in workers
engaged at a HCH manufacturing plant. Int Arch Occup Environ Health 57:315-320.
NIOSH. 1994. Lindane. Immediately dangerous to life or health concentrations (IDLH). National
Institute for Occupational Safety and Health. https://www.cdc.gov/niosh/idlh/58899.html. March
23, 2021.
NIOSH. 2019. Lindane. NIOSH pocket guide to chemical hazards. National Institute for Occupational
Safety and Health. https://www.cdc.gov/niosh/npg/npgd0370.html. March 23, 2021.
NLM. 2021. PubChem: Lindane. National Library of Medicine.
https://pubchem.ncbi.nlm.nih.gov/compound/727. May 11, 2021.
Noegrohati S, Hammers WE. 1992. Sorption-desorption kinetics of some organochlorine insecticides in
silt-water suspensions. Toxicol Environ Chem 34:187-206.
Nordmeyer H, Pestemer W, Rahman A. 1992. Sorption and transport behavior of some pesticides in
groundwater sediments. Stygologia 7:5-11.
Nordt SP, Chew G. 2000. Acute lindane poisoning in three children. J Emerg Med 18(1):51-53.
NTP. 1984. National Toxicology Program fiscal year 1984 annual plan. Research Triangle Park, NC:
National Toxicology Program.
NTP. 2013. Draft OHAT approach for systematic review and evidence integration for literature-based
health assessments – February 2013. National Toxicology Program.
https://ntp.niehs.nih.gov/ntp/ohat/evaluationprocess/draftohatapproach_february2013.pdf. May 5,
2021.
NTP. 2015. OHAT risk of bias rating tool for human and animal studies. National Toxicology Program.
https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf. March 19, 2019.
NTP. 2021. Lindane, hexachlorocyclohexane (technical grade), and other hexachlorocyclohexane
isomers. In: Report on carcinogens. 15
th
ed. National Toxicology Program,
https://ntp.niehs.nih.gov/sites/default/files/ntp/roc/content/profiles/lindane.pdf. July 16, 2023.
Nybom N, Knutsson B. 1947. Investigations on C-mitosis in Allium cepa. Hereditus 33:220-234.
Nyholm N, Jacobsen BN, Pedersen BM, et al. 1992. Removal of organic micropollutants at ppb levels in
laboratory activated-sludge reactors under various operating-conditions: biodegradation. Water Res
26:339-353.
Ociepa-Zawal M, Rubis B, Wawrzynczak D, et al. 2010. Accumulation of environmental estrogens in
adipose tissue of breast cancer patients. J Environ Sci Health A Tox Hazard Subst Environ Eng
45(3):305-312. https://doi.org/10.1080/10934520903468038.
Oesch F, Friedberg T, Herbst M, et al. 1982. Effects of lindane treatment on drug metabolizing enzymes
and liver weight of CF1 mice in which it evoked hepatomas and in non-susceptible rodents. Chem
Biol Interact 40:1-14.
HEXACHLOROCYCLOHEXANE (HCH) 334
8. REFERENCES
Oldiges H, Takenaka S, Hochrainer D. 1980. Inhalation study with lindane (γ-hexachlorocyclohexane)
to determine the LC50. Schmallenberg, Germany: Fraunhofer-Institut, Institute for Toxicology and
Aerosol Research. Celamerck Document No. 111AA-423-002.
Oldiges H, Hertel R, Kördel W, et al. 1983. 90-day inhalation study with lindane. Schmallenberg,
Germany: Fraunhofer-Institut, Institute for Toxicology and Aerosol Research. Celamerck
Document No. 111AC-435-005.
Olgun S, Misra HP. 2006. Pesticides induced oxidative stress in thymocytes. Mol Cell Biochem 290(1-
2):137-144. https://doi.org/10.1007/s11010-006-9178-7.
Olgun S, Gogal RM, Adeshina F, et al. 2004. Pesticide mixtures potentiate the toxicity in murine
thymocytes. Toxicology 196(3):181-195. https://doi.org/10.1016/j.tox.2003.09.007.
Oliver BG, Charlton MN. 1984. Chlorinated organic contaminants on settling particulates in the Niagara
River vicinity of Lake Ontario. Environ Sci Technol 18:903-908.
Orr JW. 1948. Absence of carcinogenic activity of benzene hexachloride (‘gammexane'). Nature
162(4109):189-189. https://doi.org/10.1038/162189a0.
Ortega P, Hayes WJ, Durham WF. 1957. Pathologic changes in the liver of rats after feeding low levels
of various insecticides. AMA Arch Pathol 64:614-622.
OSHA. 2021a. Occupational safety and health standards. Subpart Z - Toxic and hazardous substances.
Air contaminants. Table Z-1: Limits for air contaminants. Occupational Safety and Health
Administration. Code of Federal Regulations. 29 CFR 1910.1000.
https://www.govinfo.gov/content/pkg/CFR-2021-title29-vol6/pdf/CFR-2021-title29-vol6-sec1910-
1000.pdf. August 28, 2022.
OSHA. 2021b. Occupational safety and health standards for shipyard employment. Subpart Z - Toxic
and hazardous substances. Air contaminants. Occupational Safety and Health Administration.
Code of Federal Regulations. 29 CFR 1915.1000. https://www.govinfo.gov/content/pkg/CFR-2021-
title29-vol7/pdf/CFR-2021-title29-vol7-sec1915-1000.pdf. August 28, 2022.
OSHA. 2021c. Safety and health regulations for construction. Subpart D - Occupational health and
environment controls. Gases, vapors, fumes, dusts, and mists. Occupational Safety and Health
Administration. Code of Federal Regulations. 29 CFR 1926.55.
https://www.govinfo.gov/content/pkg/CFR-2021-title29-vol8/pdf/CFR-2021-title29-vol8-sec1926-
55.pdf. August 28, 2022.
Padma TV, Dickhut RM. 2002. Spatial and temporal variation in hexachlorocyclohexane isomers in a
temperate estuary. Mar Pollut Bull 44(12):1345-1353. https://doi.org/10.1016/s0025-
326x(02)00171-6.
Page GW. 1981. Comparison of groundwater and surface water for patterns and levels of contamination
by toxic substances. Environ Sci Technol 15:1475-1481.
Pages N, diBlasi-Vouvet S, Schlatter J, et al. 2000. Hormone disruptive effects of residual doses of
lindane in male rats exposed at prenatal and postnatal periods [Abstract]. Hum Exp Toxicol
19(8):479.
Pahwa P, Karunanayake CP, Dosman JA, et al. 2011. Soft-tissue sarcoma and pesticides exposure in
men: results of a Canadian case-control study. J Occup Environ Med 53(11):1279-1286.
https://doi.org/10.1097/JOM.0b013e3182307845.
Palmer AK, Bottomley AM, Worden AN, et al. 1978. Effect of lindane on pregnancy in the rabbit and
rat. Toxicology 9:239-247. https://doi.org/10.1016/0300-483X(78)90006-9.
Pankow JF, Isabelle LM, Asher WE. 1984. Trace organic compounds in rain. 1. Sampler design and
analysis by adsorption/thermal desorption (ATD). Environ Sci Technol 18:310-318.
Park EY, Kim J, Park E, et al. 2021. Serum concentrations of persistent organic pollutants and colorectal
cancer risk: A case-cohort study within Korean National Cancer Center Community (KNCCC)
cohort. Chemosphere 271:129596. https://doi.org/10.1016/j.chemosphere.2021.129596.
Parmar D, Yadav S, Dayal M, et al. 2003. Effect of lindane on hepatic and brain cytochrome P450s and
influence of P450 modulation in lindane induced neurotoxicity. Food Chem Toxicol 41(8):1077-
1087. https://doi.org/10.1016/s0278-6915(03)00045-0.
HEXACHLOROCYCLOHEXANE (HCH) 335
8. REFERENCES
Paul R, Talukdar A, Bhattacharya R, et al. 2013. γ-Benzene hexachloride poisoning leading to acute
hepatorenal decompensation. BMJ Case Rep 2013:9851. https://doi.org/10.1136/bcr-2013-009851.
Pestana D, Fernandes V, Faria G, et al. 2011. Persistent organic pollutant (POPs) levels in human
visceral and subcutaneous adipose tissue on an obese Portuguese population-Metabolic improvement
after bariatric surgery versus POPs burden. Toxicology Letters 205:S75-S76.
https://doi.org/10.1016/j.toxlet.2011.05.284.
Petersen MS, Halling J, Bech S, et al. 2008. Impact of dietary exposure to food contaminants on the risk
of Parkinson's disease. Neurotoxicology 29(4):584-590.
https://doi.org/10.1016/j.neuro.2008.03.001.
Philip GH, Sriraman PK, Ramamurthi R. 1989. Histopathological changes in liver and kidney of Mus
booduga following oral benzenehexachloride (BHC) feeding. Bull Environ Contam Toxicol
42(4):499-502. https://doi.org/10.1007/BF01700228.
Pi N, Chia SE, Ong CN, et al. 2016. Associations of serum organohalogen levels and prostate cancer
risk: Results from a case-control study in Singapore. Chemosphere 144:1505-1512.
https://doi.org/10.1016/j.chemosphere.2015.10.020.
Piccoli C, Cremonese C, Koifman RJ, et al. 2016. Pesticide exposure and thyroid function in an
agricultural population in Brazil. Environ Res 151:389-398.
https://doi.org/10.1016/j.envres.2016.08.011.
Pierik FH, Klebanoff MA, Brock JW, et al. 2007. Maternal pregnancy serum level of heptachlor
epoxide, hexachlorobenzene, and β-hexachlorocyclohexane and risk of cryptorchidism in offspring.
Environ Res 105(3):364-369. https://doi.org/10.1016/j.envres.2007.04.005.
Pius J, Shivanandappa T, Krishnakumari MK. 1990. Protective role of vitamin A in the male
reproductive toxicity of hexachlorocyclohexane (HCH) in the rat. Reprod Toxicol 4:325-330.
Ploteau S, Cano-Sancho G, Volteau C, et al. 2017. Associations between internal exposure levels of
persistent organic pollutants in adipose tissue and deep infiltrating endometriosis with or without
concurrent ovarian endometrioma. Environ Int 108:195-203.
https://doi.org/10.1016/j.envint.2017.08.019.
Pollack AZ, Krall JR, Kannan K, et al. 2021. Adipose to serum ratio and mixtures of persistent organic
pollutants in relation to endometriosis: Findings from the ENDO Study. Environ Res 195:110732.
https://doi.org/10.1016/j.envres.2021.110732.
Pool-Zobel BL, Lotzman N, Knoll M, et al. 1994. Detection of genotoxic effects in human gastric and
nasal mucosa cells isolated from biopsy samples. Environ Mol Mutagen 24:23-45.
Porta M, Jariod M, López T, et al. 2009. Correcting serum concentrations of organochlorine compounds
by lipids: alternatives to the organochlorine/total lipids ratio. Environ Int 35(7):1080-1085.
https://doi.org/10.1016/j.envint.2009.06.004.
Porta M, Gasull M, Pumarega J, et al. 2022. Plasma concentrations of persistent organic pollutants and
pancreatic cancer risk. Int J Epidemiol 51(2):479-490. https://doi.org/10.1093/ije/dyab115.
Portig J, Stein K, Vohland HW. 1989. Preferential distribution of α-hexachlorocyclohexane into cerebral
white matter. Xenobiotica 19:123-130.
Powell GM. 1980. Toxicity of lindane [letter]. Central Afr J Med 26:170.
Prasad AK, Pant N, Srivastava SC, et al. 1995. Effect of dermal application of hexachlorocyclohexane
(HCH) on male reproductive system of rat. Hum Exp Toxicol 14(6):484-488.
https://doi.org/10.1177/096032719501400603.
Prasad WL, Srilatha C, Sailaja N, et al. 2016. Amelioration of gamma-hexachlorocyclohexane (lindane)
induced renal toxicity by Camellia sinensis in Wistar rats. Vet World 9(11):1331-1337.
https://doi.org/10.14202/vetworld.2016.1331-1337.
Presutti R, Harris SA, Kachuri L, et al. 2016. Pesticide exposures and the risk of multiple myeloma in
men: An analysis of the North American Pooled Project. Int J Cancer 139(8):1703-1714.
https://doi.org/10.1002/ijc.30218.
HEXACHLOROCYCLOHEXANE (HCH) 336
8. REFERENCES
Purdue MP, Hoppin JA, Blair A, et al. 2007. Occupational exposure to organochlorine insecticides and
cancer incidence in the Agricultural Health Study. Int J Cancer 120(3):642-649.
https://doi.org/10.1002/ijc.22258.
Quadroni S, Bettinetti R. 2019. An unnoticed issue: Organochlorine pesticides in tobacco products
around the world. Chemosphere 219:54-57. https://doi.org/10.1016/j.chemosphere.2018.12.009.
Quansah R, Bend JR, Abdul-Rahaman A, et al. 2016. Associations between pesticide use and respiratory
symptoms: A cross-sectional study in Southern Ghana. Environ Res 150:245-254.
https://doi.org/10.1016/j.envres.2016.06.013.
Quintana PJ, Delfino RJ, Korrick S, et al. 2004. Adipose tissue levels of organochlorine pesticides and
polychlorinated biphenyls and risk of non-Hodgkin's lymphoma. Environ Health Perspect
112(8):854-861. https://doi.org/10.1289/ehp.6726.
Quintero JC, Moreira MT, Feijoo G, et al. 2005. Effect of surfactants on the soil desorption of
hexachlorocyclohexane (HCH) isomers and their anaerobic biodegradation. Journal of Chemical
Technology and Biotechnology 80(9):1005-1015. https://doi.org/10.1002/jctb.1277.
Raaschou-Nielsen O, Pavuk M, Leblanc A, et al. 2005. Adipose organochlorine concentrations and risk
of breast cancer among postmenopausal Danish women. Cancer Epidemiol Biomarkers Prev
14(1):67-74.
Radomski JL, Astolfi E, Deichmann WB, et al. 1971a. Blood levels of organochlorine pesticides in
Argentina: Occupationally and nonoccupationally exposed adults, children and newborn infants.
Toxicol Appl Pharmacol 20:186-193.
Radomski JL, Deichmann WB, Rey AA, et al. 1971b. Human pesticide blood levels as a measure of
body burden and pesticide exposure. Toxicol Appl Pharmacol 20:175-185.
Radosavljević T, Mladenović D, Vucević D, et al. 2008. Effect of acute lindane and alcohol intoxication
on serum concentration of enzymes and fatty acids in rats. Food Chem Toxicol 46(5):1739-1743.
https://doi.org/10.1016/j.fct.2008.01.011.
Rafeeinia A, Asadikaram G, Karimi Darabi M, et al. 2023. Organochlorine pesticides, oxidative stress
biomarkers, and leukemia: a case-control study. J Investig Med 71(3):295-306.
https://doi.org/10.1177/10815589221145043.
Raizada RB, Misra P, Saxena I, et al. 1980. Weak estrogenic activity of lindane in rats. J Toxicol
Environ Health 6:483-492.
Raizada RB, Srivastava MK, Sarin S. 1993. Impact of technical hexachlorocyclohexane (HCH) on
biogenic amines and locomotor activity of rat. Natl Acad Sci Letts Indian 16(2):73-76.
Ramabhatta S, Sunilkumar GR, Somashekhar C. 2014. Lindane toxicity following accidental oral
ingestion. Indian J Dermatol Venereol Leprol 80(2):181-182. https://doi.org/10.4103/0378-
6323.129419.
Ramachandran M, Banerjee BD, Gulati M, et al. 1984. DDT and HCH residues in the body fat and
blood samples from some Delhi hospitals. Indian J Med Res 80:590-593.
Ramamoorthy S. 1985. Competition of fate processes in the bioconcentration of lindane. Bull Environ
Contam Toxicol 34:349-358.
Ramchander V, Cameron ES, Reid HF. 1991. Lindane toxicity in an infant. West Indian Med J 40:41-
43.
Rauch AE, Kowalsky SF, Lesar TS, et al. 1990. Lindane (Kwell)-induced aplastic anemia. Arch Intern
Med 150:2393-2395.
Ravinder P, Srinivasan K, Radhakrishnamurty R. 1989. Biochemical toxicity of hexachlorocyclohexane
and its γ-isomer in albino mice. Indian J Exp Biol 27(3):248-251.
Ravinder P, Srinivasan K, Radhakrishnamurty R. 1990. Dietary hexachlorocyclohexane induced changes
in blood and liver lipids in albino mice. Indian J Exp Biol 28(2):155-157.
Reina-Pérez I, Artacho-Cordón F, Mustieles V, et al. 2023. Cross-sectional associations of persistent
organic pollutants measured in adipose tissue and metabolic syndrome in clinically diagnosed
middle-aged adults. Environ Res 222:115350. https://doi.org/10.1016/j.envres.2023.115350.
HEXACHLOROCYCLOHEXANE (HCH) 337
8. REFERENCES
Reinhart DR, Pohland FG. 1991. The assimilation of organic hazardous wastes by municipal solid waste
landfills. J Ind Microbiol 8:193-200.
Reinhart DR, Pohland FG, Gould JP, et al. 1991. The fate of selected organic pollutants codisposed with
municipal refuse. Res J Water Pollut Control Fed 63:780-788.
RePORTER. 2023. Hexachlorocyclohexanes. National Institutes of Health, Research Portfolio Online
Reporting Tools. http://projectreporter.nih.gov/reporter.cfm. March 15, 2021.
Ribas-Fito N, Sala M, Cardo E, et al. 2003. Organochlorine compounds and concentrations of thyroid
stimulating hormone in newborns. Occup Environ Med 60(4):301-303.
https://doi.org/10.1136/oem.60.4.301.
Richardson JR, Shalat SL, Buckley B, et al. 2009. Elevated serum pesticide levels and risk of Parkinson
disease. Arch Neurol 66(7):870-875. https://doi.org/10.1001/archneurol.2009.89.
Richardson JR, Roy A, Shalat SL, et al. 2011. β-Hexachlorocyclohexane levels in serum and risk of
Parkinson's disease. Neurotoxicology 32(5):640-645. https://doi.org/10.1016/j.neuro.2011.04.002.
Rippen G, Ilgenstein M, Klöpffer W, et al. 1982. Screening of the adsorption behavior of new
chemicals: Natural soils and model adsorbents. Ecotoxicology and Environmental Safety 6(3):236-
245. https://doi.org/10.1016/0147-6513(82)90014-8.
Rivera S, Sanfeliu C, Sunol C, et al. 1991. Regional effects on the cerebral concentration of
noradrenaline, serotonin, and dopamine in suckling rats after a single dose of lindane. Toxicology
69:43-54. https://doi.org/10.1016/0300-483X(91)90152-Q.
Rivera S, Rosa R, Marinez E, et al. 1998. Behavioral and monoaminergic changes after lindane exposure
in developing rats. Neurotoxicol Teratol 20(2):155-160. https://doi.org/10.1016/S0892-
0362(97)00079-2.
Rivett KF, Chesterman H, Kellett DN, et al. 1978. Effects of feeding lindane to dogs for periods of up to
2 years. Toxicology 9:273-289. https://doi.org/10.1016/0300-483X(78)90010-0.
Rocchi P, Perocco P, Alberghini W, et al. 1980. Effect of pesticides on scheduled and unscheduled DNA
synthesis of rat thymocytes and human lymphocytes. Arch Toxicol 45:101-108.
Rogers WM, Kendall DC, Salmon GD, et al. 1995. Accumulated pesticide and industrial chemical
findings from a ten-year study of ready-to-eat foods. J AOAC Int 78:614-631.
Rooney AA, Boyles AL, Wolfe MS, et al. 2014. Systematic review and evidence integration for
literature-based environmental health science assessments. Environ Health Perspect 122(7):711-718.
https://doi.org/10.1289/ehp.1307972.
Roy RR, Wilson P, Laski RR, et al. 1997. Monitoring of domestic and imported apples and rice by the
U.S. Food and Drug Administration Pesticide Program. J AOAC Int 80:883-894.
Roy Chowdhury A, Gautam AK. 1990. BHC induced testicular impairments in rats. Indian J Physiol
Pharmacol 34:215-217.
Rüdel H. 1997. Volatilisation of pesticides from soil and plant surfaces. Chemosphere 38(1-2):143-
152.
Rugman FP, Cosstick R. 1990. Aplastic anaemia associated with organochlorine pesticide: Case reports
and review of evidence. J Clin Pathol 43:98-101.
Ruiz P, Ray M, Fisher J, et al. 2011. Development of a human Physiologically Based Pharmacokinetic
(PBPK) Toolkit for environmental pollutants. Int J Mol Sci 12(11):7469-7480.
https://doi.org/10.3390/ijms12117469.
Rupa DS, Rita P, Reddy PP, et al. 1988. Screening of chromosomal aberrations and sister chromatid
exchanges in peripheral lymphocytes of vegetable garden workers. Hum Toxicol 7:333-336.
Rupa DS, Reddy PP, Reddi OS. 1989a. Analysis of sister-chromatid exchanges, cell kinetics and mitotic
index in lymphocytes of smoking pesticide sprayers. Mutat Res 223:253-258.
Rupa DS, Reddy PP, Reddi OS. 1989b. Chromosomal aberrations in peripheral lymphocytes of cotton
field workers exposed to pesticides. Environ Res 49:1-6.
Rupa DS, Reddy PP, Reddi OS. 1989c. Frequencies of chromosomal aberrations in smokers exposed to
pesticides in cotton fields. Mutat Res 222:37-41.
HEXACHLOROCYCLOHEXANE (HCH) 338
8. REFERENCES
Rupa DS, Reddy PP, Reddi OS. 1989d. Genotoxic effect of benzene hexachloride in cultured human
lymphocytes. Hum Genet 83:271-273.
Rylander C, Sandanger TM, Nost TH, et al. 2015. Combining plasma measurements and mechanistic
modeling to explore the effect of POPs on type 2 diabetes mellitus in Norwegian women. Environ
Res 142:365-373. https://doi.org/10.1016/j.envres.2015.07.002.
Ryu DH, Yu HT, Kim SA, et al. 2018. Is chronic exposure to low-dose organochlorine pesticides a new
risk factor of t-cell immunosenescence? Cancer Epidemiol Biomarkers Prev 27(10):1159-1167.
https://doi.org/10.1158/1055-9965.Epi-17-0799.
Safe SH. 1993. Toxic aromatics. In: Kroschwitz JI, Howe-Grant M, eds. Kirk-Othmer’s encyclopedia
of chemical technology. New York, NY: John Wiley & Sons, 127-139.
Sahaya K, Mahajan P, Mediratta PK, et al. 2007. Reversal of lindane-induced impairment of step-down
passive avoidance and oxidative stress by neurosteroids in rats. Toxicology 239(1-2):116-126.
https://doi.org/10.1016/j.tox.2007.07.002.
Sahoo A, Samanta L, Das A, et al. 1999. Hexachlorocyclohexane-induced behavioral and neurochemical
changes in rat. J Appl Toxicol 19(1):13-18. https://doi.org/10.1002/(SICI)1099-
1263(199901/02)19:1<13::AID-JAT531>3.0.CO;2-E.
Sahu SK, Patnaik KK, Bhuyan S, et al. 1993. Degradation of soil-applied isomers of
hexachlorocyclohexane by a Pseudomonas SP. Soil Biol Biochem 25(3):387-391.
https://doi.org/10.1016/0038-0717(93)90139-3.
Saleh FY, Lee GF, Butler JS. 1978. Kepone and other selected chlorinated hydrocarbon pesticides and
PCBs behavior during hydraulic dredging of the James River near Hopewell, Virginia. J Environ Sci
Health A 13:261-294.
Saleh FY, Dickson KL, Rodgers JH. 1982. Fate of lindane in the aquatic environment: Rate constants of
physical and chemical processes. Environ Toxicol Chem 1:289-297.
Saleh IA, Zouari N, Al-Ghouti MA. 2020. Removal of pesticides from water and wastewater: Chemical,
physical and biological treatment approaches. Environ Tech Innov 19:101026.
https://doi.org/10.1016/j.eti.2020.101026.
Salimi F, Asadikaram G, Abolhassani M, et al. 2023. Organochlorine pesticides induce thyroid tumors
through oxidative stress; an in vivo and in silico study. Environ Sci Pollut Res Int 30(15):45046-
45066. https://doi.org/10.1007/s11356-023-25304-1.
Salo HM, Koponen J, Kiviranta H, et al. 2019. No evidence of the role of early chemical exposure in the
development of β-cell autoimmunity. Environmental Science and Pollution Research 26(2):1370-
1378. https://doi.org/10.1007%2Fs11356-018-3659-6.
Samanta L, Chainy GBN. 1997. Comparison of hexachlorocyclohexane-induced oxidative stress in the
testis of immature and adult rats. Comp Biochem Physiol 118C(3):319-327.
Samanta L, Sahoo A, Chainy GB. 1999. Age-related changes in rat testicular oxidative stress parameters
by hexachlorocyclohexane. Arch Toxicol 73(2):96-107. https://doi.org/10.1007/s002040050593.
Samuel T, Pillai MKK. 1990. Effect of temperature and sunlight exposure on the fate of soil-applied
[
14
C]-gamma-hexachlorocyclohexane. Arch Environ Contam Toxicol 19:214-220.
Sandhu SS, Warren WJ, Nelson P. 1978. Pesticidal residue in rural potable water. J Am Water Works
Assoc 70:41-45.
Saradha B, Mathur PP. 2006. Induction of oxidative stress by lindane in epididymis of adult male rats.
Environ Toxicol Pharmacol 22(1):90-96. https://doi.org/10.1016/j.etap.2005.12.008.
Sasaki K, Ishizaka T, Suzuki T, et al. 1991. Organochlorine chemicals in skin lipids as an index of their
accumulation in the human body. Archives of Environmental Contamination and Toxicology
21(2):190-194. https://doi.org/10.1007/bf01055336.
Sauviat MP, Bouvet S, Godeau G, et al. 2005. Electrical activity alterations induced by chronic
absorption of lindane (γ-hexachlorocyclohexane) trace concentrations in adult rat heart. Can J
Physiol Pharmacol 83(3):243-251. https://doi.org/10.1139/y04-132.
Sauviat M-P, Godeau G, Pages N. 2007. Alterations of offspring heart muscle electrical activity
transferred by rat male genitors chronically treated with lindane (γ-hexachlorocyclohexane) trace
HEXACHLOROCYCLOHEXANE (HCH) 339
8. REFERENCES
concentrations. Pesticide Biochemistry and Physiology 87(2):131-137.
https://doi.org/10.1016/j.pestbp.2006.07.002.
Sawada N, Iwasaki M, Inoue M, et al. 2010. Plasma organochlorines and subsequent risk of prostate
cancer in Japanese men: a nested case-control study. Environ Health Perspect 118(5):659-665.
https://doi.org/10.1289/ehp.0901214.
Sawyer ME, Evans MV, Wilson CA, et al. 2016. Development of a human physiologically based
pharmacokinetic (PBPK) model for dermal permeability for lindane. Toxicology Letters 245:106-
109. https://doi.org/10.1016/j.toxlet.2016.01.008.
Saxena MC, Siddiqui MKJ, Bhargava AK, et al. 1980. Role of chlorinated hydrocarbon pesticides in
abortions and premature labour. Toxicology 17:323-331.
Saxena MC, Siddiqui MKJ, Seth TD, et al. 1981a. Organochlorine pesticides in specimens from women
undergoing spontaneous abortion, premature or full-term delivery. J Anal Toxicol 5:6-9.
Saxena MC, Siddiqui MKJ, Bhargava AK, et al. 1981b. Placental transfer of pesticides in humans. Arch
Toxicol 48:127-134.
Saxena DK, Murthy RC, Chandra SV. 1986. Embryotoxic and teratogenic effects of interaction of
cadmium and lindane in rats. Acta Pharmacol Toxicol 59:175-178.
Scascitelli M, Pacchierotti F. 2003. Effects of lindane on oocyte maturation and preimplantation
embryonic development in the mouse. Reprod Toxicol 17(3):299-303.
https://doi.org/10.1016/s0890-6238(03)00008-x.
Schattenberg HJ, Hsu JP. 1992. Pesticide residue survey of produce from 1989 to 1991. J AOAC Int
75:925-933.
Schimmel SC, Patrick JM, Forester J. 1977. Toxicity and bioconcentration of BHC and lindane in
selected estuarine animals. Arch Environ Contam Toxicol 6:355-363.
Schmitt CJ, Zajicek JL, Ribick MA. 1985. National pesticide monitoring program: Residues of
organochlorine chemicals in freshwater fish, 1980-1981. Arch Environ Contam Toxicol 14:225-260.
Schmitt CJ, Zajicek JL, Peterman PH. 1990. National contaminant biomonitoring program: Residues of
organochlorine chemicals in U.S. Freshwater Fish, 1976-1984. Arch Environ Contam Toxicol
19(5):748-781. https://doi.org/10.1007/bf01183992.
Schmitt M, Gellert G, Ludwig J, et al. 2005. Assessment of cyto- and genotoxic effects of a variety of
chemicals using Saccharomyces cerevisiae. Acta Hydrochim Hydrobiol 33(1):56-63.
https://doi.org/10.1002/aheh.200300554.
Schröter C, Parzefall W, Schröter H, et al. 1987. Dose-response studies on the effects of α-, β-, and g-
hexachlorocyclohexane on putative preneoplastic foci, monooxygenases, and growth in rat liver.
Cancer Res 47:80-88.
Schwarz M, Wolf K, Schneider A, et al. 2021. Association of persistent organic pollutants with
sensorimotor neuropathy in participants with and without diabetes or prediabetes: Results from the
population-based KORA FF4 study. Int J Hyg Environ Health 235:113752.
https://doi.org/10.1016/j.ijheh.2021.113752.
Seiler P, Fischer B, Lindenau A, et al. 1994. Effects of persistent chlorinated hydrocarbons on fertility
and embryonic development in the rabbit. Hum Reprod 9(10):1920-1926.
https://doi.org/10.1093/oxfordjournals.humrep.a138359.
Seo SH, Choi SD, Batterman S, et al. 2022. Health risk assessment of exposure to organochlorine
pesticides in the general population in Seoul, Korea over 12 years: A cross-sectional epidemiological
study. J Hazard Mater 424(Pt B):127381. https://doi.org/10.1016/j.jhazmat.2021.127381.
Sericano JL, Atlas EL, Wade TL, et al. 1990. NOAA's status and trends mussel watch program:
Chlorinated pesticides and PCBs in oysters (Crassostrea virginica) and sediments from the Gulf of
Mexico, 1986-1987. Mar Environ Res 29:161-203.
Serrano MT, Vendrell M, Rivera S, et al. 1990. Effect of lindane on the myelination process in the rat.
Neurotoxicol Teratol 12:577-583. https://doi.org/10.1016/0892-0362(90)90065-K.
Seth V, Ahmad RS, Suke SG, et al. 2005. Lindane-induced immunological alterations in human
poisoning cases. Clin Biochem 38(7):678-680. https://doi.org/10.1016/j.clinbiochem.2005.03.009.
HEXACHLOROCYCLOHEXANE (HCH) 340
8. REFERENCES
Sexton K, Ryan AD. 2012. Using exposure biomarkers in children to compare between-child and
within-child variance and calculate correlations among siblings for multiple environmental
chemicals. J Expo Sci Environ Epidemiol 22(1):16-23. https://doi.org/10.1038/jes.2011.30.
Shah HK, Sharma T, Banerjee BD. 2020. Organochlorine pesticides induce inflammation, ROS
production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere
246:125691. https://doi.org/10.1016/j.chemosphere.2019.125691.
Sexton K, Ryan AD, Adgate JL, et al. 2011. Biomarker measurements of concurrent exposure to
multiple environmental chemicals and chemical classes in children. J Toxicol Environ Health A
74(14):927-942. https://doi.org/10.1080/15287394.2011.573745.
Shah PR, Kute VB, Gumber MR, et al. 2013. Benzene hexachloride poisoning with rhabdomyolysis and
acute kidney injury. Indian J Nephrol 23(1):80-81. https://doi.org/10.4103/0971-4065.107222.
Shahin MM, von Borstel RC. 1977. Mutagenic and lethal effects of α-benzene hexachloride, dibutyl
phthalate, and trichloroethylene in Saccharomyces cerevisiae. Mutat Res 48:173-180.
Sharma P, Singh R. 2010. Protective role of curcumin on lindane induced reproductive toxicity in male
Wistar rats. Bull Environ Contam Toxicol 84(4):378-384. https://doi.org/10.1007/s00128-010-
9942-y.
Sharma H, Zhang P, Barber DS, et al. 2010. Organochlorine pesticides dieldrin and lindane induce
cooperative toxicity in dopaminergic neurons: role of oxidative stress. Neurotoxicology 31(2):215-
222. https://doi.org/10.1016/j.neuro.2009.12.007.
Sharma E, Mustafa M, Pathak R, et al. 2012. A case control study of gene environmental interaction in
fetal growth restriction with special reference to organochlorine pesticides. Eur J Obstet Gynecol
Reprod Biol 161(2):163-169. https://doi.org/10.1016/j.ejogrb.2012.01.008.
Sharma T, Banerjee BD, Mustafa M, et al. 2013. Gene environment interaction in preterm delivery with
special reference to organochlorine pesticide: a case control study. Int J Biochem Mol Biol
4(4):209-214.
Sharma T, Banerjee BD, Thakur GK, et al. 2019. Polymorphism of xenobiotic metabolizing gene and
susceptibility of epithelial ovarian cancer with reference to organochlorine pesticides exposure. Exp
Biol Med 244(16):1446-1453. https://doi.org/10.1177/1535370219878652.
Sharom MS, Miles JRW, Harris CR, et al. 1980. Persistence of 12 insecticides in water. Water Res
14:1089-1093.
Shearer JJ, Sandler DP, Andreotti G, et al. 2021. Pesticide use and kidney function among farmers in the
Biomarkers of Exposure and Effect in Agriculture study. Environ Res 199:111276.
https://doi.org/10.1016/j.envres.2021.111276.
Shen L, Wania F, Lei YD, et al. 2004. Hexachlorocyclohexanes in the North American atmosphere.
Environ Sci Technol 38(4):965-975. https://doi.org/10.1021/es034998k.
Shen H, Main KM, Virtanen HE, et al. 2007. From mother to child: investigation of prenatal and
postnatal exposure to persistent bioaccumulating toxicants using breast milk and placenta
biomonitoring. Chemosphere 67(9):S256-262. https://doi.org/10.1016/j.chemosphere.2006.05.106.
Shimazu H, Shiraishi N, Akematsu T, et al. 1972. Carcinogenicity screening tests on induction of
chromosomal aberrations in rat bone marrow cells in vivo [abstract]. Mutat Res 38:347.
Shirasu Y, Moriya M, Kato K, et al. 1976. Mutagenicity screening of pesticides in the microbial system.
Mutat Res 40:19-30.
Shivanandappa T, Krishnakumari MK. 1983. Hexachlorocyclohexane-induced testicular dysfunction in
rats. Acta Pharmacol Toxicol 52:12-17.
Shrestha S, Umbach DM, Beane Freeman LE, et al. 2021. Occupational pesticide use and self-reported
olfactory impairment in US farmers. Occup Environ Med 78(3):179-191.
https://doi.org/10.1136/oemed-2020-106818.
Siddarth M, Datta SK, Mustafa M, et al. 2014. Increased level of organochlorine pesticides in chronic
kidney disease patients of unknown etiology: role of GSTM1/GSTT1 polymorphism. Chemosphere
96:174-179. https://doi.org/10.1016/j.chemosphere.2013.10.029.
HEXACHLOROCYCLOHEXANE (HCH) 341
8. REFERENCES
Siddiqui MKJ, Saxena M, Krishna MC. 1981. Storage of DDT and BHC in adipose tissue of Indian
males. Int J Environ Anal Chem 10:197-204.
Siddiqui MK, Srivastava S, Srivastava SP, et al. 2003. Persistent chlorinated pesticides and intra-uterine
foetal growth retardation: a possible association. Int Arch Occup Environ Health 76(1):75-80.
https://doi.org/10.1007/s00420-002-0393-6.
Sidorova TS, Matesic DF. 2008. Protective effect of the natural product, chaetoglobosin K, on lindane-
and dieldrin-induced changes in astroglia: identification of activated signaling pathways. Pharm Res
25(6):1297-1308. https://doi.org/10.1007/s11095-007-9487-x.
Simon-Giavarotti KA, Giavarotti L, Gomes LF, et al. 2002. Enhancement of lindane-induced liver
oxidative stress and hepatotoxicity by thyroid hormone is reduced by gadolinium chloride. Free
Radic Res 36(10):1033-1039. https://doi.org/10.1080/1071576021000028280.
Sineli P, Benimeli CS, Amoroso MJ, et al. 2014. Biodegradation of alpha- and beta-
Hexachlorocyclohexane by Indigenous Actinobacteria. In: Alvarez A, Polti M, eds. Bioremediation
in Latin America. Switzerland: Springer International Publishing, 279-288.
https://doi.org/10.1007/978-3-319-05738-5_18.
Singh R, Sharma P. 2011. Hepatoprotective effect of curcumin on lindane-induced oxidative stress in
male Wistar rats. Toxicol Int 18(2):124-129. https://doi.org/10.4103/0971-6580.84264.
Singh G, Kathpal TS, Spencer WF, et al. 1991. Dissipation of some organochlorine insecticides in
cropped and uncropped soil. Environ Pollut 70:219-240.
Singh NK, Chhillar N, Banerjee BD, et al. 2012. Gene-environment interaction in Alzheimer's disease.
Am J Alzheimers Dis Other Demen 27(7):496-503. https://doi.org/10.1177/1533317512456067.
Singh N, Chhillar N, Banerjee B, et al. 2013. Organochlorine pesticide levels and risk of Alzheimer's
disease in north Indian population. Hum Exp Toxicol 32(1):24-30.
https://doi.org/10.1177/0960327112456315.
Singh NK, Banerjee BD, Bala K, et al. 2014. Polymorphism in cytochrome P450 2D6, glutathione S-
transferases Pi I Genes, and organochlorine pesticides in Alzheimer disease: A case-control study in
north Indian population. J Geriatr Psychiatry Neurol 27(2):119-127.
https://doi.org/10.1177/0891988714522698.
Sinha C, Shukla GS. 2003. Species variation in pesticide-induced blood-brain barrier dysfunction. Hum
Exp Toxicol 22(12):647-652. https://doi.org/10.1191/0960327103ht405oa.
Sipes IG, Gandolfi AJ. 1991. Biotransformation of toxicants. In: Amdur MO, Doull J, Klaassen CD,
eds. Toxicology: The basic science of poisons. 4
th
ed. New York, NY: Pergamon Press, 86-126.
Sircar S, Lahiri P. 1989. Lindane (g-HCH) causes reproductive failure and fetotoxicity in mice.
Toxicology 59:171-177.
Sisto R, Moleti A, Palkovicova Murinova L, et al. 2015. Environmental exposure to organochlorine
pesticides and deficits in cochlear status in children. Environ Sci Pollut Res Int 22(19):14570-
14578. https://doi.org/10.1007/s11356-015-4690-5.
Šmídová K, Hofman J, Ite AE, et al. 2012. Fate and bioavailability of ¹⁴C-pyrene and ¹⁴C-lindane in
sterile natural and artificial soils and the influence of aging. Environ Pollut 171:93-98.
https://doi.org/10.1016/j.envpol.2012.07.031.
Šmídová K, Šerá J, Bielská L, et al. 2015. Influence of feeding and earthworm density on compound
bioaccumulation in earthworms Eisenia andrei. Environ Pollut 207:168-175.
https://doi.org/10.1016/j.envpol.2015.09.025.
Smith AG. 1991. Chlorinated hydrocarbon insecticides. In: Handbook of pesticide toxicology. Vol. 2.
San Diego, CA: Academic Press, 731-743, 791-816, 868-915.
Smith MT, Guyton KZ, Gibbons CF, et al. 2016. Key characteristics of carcinogens as a basis for
organizing data on mechanisms of carcinogenesis. Environmental Health Perspectives 124(6):713-
721. https://doi.org/10.1289/ehp.1509912.
Smith-Baker C, Saleh MA. 2011. Hair as a marker for pesticides exposure. J Environ Sci Health B
46(7):648-653. https://doi.org/10.1080/03601234.2012.597701.
HEXACHLOROCYCLOHEXANE (HCH) 342
8. REFERENCES
Solomon L, Faherer L, West D. 1977a. Gamma benzene hexachloride toxicity. Arch Dermatol 113:353-
357.
Solomon L, West D, Fitzloff J, et al. 1977b. Gamma benzene hexachloride in guinea-pig brain after
topical application. J Invest Dermatol 68:310-312.
Solomon BA, Haut SR, Carr EM, et al. 1995. Neurotoxic reaction to lindane in an HIV-seropositive
patient: An old medication's new problem. J Fam Pract 40:291-295.
Song S, Ma X, Pan M, et al. 2018. Excretion kinetics of three dominant organochlorine compounds in
human milk within the first 6 months postpartum. Environ Monit Assess 190(8):457.
https://doi.org/10.1007/s10661-018-6850-9.
Srinivasan K, Radhakrishnamurty R. 1983. Studies on the distribution of β- and g-isomers of
hexachlorocyclohexane in rat tissues. J Environ Sci Health B 18:401-418.
Srinivasan K, Radhakrishnamurty R. 1988. Biochemical changes produced by β- and g-
hexachlorocyclohexane isomers in albino rats. J Environ Sci Health 23:367-386.
Srinivasan K, Ramesh HP, Radhakrishnamurty R. 1984. Renal tubular dysfunction caused by dietary
hexachlorocyclohexane (HCH) isomers. J Environ Sci Health 19:453-466.
https://doi.org/10.1080/03601238409372443.
Srinivasan K, Mahadevappa KL, Radhakrishnamurty R. 1991. Effect of maternal dietary
hexachlorocyclohexane exposure on pup survival and growth in albino rats. J Environ Sci Health B
26(3):339-349. https://doi.org/10.1080/03601239109372740.
Srivastava MK, Raizada RB. 1993. Prenatal effects of technical hexachlorocyclohexane in mice.
Journal of Toxicology and Environmental Health 40(1):105-115.
https://doi.org/10.1080/15287399309531778.
Srivastava MK, Raizada RB. 2000. A limited three-generation reproduction study on
hexachlorocyclohexane (HCH) in rats. Food Chem Toxicol 38:195-201.
Srivastava A, Shivanandappa T. 2014. Prevention of hexachlorocyclohexane-induced neuronal oxidative
stress by natural antioxidants. Nutr Neurosci 17(4):164-171.
https://doi.org/10.1179/1476830513y.0000000075.
Srivastava A, Srivastava AK, Mishra M, et al. 2019. A proteomic approach to investigate enhanced
responsiveness in rechallenged adult rats prenatally exposed to lindane. Neurotoxicology 74:184-
195. https://doi.org/10.1016/j.neuro.2019.07.004.
Stachel B, Dougherty RC, Lahl U, et al. 1989. Toxic environmental chemicals in human semen:
Analytical method and case studies. Andrologia 21:282-291.
Stanley CW, Barney JE, Helton MR, et al. 1971. Measurement of atmospheric levels of pesticides.
Environ Sci Technol 5:430-435.
Staples CA, Werner A, Hoogheem T. 1985. Assessment of priority pollutant concentrations in the
United States using STORET database. Environ Toxicol Chem 4:131-142.
Starr HJ, Clifford NJ. 1972. Acute lindane intoxication. A case study. Arch Environ Health 25:374-
375.
Starr HG, Aldrich FD, McDougall WD, et al. 1974. Contribution of household dust to the human
exposure to pesticides. Pest Monit J 8:209-212.
Steenland K, Mora AM, Barr DB, et al. 2014. Organochlorine chemicals and neurodegeneration among
elderly subjects in Costa Rica. Environ Res 134:205-209.
https://doi.org/10.1016/j.envres.2014.07.024.
Storen G. 1955. Lethal poisoning with the moth- and insecticide 'Jacutin'. Nord J Hyg 36:77-81.
Strachan WMJ. 1988. Toxic contaminants in rainfall in Canada: 1984. Environ Toxicol Chem 7:871-
877.
Sturgeon S, Broxk J, Potischman N, et al. 1998. Serum concentrations of organochlorine compounds and
endometrial cancer risk (United States). Cancer Causes Control 9:417-424.
Sudakin DL. 2007. Fatality after a single dermal application of lindane lotion. Arch Environ Occup
Health 62(4):201-203. https://doi.org/10.3200/aeoh.62.4.201-203.
HEXACHLOROCYCLOHEXANE (HCH) 343
8. REFERENCES
Sulik M, Deregowski K, Kemona A. 1988. Distribution and excretion of lindane-14C in acute
intoxication in rats. Mater Med Pol 20:92-94.
Sullivan K, Krengel M, Bradford W, et al. 2018. Neuropsychological functioning in military pesticide
applicators from the Gulf War: Effects on information processing speed, attention and visual
memory. Neurotoxicol Teratol 65:1-13. https://doi.org/10.1016/j.ntt.2017.11.002.
Sumida K, Saito K, Oeda K, et al. 2007. A comparative study of gene expression profiles in rat liver
after administration of alpha-hexachlorocyclohexane and lindane. J Toxicol Sci 32(3):261-288.
https://doi.org/10.2131/jts.32.261.
Sunder Ram Rao CV, Shreenivas R, Singh V, et al. 1988. Disseminated intravascular coagulation in a
case of fatal lindane poisoning. Vet Hum Toxicol 30:132-134.
Sunyer J, Alvarez-Pedrerol M, To-Figueras J, et al. 2008. Urinary porphyrin excretion in children is
associated with exposure to organochlorine compounds. Environ Health Perspect 116(10):1407-
1410. https://doi.org/10.1289/ehp.11354.
Suter P. 1983. Three months toxicity study in rats with lindane. Itingen, Switzerland: Research and
Consulting Company AG. RCC project no. 005220.
Sweeney RA. 1969. Metabolism of lindane by unicellular algae. In: Proceedings of the 12
th
Conference
on Great Lakes Research. International Association for Great Lakes Research, 98-102.
Sweeney LM, Gearhart JM. 2020. Examples of physiologically based pharmacokinetic modeling applied
to risk assessment. In: Fisher JW, Gearhart JM, Lin Z, eds. Physiologically based pharmacokinetic
(PBPK) modeling. Academic Press, 281-299. https://doi.org/10.1016/B978-0-12-818596-4.00011-
4.
Szeto SY, Price PM. 1991. Persistence of pesticide residues in mineral and organic soils in the Fraser
Valley of British Columbia. J Agric Food Chem 39:1679-1684.
Szokolay A, Rosival L, Uhnak J, et al. 1977. Dynamics of benzene hexachloride (BHC) isomers and
other chlorinated pesticides in the food chain and in human fat. Ecotoxicol Environ Safety 1:349-
359.
Szymczynski GA, Waliszewski SM. 1981. Comparison of the content of chlorinated pesticide residues
in human semen, testicles and fat tissues. Andrologia 13:250-252.
Takahashi W, Saidin D, Takei G, et al. 1981. Organochlorine pesticide residues in human milk in
Hawaii, 1979-1980. Bull Environ Contam Toxicol 27:506-511.
Tan J, Loganath A, Chong YS, et al. 2009. Exposure to persistent organic pollutants in utero and related
maternal characteristics on birth outcomes: a multivariate data analysis approach. Chemosphere
74(3):428-433. https://doi.org/10.1016/j.chemosphere.2008.09.045.
Tan YM, Chan M, Chukwudebe A, et al. 2020. PBPK model reporting template for chemical risk
assessment applications. Regul Toxicol Pharmacol 115:104691.
https://doi.org/10.1016/j.yrtph.2020.104691.
Tanabe S, Tatsukawa R, Kawano M, et al. 1982. Global distribution and atmospheric transport of
chlorinated hydrocarbons: HCH (BHC) isomers and DDT compounds in the western Pacific, eastern
Indian and Antarctic Oceans. J Oceanogr Soc Jpn 38:137-148.
Tanaka K, Kurihara N, Nakajima N. 1979. Oxidative metabolism of lindane and its isomers with
microsomes from rat liver and house fly abdomen. Pestic Biochem Physiol 10:96-103.
Tao S, Lu Y, Zhang D, et al. 2009. Assessment of oral bioaccessibility of organochlorine pesticides in
soil using an in vitro gastrointestinal model. Environ Sci Technol 43(12):4524-4529.
https://doi.org/10.1021/es900188c.
Tawar N, Banerjee BD, Madhu SV, et al. 2022. Association of organochlorine pesticides with genetic
markers of endoplasmic reticulum stress in Type 2 diabetes mellitus: A case-control study among the
North-Indian population. Front Endocrinol (Lausanne) 13:841463.
https://doi.org/10.3389/fendo.2022.841463.
Telch J, Jarvis DA. 1982. Acute intoxication with lindane (gamma benzene hexachloride). Can Med
Assoc J 126:662-663.
Tenenbein M. 1991. Seizures after lindane therapy. J Am Geriatr Soc 39:394-395.
HEXACHLOROCYCLOHEXANE (HCH) 344
8. REFERENCES
Tewari A, Sethi RS, Banga HS, et al. 2017. Concomitant effect of low dose of lindane and intranasal
lipopolysaccharide on respiratory system of mice. Hum Exp Toxicol 36(11):1201-1211.
https://doi.org/10.1177/0960327116685889.
Tezak Z, Simic B, Kniewald J. 1992. Effect of pesticides on oestradiol-receptor complex formation in
rat uterus cytosol. Food Chem Toxicol 30:879-885.
Thakore KN, Karnik AB, Nigam SK, et al. 1981. Sequential changes in lactate, isocitrate, and malate
dehydrogenases in mice exposed to technical grade hexachlorocyclohexane (BHC) and their possible
relationship to liver tumors. Pestic Biochem Physiol 15:262-266. https://doi.org/10.1016/0048-
3575(81)90009-2.
Thomas JC, Berger F, Jucquier M, et al. 1996. Isolation and characterization of a novel g-
hexachlorocyclohexane-degrading bacterium. J Bacteriol 178(20):6049-6055.
Thorpe E, Walker AIT. 1973. The toxicology of dieldrin (HEOD). II. Comparative long-term oral
toxicity studies in mice with dieldrin, DDT, phenobarbitone, β-BHC, and γ-BHC. Food Cosmet
Toxicol 11:433-442. https://doi.org/10.1016/0015-6264(73)90008-4.
Tilson HA, Shaw S, McLamb RL. 1987. The effects of lindane, DDT, and chlordecone on avoidance
responding and seizure activity. Toxicol Appl Pharmacol 88:57-65.
Tochman AM, Kamiński R, Turski WA, et al. 2000. Protection by conventional and new antiepileptic
drugs against lindane-induced seizures and lethal effects in mice. Neurotox Res 2(1):63-70.
https://doi.org/10.1007/bf03033328.
Tomczak S, Baumann K, Lehnert G. 1981. Occupational exposure to hexachlorocyclohexane. IV. Sex
hormone alterations in HCH-exposed workers. Int Arch Occup Environ Health 48:283-287.
Torres-Arreola L, Berkowitz G, Torres-Sanchez L, et al. 2003. Preterm birth in relation to maternal
organochlorine serum levels. Annals of Epidemiology 13(3):158-162.
https://doi.org/10.1016/s1047-2797(02)00424-6.
Traina ME, Rescia M, Urbani E, et al. 2003. Long-lasting effects of lindane on mouse spermatogenesis
induced by in utero exposure. Reprod Toxicol 17(1):25-35. https://doi.org/10.1016/s0890-
6238(02)00101-6.
TRI21. 2022. TRI explorer: Providing access to EPA’s toxics release inventory data. Washington, DC:
Toxics Release Inventory. U.S. Environmental Protection Agency.
https://enviro.epa.gov/triexplorer. December 19, 2022.
Trivedi NP, Rawal UM, Patel BP. 2007. Hepatoprotective effect of andrographolide against
hexachlorocyclohexane-induced oxidative injury. Integr Cancer Ther 6(3):271-280.
https://doi.org/10.1177/1534735407305985.
Trivedi NP, Rawal UM, Patel BP. 2009. Potency of andrographolide as an antitumor compound in BHC-
induced liver damage. Integr Cancer Ther 8(2):177-189.
https://doi.org/10.1177/1534735409335606.
Tryphonas L, Iverson F. 1983. Sequential histopathologic analysis of α-hexachlorocyclohexane-induced
hepatic megalocytosis and adenoma formation in the HPB mouse. J Natl Cancer Inst 71:1307-1318.
https://doi.org/10.1093/jnci/71.6.1307.
Tsatsakis AM, Tzatzarakis MN, Tutudaki M, et al. 2008. Assessment of levels of organochlorine
pesticides and their metabolites in the hair of a Greek rural human population. Hum Exp Toxicol
27(12):933-940. https://doi.org/10.1177/0960327108102047.
Tsezos M, Wang X. 1991a. Study on the kinetics of hazardous pollutants adsorption and desorption by
biomass: Mechanistic considerations. J Chem Technol Biotechnol 50:507-521.
Tsezos M, Wang X. 1991b. A study on lindane: Biosorption and biodegradation interactions. Biotech
Forum Europe 8:120-125.
Tsukada H, Gotoh M, Mochizuki Y, et al. 1979. Changes in peroxisomes in preneoplastic liver and
hepatoma of mice induced by α-benzene hexachloride. Cancer Res 39(5):1628-1634.
Tu CM. 1976. Utilization and degradation of lindane by soil microorganisms. Arch Microbiol 108:259-
263.
HEXACHLOROCYCLOHEXANE (HCH) 345
8. REFERENCES
Turner JC, Shanks V. 1980. Absorption of some organochlorine compounds by the rat small intestine -
in vivo. Bull Environ Contam Toxicol 24:652-655.
Tusell JM, Sunol C, Gelpi E, et al. 1988. Effect of lindane at repeated low doses. Toxicology 49:375-
379. https://doi.org/10.1016/0300-483X(88)90021-2.
Tyagi V, Mustafa MD, Sharma T, et al. 2016. Association of organochlorine pesticides with the mRNA
expression of tumour necrosis factor-alpha (TNF-α) & cyclooxygenase-2 (COX-2) genes in
idiopathic preterm birth. Indian J Med Res 143(6):731-738. https://doi.org/10.4103/0971-
5916.191986.
Tyagi S, Siddarth M, Mishra BK, et al. 2021. High levels of organochlorine pesticides in drinking water
as a risk factor for type 2 diabetes: A study in north India. Environ Pollut 271:116287.
https://doi.org/10.1016/j.envpol.2020.116287.
Ukropec J, Radikova Z, Huckova M, et al. 2010. High prevalence of prediabetes and diabetes in a
population exposed to high levels of an organochlorine cocktail. Diabetologia 53(5):899-906.
https://doi.org/10.1007/s00125-010-1683-2.
Ullmann L. 1986a. Acute dermal toxicity study with lindane in rats. Itingen, Switzerland: Research and
Consulting Company AG. RCC Project No. 061648.
Ullmann L. 1986b. 4-Hour acute aerosol inhalation toxicity study with lindane in rats. Itingen,
Switzerland: Research and Consulting Company AG. RCC Project No. 061637.
Ullmann L. 1986c. Primary eye irritation study with lindane in rabbits. Itingen, Switzerland: Research
and Consulting Company AG. RCC Project No. 061672.
Ullmann L. 1986d. Primary skin irritation study with lindane in rabbits (4-hour occlusive application).
Itingen, Switzerland: Research and Consulting Company AG. RCC Project No. 061661.
Uphouse L, Williams J. 1989. Diestrous treatment with lindane disrupts the female rat reproductive
cycle. Toxicology Letters 48:21-28. https://doi.org/10.1016/0378-4274(89)90181-1.
Upson K, De Roos AJ, Thompson ML, et al. 2013. Organochlorine pesticides and risk of endometriosis:
findings from a population-based case-control study. Environ Health Perspect 121(11-12):1319-
1324. https://doi.org/10.1289/ehp.1306648.
USGS. 2003. Methods of analysis of the U.S. Geological Survey National Water Quality Laboratory -
Determination of organochlorine pesticides and polychlorinated biphenyls in bottom and suspended
sediment by gas chromatography with electron-capture detection. Denver, CO: U.S. Geological
Survey. Resources Investigations Report 03-4293.
https://www.nemi.gov/methods/method_summary/8946/. May 5, 2021.
Valera B, Ayotte P, Poirier P, et al. 2013a. Associations between plasma persistent organic pollutant
levels and blood pressure in Inuit adults from Nunavik. Environ Int 59:282-289.
https://doi.org/10.1016/j.envint.2013.06.019.
Valera B, Jorgensen ME, Jeppesen C, et al. 2013b. Exposure to persistent organic pollutants and risk of
hypertension among Inuit from Greenland. Environ Res 122:65-73.
https://doi.org/10.1016/j.envres.2012.12.006.
Van Velsen FL, Danse LHJC, Van LFXR, et al. 1986. The subchronic oral toxicity of the β-isomer of
hexachlorocyclohexane in rats. Fundam Appl Toxicol 6:697-712. https://doi.org/10.1016/0272-
0590(86)90183-1.
Vargas-Gonzalez HH, Mendez-Rodriguez LC, Garcia-Hernandez J, et al. 2016. Persistent organic
pollutants (POPs) in populations of the clam Chione californiensis in coastal lagoons of the Gulf of
California. J Environ Sci Health B 51(7):435-445. https://doi.org/10.1080/03601234.2016.1159455.
Varona-Uribe ME, Torres-Rey CH, Diaz-Criollo S, et al. 2016. Exposure to pesticide mixtures and DNA
damage among rice field workers. Arch Environ Occup Health 71(1):3-9.
https://doi.org/10.1080/19338244.2014.910489.
Veith G, Defoe D, Bergstedt B. 1979. Measuring and estimating the bioconcentration factor of
chemicals in fish. J Fish Res Board Can 36:1040-1048.
Vendrell M, Tusell JM, Serratosa J. 1992a. c-fos Expression as a model for studying the action of
hexachlorocyclohexane isomers in the CNS. J Neurochem 58:862-869.
HEXACHLOROCYCLOHEXANE (HCH) 346
8. REFERENCES
Vendrell M, Tusell JM, Serratosa J. 1992b. Effect of γ-hexachlorocyclohexane and its isomers on proto-
oncogene c-fos expression in brain. Neurotoxicology 13:301-308.
Verbrugge DA, Othoudt RA, Grzyb KR, et al. 1991. Concentrations of inorganic and organic
contaminants in sediments of six harbors on the North American Great Lakes. Chemosphere 22:809-
820.
Verma A, Pillai MK. 1991. Bioavailability of soil-bound residues of DDT and HCH to certain plants.
Soil Biology and Biochemistry 23(4):347-351. https://doi.org/10.1016/0038-0717(91)90190-u.
Verner M, Charbonneau M, López-Carrillo L, et al. 2008. Physiologically based pharmacokinetic
modeling of persistent organic pollutants for lifetime exposure assessment: A new tool in breast
cancer epidemiologic studies. Environmental Health Perspectives 116(7):886-892.
https://doi.org/10.1289/ehp.10917.
Verner MA, Ayotte P, Muckle G, et al. 2009. A physiologically based pharmacokinetic model for the
assessment of infant exposure to persistent organic pollutants in epidemiologic studies. Environ
Health Perspect 117(3):481-487. https://doi.org/10.1289/ehp.0800047.
Verschueren K. 1983. alpha-Hexachlorocyclohexane. In: Handbook of environmental data of organic
chemicals. 2
nd
ed. New York, NY: Van Nostrand Reinhold Co, 718-725.
Viel JF, Floret N, Deconinck E, et al. 2011. Increased risk of non-Hodgkin lymphoma and serum
organochlorine concentrations among neighbors of a municipal solid waste incinerator. Environ Int
37(2):449-453. https://doi.org/10.1016/j.envint.2010.11.009.
Vijaya Padma V, Sowmya P, Arun Felix T, et al. 2011. Protective effect of gallic acid against lindane
induced toxicity in experimental rats. Food Chem Toxicol 49(4):991-998.
https://doi.org/10.1016/j.fct.2011.01.005.
Vijaya Padma V, Poornima P, Prakash C, et al. 2013. Oral treatment with gallic acid and quercetin
alleviates lindane-induced cardiotoxicity in rats. Can J Physiol Pharmacol 91(2):134-140.
https://doi.org/10.1139/cjpp-2012-0279.
Vijgen J, Abhilash PC, Li YF, et al. 2011. Hexachlorocyclohexane (HCH) as new Stockholm
Convention POPs-a global perspective on the management of lindane and its waste isomers. Environ
Sci Pollut Res Int 18(2):152-162. https://doi.org/10.1007/s11356-010-0417-9.
Viswanathan R, Ray S, Scheunert I, et al. 1988. Investigations on accumulation and biotransformation
by earthworms of lindane occurring as soil contaminant. In: Abbou R, ed. Hazard Waste:
Detection, Control, Treatment. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.,
759-765.
Vizcaino E, Grimalt JO, Carrizo D, et al. 2011. Assessment of prenatal exposure to persistent
organohalogen compounds from cord blood serum analysis in two Mediterranean populations
(Valencia and Menorca). J Environ Monit 13(2):422-432. https://doi.org/10.1039/c0em00483a.
Vodopick H. 1975. Cherchez la Chienne: Erythropoietic hypoplasia after exposure to g-benzene
hexachloride. JAMA 24(8):850-851.
Wadaskar JV, Ekhe JD, Kale SP. 2006. Adsorption-desorption of HCH and endosulfan on a soil.
Environ Technol 27(9):1011-1017. https://doi.org/10.1080/09593332708618709.
Waite DT, Gurprasad NP, Sproull JF, et al. 2001. Atmospheric movements of lindane (γ-
hexachlorocyclohexane) from canola fields planted with treated seed. J Environ Qual 30:768-775.
Waite DT, Cabalo E, Chau D, et al. 2007. A comparison of flux chambers and ambient air sampling to
measure γ-hexachlorocyclohexane volatilisation from canola (Brassica napus) fields. Chemosphere
68(6):1074-1081. https://doi.org/10.1016/j.chemosphere.2007.01.085.
Waliszewski SM. 1993. Residues of lindane, HCH isomers and HCB in the soil after lindane
application. Environ Pollut 82:289-292.
Waliszewski SM, Bermudez MT, Infanzon RM, et al. 2005. Persistent organochlorine pesticide levels in
breast adipose tissue in women with malignant and benign breast tumors. Bull Environ Contam
Toxicol 75(4):752-759. https://doi.org/10.1007/s00128-005-0815-8.
HEXACHLOROCYCLOHEXANE (HCH) 347
8. REFERENCES
Waliszewski SM, Melo-Santiesteban G, Villalobos-Pietrini R, et al. 2009. Breast milk excretion kinetic
of β-HCH, pp'DDE and pp'DDT. Bull Environ Contam Toxicol 83(6):869-873.
https://doi.org/10.1007/s00128-009-9796-3.
Wang TC, Hoffman ME, David J, et al. 1992. Chlorinated pesticide residue occurrence and distribution
in mosquito control impoundments along the Florida Indian River Lagoon. Bull Environ Contam
Toxicol 49:217-223.
Wang F, Xu ZR, Sun JH. 2006. Effect of HCH contamination of diet on the growth performance and
immune and antioxidant ability in growing/finishing pigs. Vet Res Commun 30(6):645-654.
https://doi.org/10.1007/s11259-006-3327-z.
Wang X, Gao M, Tan Y, et al. 2021a. Associations of dietary exposure to organochlorine pesticides
from plant-origin foods with lipid metabolism and inflammation in women: A multiple follow-up
study in North China. Bull Environ Contam Toxicol 107(2):289-295.
https://doi.org/10.1007/s00128-021-03224-5.
Wang S, Hu C, Lu A, et al. 2021b. Association between prenatal exposure to persistent organic
pollutants and neurodevelopment in early life: A mother-child cohort (Shanghai, China). Ecotoxicol
Environ Saf 208:111479. https://doi.org/10.1016/j.ecoenv.2020.111479.
Wang SS, Lu AX, Cao LL, et al. 2022a. Effects of prenatal exposure to persistent organic pollutants on
neonatal outcomes: A mother-child cohort (Shanghai, China). Environ Res 203:111767.
https://doi.org/10.1016/j.envres.2021.111767.
Wang J, Cao LL, Gao ZY, et al. 2022b. Relationship between thyroid hormone parameters and exposure
to a mixture of organochlorine pesticides, mercury and nutrients in the cord blood of newborns.
Environ Pollut 292(Pt A):118362. https://doi.org/10.1016/j.envpol.2021.118362.
Ward E, Sheulte P, Grajewski B, et al. 2000. Serum organochlorine levels with breast cancer: A nested
case-control study of Norwegian women. Cancer Epidemiol Biomarkers Prev 9:1357-1367.
Warembourg C, Debost-Legrand A, Bonvallot N, et al. 2016. Exposure of pregnant women to persistent
organic pollutants and cord sex hormone levels. Hum Reprod 31(1):190-198.
https://doi.org/10.1093/humrep/dev260.
Warner M, Rauch S, Coker ES, et al. 2018. Obesity in relation to serum persistent organic pollutant
concentrations in CHAMACOS women. Environ Epidemiol 2(4):e032.
https://doi.org/10.1097/ee9.0000000000000032.
Watanabe KH, Desimone FW, Thiyagarajah A, et al. 2003. Fish tissue quality in the lower Mississippi
River and health risks from fish consumption. Sci Total Environ 302(1-3):109-126.
https://doi.org/10.1016/s0048-9697(02)00396-0.
Wattigney WA, Irvin-Barnwell E, Li Z, et al. 2022. Biomonitoring of toxic metals, organochlorine
pesticides, and polybrominated biphenyl 153 in Michigan urban anglers. Environ Res 203:111851.
https://doi.org/10.1016/j.envres.2021.111851.
Weber L, Song K, Boyle T, et al. 2018. Organochlorine levels in plasma and risk of multiple myeloma.
J Occup Environ Med 60(10):911-916. https://doi.org/10.1097/jom.0000000000001387.
Weiss G. 1986. Benzene hexachloride. In: Hazardous chemicals data book. 2
nd
ed. Park Ridge, NJ:
Noyes Data Corporation, 153.
Weisse I, Herbst M. 1977. Carcinogenicity study of lindane in the mouse. Toxicology 7(2):233-238.
https://doi.org/10.1016/0300-483x(77)90069-5.
Wheatley GA, Hardman JA. 1965. Indications of the presence of organochlorine insecticides in
rainwater in Central England. Nature 207(4996):486-487. https://doi.org/10.1038/207486a0.
Wheeler M. 1977. Gamma benzene hexachloride (Kwell) in a child. Western Journal of Medicine
127(6):518-521.
Whitehead TP, Crispo Smith S, Park JS, et al. 2015. Concentrations of persistent organic pollutants in
California women's serum and residential dust. Environ Res 136:57-66.
https://doi.org/10.1016/j.envres.2014.10.009.
WHO. 2010. Guidelines for indoor air quality: Selected pollutants. World Health Organization.
https://www.who.int/publications/i/item/9789289002134. July 16, 2023.
HEXACHLOROCYCLOHEXANE (HCH) 348
8. REFERENCES
WHO. 2022. Guidelines for drinking-water quality. Fourth edition incorporating the first and second
addenda. World Health Organization. https://www.who.int/publications/i/item/9789240045064.
July 16, 2023.
W
iberg K, Brorstrom-Lunden E, Wangberg I, et al. 2001. Concentrations and fluxes of hexachloro-
c
yclohexanes and chiral composition of α-HCH in environmental samples from Southern Baltic Sea.
Environ Sci Technol 35(24):4739-4746.
W
ielsoe M, Kern P, Bonefeld-Jorgensen EC. 2017. Serum levels of environmental pollutants is a risk
factor for breast cancer in Inuit: a case control study. Environ Health 16(1):56.
https://doi.org/10.1186/s12940-017-0269-6.
Wild SR, Jones KC. 1992. Organic chemicals entering agricultural soils in sewage sludges: Screening
for their potential to transfer to crop plants and livestock. Sci Total Environ 119:85-119.
Wiles DA, Russell JL, Olson KR, et al. 2015. Massive lindane overdose with toxicokinetics analysis. J
Med Toxicol 11(1):106-109. https://doi.org/10.1007/s13181-014-0403-6.
Wittlinger R, Ballschmiter K. 1990. Studies of the global baseline pollution. XIII. C6-C14
organohalogens (α- and g-HCH, HCB, PCB 4,4'-DDT, 4,4'-DDE, cis- and trans-chlordane, trans-
nonachlor, anisols) in the lower troposphere of the southern Indian Ocean. Fresenius J Anal Chem
336:193-200.
Wolff G, Roberts D, Morrissey R, et al. 1987. Tumorigenic responses to lindane in mice: Potentiation by
a dominant mutation. Carcinogenesis 8(12):1889-1897. https://doi.org/10.1093/carcin/8.12.1889.
Wong F, Hung H, Dryfhout-Clark H, et al. 2021. Time trends of persistent organic pollutants (POPs)
and chemicals of emerging Arctic concern (CEAC) in Arctic air from 25 years of monitoring. Sci
Total Environ 775:145109. https://doi.org/10.1016/j.scitotenv.2021.145109.
Woodliff HJ, Connor PM, Scopa J. 1966. Aplastic anemia associated with insecticides. Med J Aust
1:628-629.
Woolley DE, Griffith JA. 1989. Kinetics and thresholds of several indices of lindane-induced toxicity.
Pharmacol Biochem Behav 33:787-792. https://doi.org/10.1016/0091-3057(89)90471-1.
WQP. 2023. Water Quality Portal data: alpha-Hexachlorocyclohexane, beta-hexachlorocyclohexane,
1,2,3,4,5,6 -Hexachlorocyclohexane. National Water Quality Monitoring Council.
https://www.waterqualitydata.us/portal/. May 6, 2021.
Wu WZ, Xu Y, Schramm KW, et al. 1997. Study of sorption, biodegradation, and isomerization of HCH
in stimulated sediment/water system. Chemosphere 35(9):1887-1894.
Wu Z, Lin T, Hu L, et al. 2020. Atmospheric legacy organochlorine pesticides and their recent exchange
dynamics in the Northwest Pacific Ocean. Sci Total Environ 727:138408.
https://doi.org/10.1016/j.scitotenv.2020.138408.
Xu X, Dailey AB, Talbott EO, et al. 2010. Associations of serum concentrations of organochlorine
pesticides with breast cancer and prostate cancer in U.S. adults. Environ Health Perspect 118(1):60-
66. https://doi.org/10.1289/ehp.0900919.
Xu S, Yang X, Qian Y, et al. 2022. Analysis of serum levels of organochlorine pesticides and related
factors in Parkinson's disease. Neurotoxicology 88:216-223.
https://doi.org/10.1016/j.neuro.2021.12.001.
Xue M, Shen G, Yu J, et al. 2010. Dynamic changes of α-hexachlorocyclohexane and its enantiomers in
various tissues of Japanese Rabbits (Oyctolagus cuniculus) after oral or dermal exposure.
Chemosphere 81(11):1486-1491. https://doi.org/10.1016/j.chemosphere.2010.08.046.
Yaduvanshi SK, Srivastava N, Marotta F, et al. 2012. Evaluation of micronuclei induction capacity and
mutagenicity of organochlorine and organophosphate pesticides. Drug Metab Lett 6(3):187-197.
https://doi.org/10.2174/1872312811206030006.
Yalcin SS, Orun E, Yalcin S, et al. 2015. Organochlorine pesticide residues in breast milk and maternal
psychopathologies and infant growth from suburban area of Ankara, Turkey. Int J Environ Health
Res 25(4):364-372. https://doi.org/10.1080/09603123.2014.945515.
Yamazaki K, Itoh S, Araki A, et al. 2020. Associations between prenatal exposure to organochlorine
pesticides and thyroid hormone levels in mothers and infants: The Hokkaido study on environment
HEXACHLOROCYCLOHEXANE (HCH) 349
8. REFERENCES
and children's health. Environmental Research 189:109840.
https://doi.org/10.1016/j.envres.2020.109840.
Yang X, Jiang X, Yu G, et al. 2007. Leaf-air transfer of organochlorine pesticides from three selected
vegetables. Environ Pollut 148(2):555-561. https://doi.org/10.1016/j.envpol.2006.11.029.
Yang D, Li X, Tao S, et al. 2010. Enantioselective behavior of α-HCH in mouse and quail tissues.
Environ Sci Technol 44(5):1854-1859. https://doi.org/10.1021/es9030134.
Yang Y, Zhang YB, Sheng W, et al. 2014. Influence of soy isoflavone on lindane cumulant in Sprague-
Dawley rats. Biomed Environ Sci 27(8):637-640. https://doi.org/10.3967/bes2014.097.
Yang X, Zhang M, Lu T, et al. 2020. Metabolomics study and meta-analysis on the association between
maternal pesticide exposome and birth outcomes. Environ Res 182:109087.
https://doi.org/10.1016/j.envres.2019.109087.
Yang C, Fang J, Sun X, et al. 2021a. Prenatal exposure to organochlorine pesticides and infant growth:
A longitudinal study. Environ Int 148:106374. https://doi.org/10.1016/j.envint.2020.106374.
Yang W, Ni W, Jin L, et al. 2021b. Determination of organochlorine pesticides in human umbilical cord
and association with orofacial clefts in offspring. Chemosphere 266:129188.
https://doi.org/10.1016/j.chemosphere.2020.129188.
Yin S, Zhang J, Guo F, et al. 2019. Transplacental transfer of organochlorine pesticides: Concentration
ratio and chiral properties. Environ Int 130:104939. https://doi.org/10.1016/j.envint.2019.104939.
Yin S, Zhang J, Guo F, et al. 2020. Transplacental transfer mechanism of organochlorine pesticides: An
in vitro transcellular transport study. Environ Int 135:105402.
https://doi.org/10.1016/j.envint.2019.105402.
Yin S, Sun Y, Yu J, et al. 2021. Prenatal exposure to organochlorine pesticides is associated with
increased risk for neural tube defects. Sci Total Environ 770:145284.
https://doi.org/10.1016/j.scitotenv.2021.145284.
Yousefi F, Asadikaram G, Karamouzian S, et al. 2022. Organochlorine and organophosphorus pesticides
may induce brain cancer through oxidative stress. Toxicol Ind Health 38(11):717-732.
https://doi.org/10.1177/07482337221125954.
Yu Y, Wang B, Wang X, et al. 2013. Hexachlorocyclohexanes (HCHs) in placenta and umbilical cord
blood and dietary intake for women in Beijing, China. Environ Pollut 179:75-80.
https://doi.org/10.1016/j.envpol.2013.03.056.
Yu Y, Hung H, Alexandrou N, et al. 2015. Multiyear measurements of flame retardants and
organochlorine pesticides in air in Canada's Western sub-Arctic. Environ Sci Technol 49(14):8623-
8630. https://doi.org/10.1021/acs.est.5b01996.
Yuksel H, Karadas E, Keles H, et al. 2009. Effects of hexachlorocyclohexane (HCH-γ-isomer, lindane)
intoxication on the proliferation and apoptosis in rat testes. Acta Vet Brno 78(4):615-620.
https://doi.org/10.2754/avb200978040615.
Zeng JY, Miao Y, Liu C, et al. 2022. Serum multiple organochlorine pesticides in relation to
testosterone concentrations among Chinese men from an infertility clinic. Chemosphere
299:134469. https://doi.org/10.1016/j.chemosphere.2022.134469.
Zesch A, Nitzsche K, Lange M. 1982. Demonstration of the percutaneous resorption of a lipophilic
pesticide and its possible storage in the human body. Arch Dermatol Res 273:43-49.
Zhang N, Bashir S, Qin J, et al. 2014. Compound specific stable isotope analysis (CSIA) to characterize
transformation mechanisms of α-hexachlorocyclohexane. J Hazard Mater 280:750-757.
https://doi.org/10.1016/j.jhazmat.2014.08.046.
Zhang Y, Guo J, Zhang X, et al. 2016. Interaction effects between organochlorine pesticides and
isoflavones in vitro and in vivo. Biomed Res Int 2016:6861702.
https://doi.org/10.1155/2016/6861702.
Zhang X, Wu X, Lei B, et al. 2018. Transplacental transfer characteristics of organochlorine pesticides
in paired maternal and cord sera, and placentas and possible influencing factors. Environ Pollut
233:446-454. https://doi.org/10.1016/j.envpol.2017.10.075.
HEXACHLOROCYCLOHEXANE (HCH) 350
8. REFERENCES
Zhang J, Li C, Yin S, et al. 2021. Environmental exposure to organochlorine pesticides and its
association with the risk of hearing loss in the Chinese adult population: A case-control study. Sci
Total Environ 767:145153. https://doi.org/10.1016/j.scitotenv.2021.145153.
Zhang M, Wang L, Li X, et al. 2023. Individual and mixtures of polychlorinated biphenyls and
organochlorine pesticides exposure in relation to metabolic syndrome among Chinese adults. Sci
Total Environ 877:162935. https://doi.org/10.1016/j.scitotenv.2023.162935.
Zhao B, Shen H, Liu F, et al. 2012. Exposure to organochlorine pesticides is an independent risk factor
of hepatocellular carcinoma: a case-control study. J Expo Sci Environ Epidemiol 22(6):541-548.
https://doi.org/10.1038/jes.2011.29.
Zoeteman BCJ, Harmsen K, Linders JBHJ, et al. 1980. Persistent organic pollutants in river water and
ground water of the Netherlands. Chemosphere 9:231-249.
Zong G, Valvi D, Coull B, et al. 2018. Persistent organic pollutants and risk of type 2 diabetes: A
prospective investigation among middle-aged women in Nurses' Health Study II. Environ Int
114:334-342. https://doi.org/10.1016/j.envint.2017.12.010.
HEXACHLOROCYCLOHEXANE (HCH) A-1
APPENDIX A. ATSDR MINIMAL RISK LEVEL WORKSHEETS
MRLs are derived when reliable and sufficient data exist to identify the target organ(s) of effect or the
most sensitive health effect(s) for a specific duration for a given route of exposure. An MRL is an
estimate of the daily human exposure to a hazardous substance that is likely to be without appreciable risk
of adverse noncancer health effects over a specified route and duration of exposure. MRLs are based on
noncancer health effects only; cancer effects are not considered. These substance-specific estimates,
which are intended to serve as screening levels, are used by ATSDR health assessors to identify
contaminants and potential health effects that may be of concern at hazardous waste sites. It is important
to note that MRLs are not intended to define clean-up or action levels.
MRLs are derived for hazardous substances using the NOAEL/uncertainty factor approach. They are
below levels that might cause adverse health effects in the people most sensitive to such chemical-
induced effects. MRLs are derived for acute (1–14 days), intermediate (15–364 days), and chronic
(≥365 days) durations and for the oral and inhalation routes of exposure. Currently, MRLs for the dermal
route of exposure are not derived because ATSDR has not yet identified a method suitable for this route
of exposure. MRLs are generally based on the most sensitive substance-induced endpoint considered to
be of relevance to humans. LOAELs for serious health effects (such as irreparable damage to the liver or
kidneys, or serious birth defects) are not used as a basis for establishing MRLs. Exposure to a level above
the MRL does not mean that adverse health effects will occur.
MRLs are intended only to serve as a screening tool to help public health professionals decide where to
look more closely. They may also be viewed as a mechanism to identify those hazardous waste sites that
are not expected to cause adverse health effects. Most MRLs contain a degree of uncertainty because of
the lack of precise toxicological information on the people who might be most sensitive (e.g., infants,
elderly, nutritionally or immunologically compromised) to the effects of hazardous substances. ATSDR
uses a conservative (i.e., protective) approach to address this uncertainty consistent with the public health
principle of prevention. Although human data are preferred, MRLs often must be based on animal studies
because relevant human studies are lacking. In the absence of evidence to the contrary, ATSDR assumes
that humans are more sensitive to the effects of hazardous substances than animals and that certain
persons may be particularly sensitive. Thus, the resulting MRL may be as much as 100-fold below levels
that have been shown to be nontoxic in laboratory animals.
HEXACHLOROCYCLOHEXANE (HCH) A-2
APPENDIX A
Proposed MRLs undergo a rigorous review process: Health Effects/MRL Workgroup reviews within the
Office of Innovation and Analytics, Toxicology Section, expert panel peer reviews, and agency-wide
MRL Workgroup reviews, with participation from other federal agencies and comments from the public.
They are subject to change as new information becomes available concomitant with updating the
toxicological profiles. Thus, MRLs in the most recent toxicological profiles supersede previously
published MRLs. For additional information regarding MRLs, please contact the Office of Innovation
and Analytics, Toxicology Section, Agency for Toxic Substances and Disease Registry, 1600 Clifton
Road NE, Mailstop S106-5, Atlanta, Georgia 30329-4027.
HCH is a mixture of eight isomers, four of which are of commercial significance: α-HCH (CAS Registry
Number 319-84-6), β-HCH (CAS Registry Number 319-85-7), γ-HCH (CAS Registry Number 58-89-9),
and δ-HCH (CAS Registry Number 319-86-8). Technical HCH, which is used as an insecticide, is made
up of the various isomers at different concentrations. The wide variations in isomer composition of
technical HCH preclude the possibility of MRL derivation. MRL derivation was considered for the
isomers included in this toxicological profile: α-, β-, γ-, and δ-HCH.
HEXACHLOROCYCLOHEXANE (HCH) A-3
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration inhalation MRL for
α-HCH.
Rationale for Not Deriving an MRL: No acute-duration inhalation studies of α-HCH in humans or
animals were located, precluding derivation of an acute-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-4
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration inhalation MRL
for α-HCH.
Rationale for Not Deriving an MRL: No intermediate-duration inhalation studies of α-HCH in humans
or animals were located, precluding derivation of an intermediate-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-5
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration inhalation MRL for
α-HCH.
Rationale for Not Deriving an MRL: No chronic-duration inhalation studies of α-HCH in humans or
animals were located, precluding derivation of a chronic-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-6
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration oral MRL for α-HCH.
Rationale for Not Deriving an MRL: No adequate exposure-response data were available for humans.
Data on effects in laboratory animals exposed orally to α-HCH for acute durations are limited to a
freestanding NOAEL of 20 mg/kg/day for body weight changes during the first 14 days of a 28-day study
comparing gene expression profiles for α- and γ-HCH (Sumida et al. 2007). Increased relative liver
weight (24%) and decreased serum ALP (19%) were seen at this dose after 14 days, but histopathology
was not evaluated at this time, so reliable hepatic effect levels could not be determined for this study.
Thus, this study did not provide an adequate basis for MRL derivation.
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-7
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Intermediate
MRL: 0.002 mg/kg/day (2 µg/kg/day)
Critical Effect: Increased liver weight and liver histopathology changes
Reference: Sumida et al. 2007
Point of Departure: 2 mg/kg/day (NOAEL)
Uncertainty Factor: 100
Modifying Factor: 10
LSE Graph Key: 6
Species: Rat
MRL Summary: An intermediate-duration oral MRL of 0.002 mg/kg/day (2 µg/kg/day) was derived
based on a NOAEL of 2 mg/kg/day for liver effects in a 28-day study in rats (Sumida et al. 2007). The
NOAEL was divided by a total uncertainty factor of 100 (10 for human variability and 10 for animal to
human extrapolation) and a modifying factor of 10 (for lack of studies examining developmental and
immunological effects and limitations in available data on neurotoxicity).
Selection of the Critical Effect: No dose-response data are available for humans. In animal studies,
hepatic effects (including cancers) occurred at lower doses than effects on body weight or kidneys. A
single study found no effects on motor nerve conduction velocity in rats exposed to 106.2 mg/kg/day for
30 days (Muller et al. 1981). The α-hexachlorocyclohexane database only contains studies with body
weight, renal, hepatic, neurological, and cancer endpoints; no other effects were evaluated in the available
studies. Table A-1 summarizes the hepatic effects from intermediate-duration oral studies in laboratory
animals exposed to doses up to 70 mg/kg/day.
Table A-1. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
α-Hexachlorocyclohexane
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Hepatic effects
Mouse (dd)
20–40 M
24 weeks
(F)
ND
18
33% relative liver weight
increase; hepatocellular
hypertrophy
Ito et al.
1973
Rat
(Fischer-344)
4 M
28 days
(GO)
2
20
Increased relative liver weight
(25%); centrilobular
hepatocellular hypertrophy
Sumida et al.
2007
Rat (W strain)
18–24 M
48 weeks
(F)
ND
35
Hepatocellular hypertrophy
Ito et al.
1975
Rat (Wistar)
8 M
24 weeks
(F)
ND
45
Mild liver cell hypertrophy;
2-fold increase in liver weight
Nagasaki et
al. 1975

HEXACHLOROCYCLOHEXANE (HCH) A-8
APPENDIX A
Table A-1. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
α-Hexachlorocyclohexane
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Hamster
(Golden Syrian)
6–10 M
24 weeks
(F)
ND
45
20–38% increase in liver
weight; liver cell hypertrophy
Nagasaki et
al. 1975
Rat (Wistar)
10 F, 10 M
6–9
months (F)
ND
60 M
70 F
(serious
LOAEL for
decreased
survival)
Decreased survival;
moderate histopathology
changes (focal necrosis, fatty
degeneration); >2-fold
increase in liver weight
Fitzhugh et
al. 1950
Principal study for the MRL.
(F) = feed; F = female(s); (GO) = gavage in oil vehicle; LOAEL = lowest-observed-adverse-effect level; M = male(s);
ND = not determined; NOAEL = no-observed-adverse-effect level
Selection of the Principal Study: As Table A-1 shows, the lowest effect level (18 mg/kg/day) was
associated with a relative liver weight increase of 33% and hepatocellular hypertrophy in mice exposed
via diet for 24 weeks (Ito et al. 1973). A NOAEL was not identified for this study. In a study of rats
exposed by gavage for only 28 days (Sumida et al. 2007), a slightly higher LOAEL of 20 mg/kg/day was
identified for similar hepatic effects; the NOAEL in that study was 2 mg/kg/day. All of the other studies
identified hepatic effects at the lowest doses tested (no other NOAEL was identified). The study by
Sumida et al. (2007) was selected as the principal study for MRL derivation because the NOAEL
identified in the study was lower than the LOAELs identified in all other studies and because a NOAEL
was not identified in the study by Ito et al. (1973). Although the LOAEL (18 mg/kg/day) from Ito et al.
(1973) is slightly lower than the LOAEL (20 mg/kg/day) identified by Sumida et al. (2007), use of the
LOAEL from Ito et al. (1973) as the point of departure (POD) would necessitate the use of an uncertainty
factor of 10, while use of the NOAEL (2 mg/kg/day) from Sumida et al. (2007) does not. In addition, if
the LOAEL from Ito et al. (1973) were used as the POD, it would be equivalent to the value derived
based on the NOAEL from Sumida et al. (2007) (18 mg/kg/day divided by an uncertainty factor of 10 for
use of a LOAEL yields 1.8 mg/kg/day, which rounds to 2 mg/kg/day).
Summary of the Principal Study:
Sumida K, Siato K, Oeda K, et al. 2007. A comparative study of gene expression profiles in rat liver
after administration of α-hexachlorocyclohexane and lindane. J Toxicol Sci 32(3):261-288.
Groups of four male F344 rats were administered α-HCH (99% purity, in corn oil) by gavage at doses of
0 (corn oil control), 2, or 20 mg/kg/day for 28 consecutive days. The following parameters were
evaluated: body weight, serum clinical chemistry (AST, ALT, ALP, and total bilirubin in blood collected
at necropsy), liver weight, hepatic histopathology, and gene expression in the liver. There were no effects
on body weight at any dose. After 28 days of exposure, small but statistically significant increases in
AST and ALT were seen at 2 mg/kg/day, but not at 20 mg/kg/day. At the high dose, ALP was decreased
by 19% at the end of 28 days. At 20 mg/kg/day, liver weights were increased by 20% (absolute) and 25%
(relative) compared to controls. At 2 mg/kg/day, relative liver weight was increased by a small, but
HEXACHLOROCYCLOHEXANE (HCH) A-9
APPENDIX A
statistically significant margin of 6%. Hepatocellular hypertrophy was observed in 4/4 animals at
20 mg/kg/day compared to 0/4 in control and 2 mg/kg/day groups. Based on the increase in liver weight
and histological changes at 20 mg/kg/day, this dose is a LOAEL. No exposure-related changes occurred
at the 2 mg/kg/day, indicating that this dose is a NOAEL.
Selection of the Point of Departure for the MRL: The NOAEL of 2 mg/kg/day was selected as the POD
for derivation of the intermediate-duration oral MRL for α-HCH. Although quantitative data on
histopathology findings in the liver were reported, the incidence data increased from 0/4 at 2 mg/kg/day
to 4/4 at 20 mg/kg/day, so the data were not amenable to benchmark dose (BMD) modeling. BMD
modeling of relative liver weight data was undertaken, but that dataset was also not amenable to BMD
modeling, as neither the constant nor nonconstant variance models provide an adequate fit to the variance
data. Thus, the NOAEL was selected as the POD.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty and Modifying Factor: The NOAEL of 2 mg/kg/day was divided by a total uncertainty
factor (UF) of 100 and a modifying factor (MF) of 10:
• 10 for extrapolation from animals to humans
• 10 for human variability
A modifying factor of 10 was applied to the NOAEL to account for lack of data on developmental
toxicity and immunotoxicity and limitations in available data on neurotoxicity. These are sensitive
endpoints for other HCH isomers.
MRL = NOAEL ÷ (UF x MF)
2 mg/kg/day ÷ ((10 x 10) x 10) = 0.002 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Hepatic effects
have been observed in rats, mice, and hamsters after intermediate- and chronic-duration oral exposures to
α-HCH. Observed effects include increases in absolute and relative liver weight, hepatocellular
hypertrophy and/or hyperplasia, focal necrosis, fatty degeneration, hepatomegaly, bile duct proliferation,
oval cells, nodular hyperplasia, and megalocytosis (Fitzhugh et al. 1950; Ito et al. 1973, 1975, 1976;
Nagasaki et al. 1975; Sumida et al. 2007; Tryphonas and Iverson 1983). Both rats and mice have also
developed liver tumors at higher doses of α-HCH (Hanada et al. 1973; Ito et al. 1973, 1975, 1976;
Nagasaki et al. 1975; Tryphonas and Iverson 1983; Tsukada et al. 1979).
There are no studies on the effects of α-HCH on the developing organism or on the immune system.
Studies of β- and γ-HCH have shown developmental effects (e.g., Di Consiglio et al. 2009; Hassoun and
Stohs 1996a; La Sala et al. 2009; Rivera et al. 1998; Sauviat et al. 2005; Srinivasan et al. 1991), and for
γ-HCH, these are the effects occurring at the lowest doses in animal studies. γ-HCH, and to a lesser
extent β-HCH, have been demonstrated to induce suppression of the immune system in several species
(e.g., Banerjee et al. 1996; Cornacoff et al. 1988; Desi et al. 1978; Dewan et al. 1980; Hong and Boorman
1993; Khurana et al. 1999; Koner et al. 1998; Mediratta et al. 2008; Meera et al. 1992; Van Velsen et al.
1986). Animal studies of γ-HCH indicate that these effects occur at lower doses than hepatic effects.
Thus, the lack of developmental and immunotoxicity data for α-HCH is a significant limitation of the
existing database for this isomer.
Data on the neurotoxicity of α-HCH are limited to a single study showing no change in motor nerve
conduction velocity after 30 days of exposure (Muller et al. 1981). Both β- and γ-HCH have induced
neurotoxic effects in laboratory rodents (e.g., Cornacoff et al. 1988; EPA 1999a; Gilbert and Mack 1995;
HEXACHLOROCYCLOHEXANE (HCH) A-10
APPENDIX A
Parmar et al. 2003; Van Velsen et al. 1986), and γ-HCH has been shown to induce neurotoxicity in
humans exposed orally (e.g., Davies et al. 1983; Harris et al. 1969; Munk and Nantel 1977; Nordt and
Chew 2000; Powell 1980; Starr and Clifford 1972; Storen 1955).
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-11
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: α-HCH
CAS Number: 319-84-6
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Chronic
MRL: 0.0009 mg/kg/day (0.9 µg/kg/day)
Critical Effect: Increased liver weight and liver histopathology changes
Reference: Fitzhugh et al. 1950
Point of Departure: 0.9 mg/kg/day (NOAEL)
Uncertainty Factor: 100
Modifying Factor: 10
LSE Graph Key: 14
Species: Rat
MRL Summary: A chronic-duration oral MRL of 0.0009 mg/kg/day (0.9 µg/kg/day) was derived for
α-HCH based on a NOAEL of 0.9 mg/kg/day and a LOAEL of 4 mg/kg/day for increased liver weight
and liver histopathology changes in rats exposed to α-HCH in the diet for 107 weeks (Fitzhugh et al.
1950). A total uncertainty factor of 100 (10 for extrapolation from animals to humans and 10 for human
variability) and a modifying factor of 10 (for lack of immunotoxicity data and limitations in data on
potential neurotoxicity) were applied to the NOAEL to derive the MRL.
Selection of the Critical Effect: Two chronic-duration oral studies of α-HCH were located: Fitzhugh et
al. (1950) and Ito et al. (1975). While Ito et al. (1975) examined only cancer endpoints, Fitzhugh et al.
(1950) evaluated histopathology in a large number of organs and identified the liver as the most sensitive
target of chronic-duration oral exposure.
Selection of the Principal Study: Only Fitzhugh et al. (1950) evaluated endpoints other than cancer, so
this study was selected for use in derivation of the chronic-duration oral MRL.
Summary of the Principal Study:
Fitzhugh OG, Nelson AA, Frawley JP. 1950. The chronic toxicities of technical benzene hexachloride
and its α, β, and γ isomers. J Pharmacol Exp Ther 100:59-66.
Groups of 10 male and 10 female Wistar rats were treated with 0, 10, 50, 100, or 800 ppm α-HCH in food
for life. ATSDR calculated doses corresponding to these concentrations using default food intake and
body weight values for male and female Wistar rats in chronic studies as reported in EPA (1988b).
Estimated doses were 0, 0.7, 4, 7, or 60 mg/kg/day in males and 0, 0.9, 4, 9, or 70 mg/kg/day in females.
The lifetime of the animals sacrificed at the end of the experiment was taken as 107 weeks. Endpoints
included clinical signs, body weight, food consumption, organ weights (liver, kidney, and spleen), gross
pathology, and histopathology (lung, heart, liver, spleen, pancreas, stomach, small intestine, colon,
kidney, adrenal, thyroid, leg muscles and bones, bone marrow, and testis or uterus and ovary). The
numbers of animals per group evaluated for histopathology were 10, 8, 14, and 10 in the control through
100 ppm groups.
Survival was significantly reduced at the high dose (mean survival 35.9 weeks at 800 ppm [60–
70 mg/kg/day] versus 58.3 weeks in controls), so effects in this group are considered to reflect
intermediate-duration exposure. The mean age at death in the remaining groups did not differ from
HEXACHLOROCYCLOHEXANE (HCH) A-12
APPENDIX A
controls (58.3, 54.6, 54.9, and 56.2 weeks for control through 100 ppm groups, respectively). Body
weight gain through the first 6 months on study was tabulated for the 100 ppm (7–9 mg/kg/day) and
800 ppm (60–70 mg/kg/day) groups, but not for the 10 or 50 ppm groups. However, the text of the
publication indicated that “lower experimental dosage levels had no effect on growth.” Additionally,
there was no significant difference in body weight gain in the 100 ppm (7–9 mg/kg/day) group, and no
effect on food consumption in any group.
Histopathology findings were reported for the kidney, testes, and liver. Kidney pathological effects were
not observed in groups receiving 10, 50, or 100 ppm, with the exception of slight brown pigmentation of
the convoluted tubular epithelium at 100 ppm (7–9 mg/kg/day). The 800 ppm (60–70 mg/kg/day) group
had slight to moderate kidney damage primarily consisting of tubular dilatation and/or atrophy,
glomerular fibrosis and/or atrophy, and interstitial cell infiltration. The study authors reported a
“questionable” increase in the degree of testicular atrophy in the group exposed to 800 ppm (60–
70 mg/kg/day) α-HCH, but no further information was provided.
Significant increases in relative liver weight (both sexes grouped for analysis) were seen at 50 ppm (32%)
and 100 ppm (44%). Gross and microscopic pathology findings were limited to the liver in the groups
exposed for the full duration; there were no microscopic changes in the controls. Liver histopathology
findings in treated animals were described qualitatively as very slight histological changes at 50 ppm
(4 mg/kg/day) and slight histological changes at 100 ppm (7–9 mg/kg/day). The lesions were described
as “characteristic of certain chlorinated cyclic compounds” with citation to earlier studies of dichloro-
diphenyltrichloroethane (DDT). The earlier studies (e.g., Fitzhugh and Nelson 1947; Laug et al. 1950)
characterized the histological changes as primarily centrilobular hepatocellular hypertrophy with
increased cytoplasmic “oxyphilia” of these cells along with basophilia and margination of cytoplasmic
granules and hyalinization of cytoplasm. There was evidence of increased severity in the group exposed
to 800 ppm and surviving less than a year; these animals exhibited moderate histological damage
including hepatic cell enlargement or atrophy, fatty degeneration, and focal necrosis.
Based on the increase in liver weight and histological changes at 50 ppm, this dose (4 mg/kg/day) is a
LOAEL. No exposure-related changes occurred at the low dose in either sex, indicating that the NOAEL
is 10 ppm (0.7–0.9 mg/kg/day).
Selection of the Point of Departure for the MRL: The NOAEL of 0.9 mg/kg/day (for females) was
selected as the POD for MRL derivation (ATSDR policy is to select the highest NOAEL associated with
the lowest LOAEL for the POD). BMD modeling of the liver weight data was not possible because the
study did not report the numbers of animals per group evaluated for liver weights. Liver histology
findings were reported qualitatively and without incidences, so BMD modeling was not feasible for these
effects.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty and Modifying Factor: The NOAEL of 0.9 mg/kg/day was divided by a total uncertainty
factor (UF) of 100:
• 10 for extrapolation from animals to humans
• 10 for human variability
A modifying factor of 10 was applied to the NOAEL to account for the limitations (no immune studies,
one neurotoxicity study) in the toxicological database for α-HCH. Immune and nervous system effects
are sensitive endpoints for other HCH isomers.
HEXACHLOROCYCLOHEXANE (HCH) A-13
APPENDIX A
MRL = NOAEL ÷ (UF x MF)
0.9 mg/kg/day ÷ ((10 x 10) x 10) = 0.0009 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Hepatic effects
have been observed in rats, mice, and hamsters after intermediate- and chronic-duration oral exposures to
α-HCH. Observed effects include increases in absolute and relative liver weight, hepatocellular
hypertrophy and/or hyperplasia, focal necrosis, fatty degeneration, hepatomegaly, bile duct proliferation,
oval cells, nodular hyperplasia, and megalocytosis (Fitzhugh et al. 1950; Ito et al. 1973, 1975, 1976;
Nagasaki et al. 1975; Sumida et al. 2007; Tryphonas and Iverson 1983). Both rats and mice have also
developed liver tumors at higher doses of α-HCH (Hanada et al. 1973; Ito et al. 1973, 1975, 1976;
Nagasaki et al. 1975; Tryphonas and Iverson 1983; Tsukada et al. 1979).
There are no studies on the effects of α-HCH on the immune system. Studies of γ-HCH, and a few
studies of β-HCH, have shown suppression of the immune system (e.g., Banerjee et al. 1996; Cornacoff et
al. 1988; Desi et al. 1978; Dewan et al. 1980; Hong and Boorman 1993; Khurana et al. 1999; Koner et al.
1998; Mediratta et al. 2008; Meera et al. 1992; Van Velsen et al. 1986) and for γ-HCH, these effects
occur at lower doses than hepatic effects in animal studies. Thus, the lack of immunotoxicity data for
α-HCH is a significant limitation of the existing database for this isomer.
Data on the neurotoxicity of α-HCH are limited to a single study showing no change in motor nerve
conduction velocity after 30 days of exposure (Muller et al. 1981). Both β- and γ-HCH have induced
neurotoxic effects in laboratory rodents (e.g., Cornacoff et al. 1988; EPA 1999a; Gilbert and Mack 1995;
Parmar et al. 2003; Van Velsen et al. 1986), and γ-HCH has induced neurotoxicity in humans exposed
orally (e.g., Davies et al. 1983; Harris et al. 1969; Munk and Nantel 1977; Nordt and Chew 2000; Powell
1980; Starr and Clifford 1972; Storen 1955).
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-14
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration inhalation MRL for
β-HCH.
Rationale for Not Deriving an MRL: No acute-duration inhalation studies of β-HCH in humans or
animals were located, precluding derivation of an acute-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-15
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration inhalation MRL
for β-HCH.
Rationale for Not Deriving an MRL: No intermediate-duration inhalation studies of β-HCH in humans
or animals were located, precluding derivation of an intermediate-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-16
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration inhalation MRL for
β-HCH.
Rationale for Not Deriving an MRL: No chronic-duration inhalation studies of β-HCH in humans or
animals were located, precluding derivation of a chronic-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-17
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Acute
MRL: 0.08 mg/kg/day
Critical Effect: Ataxia and hypoactivity
Reference: Van Velsen et al. 1986
Point of Departure: 8 mg/kg/day (NOAEL)
Uncertainty Factor: 100
LSE Graph Key: 2
Species: Rat
MRL Summary: An acute-duration oral MRL of 0.08 mg/kg/day was derived for β-HCH based on a
NOAEL of 8 mg/kg/day in the first few weeks of a 13-week study (Van Velsen et al. 1986); the higher
dose in this study (38 mg/kg/day) was a serious LOAEL for a neurological endpoint of ataxia and
hypoactivity progressing in some animals to coma. A total uncertainty factor of 100 (10 each for
extrapolation from animals to humans and for human variability) was applied to the NOAEL to derive the
MRL.
Selection of the Critical Effect: Of the three studies reporting acute-duration oral exposure to β-HCH,
two studies (Cornacoff et al. 1988; Van Velsen et al. 1986) identified clinical signs of neurotoxicity as the
critical effect at doses of ≥38 mg/kg/day for 1–2 weeks. Renal effects were observed in the third study
(Srinivasan et al. 1984) at a higher dose (72 mg/kg/day, the only dose tested).
Selection of the Principal Study: The lowest LOAEL for acute-duration oral exposure to β-HCH was
38 mg/kg/day for ataxia and hypoactivity signs observed in rats during for the first 2 weeks of a 13-week
study (Van Velsen et al. 1986). At a dose of 8 mg/kg/day, no clinical signs of neurotoxicity were
observed throughout the 13 weeks of exposure (NOAEL).
Summary of the Principal Study:
Van Velsen FL, Danse LHJC, Van Leeuwen FXR, et al. 1986. The subchronic oral toxicity of the
β-isomer of hexachlorocyclohexane in rats. Fundam Appl Toxicol 6:697-712.
Groups of 10 male and 10 female Wistar rats were exposed to β-HCH in diets containing 0, 2, 10, 50, or
250 mg/kg feed in a 13-week study. The animals were weanlings at study initiation. For the acute (first
2 weeks) portion of the study, ATSDR calculated dose values using food intake and body weight for male
and female weanling Wistar rats from EPA (1988b) to arrive at 0, 0.3, 1.5, 8, and 38 mg/kg/day doses.
Clinical signs of toxicity were noted in the first few weeks of the study. At the end of week 2, two male
and two female rats receiving 38 mg/kg/day in the diet exhibited ataxia and hypoactivity, progressing to
coma within 3 days. The animals were humanely sacrificed, as were five additional males and six
additional females that showed similar signs later in the study. No clinical signs were seen at lower doses
of β-HCH at any time during the 13-week exposure period, nor were there histopathology changes in the
brain, spinal cord, or sciatic nerve at any dose after 13 weeks of exposure.
HEXACHLOROCYCLOHEXANE (HCH) A-18
APPENDIX A
Selection of the Point of Departure for the MRL: The NOAEL of 8 mg/kg/day was selected as the basis
for the MRL. BMD modeling was not considered because the effects at the next highest dose (clinical
signs of toxicity) reflected a serious LOAEL.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty Factor: The NOAEL of 8 mg/kg/day was divided by a total uncertainty factor of 100:
• 10 for extrapolation from animals to humans
• 10 for human variability
MRL = NOAEL ÷ (UF)
8 mg/kg/day ÷ (10 x10) = 0.08 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Mice treated with
60 or 200 mg/kg/day β-HCH in the diet in a 30-day study developed ataxia within the first week of
treatment (Cornacoff et al. 1988). The animals receiving 60 mg/kg/day recovered within a few days,
while those receiving 200 mg/kg/day became markedly worse, leading to humane sacrifice of 80% of the
animals in this group (Cornacoff et al. 1988). Effects on peripheral nerves were reported by Muller et al.
(1981), who observed a significant delay in tail nerve conduction velocity in rats fed 66.3 mg
β-HCH/kg/day for 30 days.
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-19
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Intermediate
MRL: 0.0006 mg/kg/day (0.6 µg/kg/day)
Critical Effect: Hyalinization of centrilobular cells in the liver
Reference: Van Velsen et al. 1986
Point of Departure: 0.18 mg/kg/day (minimal LOAEL)
Uncertainty Factor: 300
LSE Graph Key: 10
Species: Rat
MRL Summary: An intermediate-duration oral MRL of 0.0006 mg/kg/day (0.6 µg/kg/day) was derived
for β-HCH based on a minimal LOAEL of 0.18 mg/kg/day for liver histopathology changes (hyalinization
of centrilobular cells) in a 13-week study of rats exposed via the diet (Van Velsen et al. 1986). A total
uncertainty factor of 300 (10 each for extrapolation from animals to humans and for human variability,
and 3 for use of a minimal LOAEL) was applied to the LOAEL to derive the MRL.
Selection of the Critical Effect: Table A-2 provides a summary of the lowest effect levels in
intermediate-duration animal studies of oral exposure to β-HCH. The lowest LOAEL was
0.18 mg/kg/day for hyalinization of centrilobular cells in the liver in male rats exposed for 13 weeks (Van
Velsen et al. 1986); these effects increased with dose and are supported by the observation of β-HCH-
induced liver effects at higher doses in other intermediate-duration oral studies in rats and mice (Hanada
et al. 1973; Ito et al. 1973, 1975).
Table A-2. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
β-Hexachlorocyclohexane
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Hepatic effects
Rat (Wistar)
10 M
13 weeks (F)
ND
0.18 M
(minimal
LOAEL)
Hyalinization of centrilobular
cells
Van Velsen
et al. 1986
Mouse (dd)
20–40 M
24 weeks (F)
ND
18
18% increase in relative liver
weight
Ito et al.
1973
Rat (W strain)
18–24 M
24–48 weeks
(F)
35
Hepatocellular hypertrophy
after 48 weeks
Ito et al.
1975
Mouse (dd)
10–11 M, 10–
11 F
32 weeks
(F)
20
60 F
50 M
Nuclear irregularities in foci
of enlarged hepatocytes
Hanada et
al. 1973

HEXACHLOROCYCLOHEXANE (HCH) A-20
APPENDIX A
Table A-2. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
β-Hexachlorocyclohexane
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Developmental effects
Rat (Wistar)
6 F
GDs 0–21
LDs 1–28
(F)
ND
5
20
(serious
LOAEL)
Increased liver weight in
pups exposed during
gestation and lactation
48% pup mortality by PND 5
Srinivasan
et al. 1991
Principal study for the MRL.
(F) = feed; F = female(s); GD = gestation day; LD = lactation day; LOAEL = lowest-observed-adverse-effect level;
M = male(s); ND = not determined; NOAEL = no-observed-adverse-effect level; PND = postnatal day
Selection of the Principal Study: Van Velsen et al. (1986) was selected as the principal study because it
identified the lowest LOAEL among intermediate-duration oral studies.
Summary of the Principal Study:
Van Velsen FL, Danse LHJC, Van Leeuwen FXR, et al. 1986. The subchronic oral toxicity of the
β-isomer of hexachlorocyclohexane in rats. Fundam Appl Toxicol 6:697-712.
Groups of 10 male and 10 female Wistar rats were exposed to β-HCH in diets containing 0, 2, 10, 50, or
250 mg/kg feed (>98% pure) for 13 weeks. ATSDR calculated doses corresponding to these
concentrations using food factor values for male and female Wistar rats in subchronic studies as reported
in EPA (1988b). Estimated dietary doses were 0, 0.18, 0.9, 4.5, or 22.5 mg/kg/day in males, and 0, 0.2,
1.0, 5, or 25 mg/kg/day in females. Clinical signs were monitored daily, and body weights and food
intake were measured weekly. At sacrifice at the end of exposure, blood was collected for hematology
and clinical chemistry. Necropsy evaluations included organ weights (liver, kidneys, spleen, thymus,
adrenals, pituitary, testes, uterus, and ovaries), gross pathology, and comprehensive histopathology.
At the end of week 2, two male and two female rats receiving the highest dose exhibited ataxia and
hypoactivity, progressing to coma within 3 days. The animals were humanely sacrificed, as were five
additional males and six additional females that showed similar signs later in the study. Terminal body
weight was significantly reduced (15.5% relative to controls in both males and females) in the animals
from this dose group that survived. Other effects seen at the highest dose (either in the early decedents or
in survivors or both), but not at lower doses, included: centrilobular hepatocytic hypertrophy, proliferation
of smooth endoplasmic reticulum, increased microsomal activity, and/or increased glycogen content in
the livers; hematologic and splenic changes indicative of anemia (decreased red blood cells and
hemoglobin, increased extramedullary hematopoiesis); depletion of splenic lymphoid tissue; thymic
cortical atrophy; adrenal cortical hypertrophy in both sexes; testicular and ovarian atrophy; and epithelial
hyperplasia, metaplasia, and dilation of endometrial glands in the uterus.
No clinical signs, and no reductions in body weight gain or food intake were seen at lower doses of
β-HCH. The lower dose groups had increased food intake and increases in body weight, but terminal
body weights did not differ significantly from controls. At doses of 0.2–5 mg/kg/day, reduced neutrophil
HEXACHLOROCYCLOHEXANE (HCH) A-21
APPENDIX A
counts were seen in females, but there were no other significant hematology changes. Clinical chemistry
did not show any effects of treatment on serum AST, ALT, ALP, urea, IgM, or IgG levels. Dose-related
trends in lower serum lactate and pyruvate were seen, but the only significant difference from controls
was for serum lactate in 4.5 mg/kg/day males. Relative, but not absolute, testes weights were reduced
(~10%) at 4.5 mg/kg/day, but the difference may have resulted from increased body weight (10% higher
than controls) at this dose. At the highest dose, both absolute and relative testes weights were markedly
reduced and accompanied by testicular atrophy. Kidney weights were significantly increased at all doses
in females, but the increase did not show dose-dependence. In males, significant increases were seen only
at ≥4.5 mg/kg/day and were accompanied by renal medullary calcinosis at 22.5 mg/kg/day; this lesion
was seen in females at ≥5 mg/kg/day. Increased absolute and/or relative liver weights occurred at
≥0.9 mg/kg/day in males and ≥1.0 mg/kg/day in females. Increased incidences of hyalinization of
centrilobular cells were observed in the livers of males at all doses, but not in females except in survivors
at the highest dose (25 mg/kg/day). At the lowest dose (0.18 mg/kg/day), the hyalinization in males was
characterized as slight. Females exhibited a low incidence of increased mitoses at 5 mg/kg/day. One
male each in the 4.5 and 22.5 mg/kg/day groups exhibited focal liver cell necrosis. Periportal fat
accumulation and/or focal liver cell necrosis occurred in males and females at ≥4.5 mg/kg/day. Based on
the slight liver histopathology changes (hyalinization of centrilobular cells) seen in males at the lowest
dose level, 0.18 mg/kg/day is considered to be a minimal LOAEL. A NOAEL could not be determined.
Selection of the Point of Departure for the MRL: The minimal LOAEL of 0.18 mg/kg/day was selected
as the POD for MRL derivation because these effects occurred at the lowest dose (increased liver weights
were seen at the next higher dose of 0.9–1.0 mg/kg/day). The histopathology findings in the liver did not
exhibit a monotonic dose-response relationship and were thus not amenable to BMD modeling.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty Factor: The LOAEL of 0.18 mg/kg/day was divided by a total uncertainty factor of 300:
• 3 for use of a minimal LOAEL
• 10 for extrapolation from animals to humans
• 10 for human variability
MRL = NOAEL ÷ (UF)
0.18 mg/kg/day ÷ (3 x 10 x 10) = 0.0006 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: The liver is an
established target of β-HCH in other intermediate- and chronic-duration oral studies in rats and mice
(Fitzhugh et al. 1950; Ito et al. 1973).
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-22
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: β-HCH
CAS Number: 319-85-7
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration oral MRL for β-HCH.
Rationale for not deriving an MRL: Two chronic-duration oral studies of β-HCH were located: Fitzhugh
et al. (1950) and Thorpe and Walker (1973). While Thorpe and Walker (1973) examined only cancer
endpoints, Fitzhugh et al. (1950) evaluated histopathology in a large number of organs and identified the
liver as the most sensitive target of chronic-duration oral exposure. In this study, the lowest dose (0.7–
0.9 mg/kg/day) was identified as a LOAEL based on increased in liver weight and histological changes.
A NOAEL was not identified. Thus, the only available chronic LOAEL (0.7–0.9 mg/kg/day) is higher
than the LOAEL (0.18 mg/kg/day based on liver effects in a study by Van Velsen et al. 1986) used as the
POD for intermediate MRL derivation. Thus, a chronic-duration MRL could not be derived based on the
study by Fitzhugh et al. (1950).
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-23
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration inhalation MRL for
γ-HCH.
Rationale for Not Deriving an MRL: No acute-duration inhalation studies of γ-HCH in humans were
located. Acute-duration inhalation studies in animals are shown in Table A-3, and include two studies of
rats exposed for 4 hours (Oldiges et al. 1980; Ullmann 1986b) and data on clinical signs in mice in the
first 2 weeks of an intermediate-duration study (Klonne and Kintigh 1988). Klonne and Kintigh (1988)
observed 16% mortality in the first week of exposure to 10 mg/m3 γ-HCH (6 hours/day, 5 days/week).
Other endpoints were not evaluated in the first few weeks of these studies (Klonne and Kintigh 1988;
Oldiges et al. 1983). The rat studies of 4-hour exposures (Oldiges et al. 1980; Ullmann 1986b) identified
freestanding LOAELs (101 or 237 mg/m
3
). The available data are not adequate to identify sensitive
targets of inhaled γ-HCH, precluding derivation of an acute-duration inhalation MRL for γ-HCH.
Table A-3. Summary of NOAELs and LOAELs from Acute-Duration Studies in
Laboratory Animals Exposed to γ-Hexachlorocyclohexane by inhalation
Species
Exposure
scenario
NOAEL
(mg/m
3
)
LOAEL
(mg/m
3
)
Effect
Reference
Mouse (CD-1)
45 M, 45 F
1 week
5 days/week
6 hours/day
ND
10
(serious
LOAEL)
16% mortality during the first
week
Klonne and
Kintigh
1988
Rat (Wistar)
5 M, 5 F
4 hours
ND
101
Sedation
Ullmann
1986b
Rat (Wistar)
5 M, 5 F
4 hours
ND
237
Clinical signs of
restlessness and
hyperactivity
Oldiges et
al. 1980
F = female(s); LOAEL = lowest-observed-adverse-effect level; M = male(s); ND = not determined; NOAEL = no-
observed-adverse-effect level
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-24
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration inhalation MRL
for γ-HCH.
Rationale for Not Deriving an MRL: No intermediate-duration inhalation studies of γ-HCH in humans
were located. Intermediate-duration inhalation studies in animals are shown in Table A-4, and include
one study of rats exposed for 90 days (Oldiges et al. 1983) and a 14-week study of mice (Klonne and
Kintigh 1988). The lowest LOAEL (0.5 mg/m
3
) was identified for renal effects in male rats; a NOAEL
for this endpoint was not identified (Oldiges et al. 1980). For the 14-week mouse study by Klonne and
Kintigh (1988), a serious LOAEL of 1 mg/m
3
was identified for concentration-related increases in
mortality. No deaths were observed at 0.3 mg/m
3
, and there were no treatment-related effects on body
weight, food and water intake, clinical chemistry, organ weight, bone marrow evaluations, ophthalmic
evaluations, or gross or microscopic pathology (Klonne and Kintigh 1988). No studies evaluating
developmental, neurological, immune system, or reproductive effects of γ-HCH in animals exposed by
inhalation were located; these have been demonstrated to be sensitive endpoints of γ-HCH toxicity after
oral exposure. The available data were not considered adequate for derivation of an intermediate-duration
inhalation MRL due to the lack of studies on sensitive endpoints and because the lowest LOAEL
(0.5 mg/m
3
in rats) is only one-half the serious LOAEL of 1 mg/mg
3
for mortality in mice.
Table A-4. Summary of NOAELs and LOAELs from Intermediate-Duration
Studies in Laboratory Animals Exposed to γ-Hexachlorocyclohexane by
inhalation
Species
Exposure
scenario
NOAEL
(mg/m
3
)
LOAEL
(mg/m
3
)
Effect
Reference
Rat (Wistar)
5 M, 5 F
90 days
day/week NS
6 hours/day
ND
0.5
Dilated renal tubules with
protein-containing contents;
proliferated tubules in males
Oldiges et
al. 1983
Mouse (CD-1)
45 M, 45 F
14 week
5 days/week
6 hours/day
0.3
1
(serious
LOAEL)
1/45 males and
1/45 females died at
1 mg/m
3
; 5/45 males and
15/45 females died at
5 mg/m
3
Klonne and
Kintigh
1988
Rat (Wistar)
5 M, 5 F
90 days
days/week NS
6 hours/day
0.5
5
Diarrhea; bone marrow
myelogram changes
Oldiges et
al. 1983
F = female(s); LOAEL = lowest-observed-adverse-effect level; M = male(s); ND = not determined; NOAEL = no-
observed-adverse-effect level; NS = not specified
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-25
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration inhalation MRL for
γ-HCH.
Rationale for Not Deriving an MRL: No chronic-duration inhalation studies of γ-HCH in humans or
animals were located, precluding derivation of a chronic-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-26
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Acute
MRL: 0.003 mg/kg/day (3 µg/kg/day)
Critical Effect: Development of male reproductive tract
Reference: Dalsenter et al. 1997b
Point of Departure: 1 mg/kg/day (LOAEL)
Uncertainty Factor: 300
LSE Graph Key: 6
Species: Rat
MRL Summary: An acute-duration oral MRL of 0.003 mg/kg/day (3 µg/kg/day) was derived for γ-HCH
based on a minimal LOAEL of 1 mg/kg/day for developmental effects (effects on developing
reproductive system in male rat pups exposed during lactation) (Dalsenter et al. 1997b). A total
uncertainty factor of 300 (10 for extrapolation from animals to humans, 10 for human variability, and
3 for use of a minimal LOAEL) was applied to the LOAEL to obtain the MRL.
Selection of the Critical Effect: Table A-5 provides a summary of the lowest effect levels in acute-
duration oral studies of γ-HCH exposure in animals. The lowest effect level was a LOAEL of
1 mg/kg/day for effects on the development of the male reproductive tract in rat pups exposed during
LDs 9–14 (Dalsenter et al. 1997b). These effects were seen during assessments on PNDs 65 and 140, as
follows. At PND 65, there was no significant effect on relative testicular weight; 7% reduction relative
epididymis weight; 29% lower spermatid count; 12% lower sperm count; and 30% lower testosterone
levels. At PND 140, the differences from control had declined: testicular weight was 6% lower than
controls; spermatid and sperm counts were 13% lower than controls; and there were no significant
differences in relative epididymal weights or serum testosterone concentration. There were no effects on
mating or fertility. There was no NOAEL associated with the study. The findings in this study are
consistent with adverse effects on developing male reproductive organs reported in other animal studies
(Agrahari et al. 2019; Dalsenter et al. 1997a, 1997b; Di Consiglio et al. 2009; La Sala et al. 2009; Traina
et al. 2003).
Table A-5. Summary of NOAELs and LOAELs from Candidate Acute-Duration
Studies in Laboratory Animals Orally Exposed to γ-Hexachlorocyclohexane
(Doses ≤10 mg/kg/day)
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Developmental effects
Rat (BOR:
spf) 9 F
LDs 9–14
(GO)
ND
1
(minimal
LOAEL)
In male pups, reduced relative
testicular and epididymis weight (6–
7%), spermatid and sperm counts
(12–29%), and testosterone levels
(8–30%) at maturity with no effect
on fertility
Dalsenter et al.
1997b

HEXACHLOROCYCLOHEXANE (HCH) A-27
APPENDIX A
Table A-5. Summary of NOAELs and LOAELs from Candidate Acute-Duration
Studies in Laboratory Animals Orally Exposed to γ-Hexachlorocyclohexane
(Doses ≤10 mg/kg/day)
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Rat (BOR:
spf) 9 F
LD 9 or
14
once
(GO)
ND
6
In male pups, reduced relative
testicular and epididymis weight
(~10%), spermatid and sperm
counts (~8–10%), testosterone
levels (~30-50%), Leydig cell
numbers, and spermatogenesis at
maturity with no effect on fertility
Dalsenter et al.
1997b
Neurological effects
Rat
(Sprague-
Dawley)
9 M
6 days
(GO)
ND
3
Increased pineal N-acetyl-
transferase, decreased serotonin
levels
Attia et al. 1991
Rat
(Sprague-
Dawley)
7–14 M
4 days
(GO)
1
3
Increased kindling acquisition
Joy et al. 1982
Rat
(Long-
Evans)
14 M
Once
(GO)
ND
5
Myoclonic jerks and single clonic
seizure in kindled animals
Gilbert and Mack
1995
Rat
(Wistar) 5
M, 5 F
3 days
(GO)
ND
5
(serious
LOAEL)
Decreased myelin in developing
brain
Serrano et al.
1990
Hepatic effects
Rat
(Wistar)
6 M
3 days
(GO)
ND
5
Fatty degeneration, vacuolation,
and necrosis of the liver
Hfaiedh et al.
2012
Hematological effects
Mouse
(B6C3F1)
7 M
10 days
(GO)
ND
10
Transient decrease in marrow
progenitor cell numbers
Hong and
Boorman 1993
Immunological effects
Rat
(Wistar)
8 M
14 days
(NS)
ND
10
Reduced delayed-type
hypersensitivity (43% decrease in
foot pad thickness)
Mediratta et al.
2008
Mouse
(B6C3F1)
7 M
10 days
(GO)
ND
10
Dose-related decrease in relative
thymus and spleen weights
Hong and
Boorman 1993
Principal study for the MRL.
F = female(s); (GO) = gavage in oil vehicle; LD = lactation day; LOAEL = lowest-observed-adverse-effect level;
M = male(s); ND = not determined; NOAEL = no-observed-adverse-effect level; NS = not specified
HEXACHLOROCYCLOHEXANE (HCH) A-28
APPENDIX A
Selection of the Principal Study: The study by Dalsenter et al. (1997b) was selected for use in deriving
the MRL because the lowest LOAEL (1 mg/kg/day) was identified for developmental effects in this
study.
Summary of the Principal Study:
Dalsenter PR, Faqi AS, Webb J, et al. 1997b. Reproductive toxicity and toxicokinetics of lindane in the
male offspring of rats exposed during lactation. Hum Exp Toxicol 16:146-153.
Reproductive toxicity was evaluated in male offspring of groups of nine Bor:spf female rats that were
administered γ-HCH in peanut oil by gavage as a single 6 mg/kg dose on day 9 or day 14 of lactation, or
as daily 1 mg/kg/day doses on days 9–14 of lactation (Dalsenter et al. 1997b). A group of nine controls
was administered the vehicle alone on days 9–14 of lactation. Male offspring (10 or 20/group) were
terminated on PND 65 (puberty) or 140 (adulthood) and evaluated for the following endpoints: testis and
epididymis weights, spermatid and sperm numbers, serum testosterone level, sexual behavior at 130 days
of age during 1:1 mating with unexposed females (mount latency, intromission and ejaculatory latency,
number and frequency of intromissions), mating index (number sperm positive females/number males
mated x100), pregnancy index (number of males that made females pregnant/number of males that made
females sperm-positive x100), fertility index (number of days elapsed until males fertilized their female
partner), pregnancy endpoints (numbers of litters, implantations/litters, fetuses/litter, resorptions), and
testicular histology (6 mg/kg offspring only).
Effects occurred in all treated groups. Findings in the 1 mg/kg/day offspring included statistically
significant (p<0.05) reductions in relative testicular weight at PND 140 (6% less than controls), relative
epididymis weight at PND 65 (7%), spermatid number at PNDs 65 and 140 (29 and 13%, respectively),
sperm number at PND 140 (13%), serum testosterone at PND 65 (30%), and increased number of
intromissions per minute up to ejaculation at PND 130 (45%). Effects were generally similar in type and
magnitude in the 6 mg/kg offspring following exposure on GDs 9 or 14, including significantly reduced
relative testicular weight at PNDs 65 and 140 (~10%), spermatid and sperm numbers at PND 140 (~8–
10%), and serum testosterone at PND 140 (~50%). There were no significant effects on sexual behavior
or fertility in the 1 or 6 mg/kg/day offspring as shown by the mating, pregnancy, and fertility indices or
other pregnancy endpoints. Thus, the statistically significant changes observed for relative organ weights,
sperm number, hormone levels, and intromission incidence are considered minimally effective for
reproduction; their associated dose levels are considered minimal LOAELs. The testicular histological
examinations of the 6 mg/kg/day offspring showed large areas of normal tissue, although some areas had
distinct changes ranging from small alterations to a pronounced effect. The most affected areas were the
tubules in which the effects included necrotic changes and reductions in Leydig cell numbers and
spermatogenesis.
Selection of the Point of Departure for the MRL: The minimal LOAEL of 1 mg/kg/day for effects on
the developing male reproductive tract (Dalsenter et al. 1997b) was selected as the POD for derivation of
the acute-duration oral MRL for γ-HCH. BMD modeling was not possible as only a single exposed group
was included in the experiment.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty Factor: The changes in relative organ weights, sperm number, hormone levels, and
intromission incidence at the LOAEL were not associated with effects on sexual behavior or fertility;
thus, the dose is considered a minimal LOAEL. Therefore, the LOAEL of 1 mg/kg/day was divided by a
total uncertainty factor of 300:
HEXACHLOROCYCLOHEXANE (HCH) A-29
APPENDIX A
• 10 for extrapolation from animals to humans
• 10 for human variability
• 3 for use of a minimal LOAEL
MRL = LOAEL ÷ (UF)
1 mg/kg/day ÷ (3 x 10 x 10) ≈ 0.003 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: In male offspring
of rats and mice exposed to γ-HCH via oral administration during gestation and/or postnatal development,
effects on preputial separation, serum hormone levels, spermatogenesis, reproductive organ weights, and
testicular histopathology have been reported (Agrahari et al. 2019; Dalsenter et al. 1997b; Di Consiglio et
al. 2009; La Sala et al. 2009; Traina et al. 2003).
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-30
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Intermediate
MRL: 8x10
-7
mg/kg/day (0.8 ng/kg/day)
Critical Effect: Cardiac effects in offspring
Reference: Sauviat et al. 2005
Point of Departure: 0.000076 mg/kg/day (NOAEL)
Uncertainty Factor: 100
LSE Graph Key: 75
Species: Rat
MRL Summary: An intermediate-duration oral MRL of 0.0000008 (8x10
-7
) mg/kg/day (0.8 ng/kg/day)
was derived for γ-HCH based on a NOAEL of 0.000076 mg/kg/day for a developmental endpoint of
cardiac effects in rat pups (Sauviat et al. 2005). A total uncertainty factor of 100 (10 for extrapolation
from animals to humans and 10 for human variability) was applied to the NOAEL to obtain the MRL.
Selection of the Critical Effect: Table A-6 provides a summary of the lowest effect levels in
intermediate-duration studies of animals exposed to γ-HCH by oral administration. The lowest effect
level (0.00015 mg/kg/day, minimal LOAEL) was identified for effects on cardiac electrophysiology in rat
pups exposed to γ-HCH in drinking water (Sauviat et al. 2005). A NOAEL of 0.000076 mg/kg/day was
identified for this study. The effects at the LOAEL in this study were minimal, but additional evidence
that the effects were adverse is provided by the serious findings of altered heart morphometry and cardiac
histopathology changes at the higher dose of 0.0003 mg/kg/day. Histopathology evaluations were not
conducted in rat pups receiving the lower doses.
Table A-6. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
γ-Hexachlorocyclohexane (Doses ≤1 mg/kg/day)
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Developmental effects
Rat
(Sprague-
Dawley)
NS F
~13 weeks
(premating, mating,
gestation, lactation,
and 3 weeks
postweaning) (W)
0.000076
0.00015
(minimal
LOAEL)
0.0003
(serious
LOAEL)
LOAEL: altered ventricular
electrophysiology.
Serious LOAEL: 21%
decrease in pup body weight;
altered heart morphometry
and electrophysiology;
cardiac histopathology
(hypertrophy in left ventricular
area, unorganized collagen
bundles and layers, fibroblast
destruction)
Sauviat et al.
2005

HEXACHLOROCYCLOHEXANE (HCH) A-31
APPENDIX A
Table A-6. Summary of NOAELs and LOAELs from Candidate Intermediate-
Duration Studies in Laboratory Animals Orally Exposed to
γ-Hexachlorocyclohexane (Doses ≤1 mg/kg/day)
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Rat
(Wistar)
13–14 F
GDs 5–21 (GO)
0.125
0.25
Persistent hyperactivity
Johri et al.
2007
Rat
(Wistar)
25 F
GDs 5–21 (GO)
ND
0.25
(serious
LOAEL)
Ultrastructural changes in the
brain (moderately distorted
mitochondria and
demyelinated neurons)
Srivastava et
al. 2019
Immunological effects
Mouse
(Swiss
albino)
6 F
24 weeks (F)
ND
0.012
Changes in cell- and
humoral-mediated immune
system
Meera et al.
1992
Principal study for the MRL.
(F) = feed; F = female(s); GD =gestation day; (GO) = gavage in oil vehicle; (W) = water; LOAEL = lowest-observed-
adverse-effect level; M = male(s); ND = not determined; NOAEL = no-observed-adverse-effect level;
PND = postnatal day
Selection of the Principal Study: The lowest LOAEL (0.00015 mg/kg/day) and NOAEL
(0.000076 mg/kg/day) for any effect of γ-HCH was identified in the study by Sauviat et al. (2005). These
doses are much lower than those associated with other effects of γ-HCH.
Summary of the Principal Study:
Sauviat MP, Bouvet S, Godeau G, et al. 2005. Electrical activity alterations induced by chronic
absorption of lindane (gamma-hexachlorocyclohexane) trace concentrations in adult rat heart. Can J
Physiol Pharmacol 83:243-251.
Groups of female Sprague-Dawley rats (number not reported) were administered γ-HCH via “beverage”
at doses of 0.5, 1, or 2 ppb. ATSDR estimated corresponding maternal doses of 0, 0.000076, 0.00015,
and 0.00030 mg/kg/day using water intake and body weight for female Sprague-Dawley rats in
subchronic studies as reported in EPA (1988b). Doses were administered prior to mating for four
estrous cycles (~2 weeks); throughout mating (~2 weeks), gestation (3 weeks), lactation (3 weeks), and
growth (3 weeks) until pups were 6 weeks of age for a total of ~13 weeks. Exposure of the pups after
weaning was not described but assumed to occur via water at the same dose as the dams. Offspring were
sacrificed at 6 weeks of age. The left ventricular papillary muscles (LVPM) were dissected from
18 control rats from 7 litters; 5 rats from 2 litters in the 0.000076 mg/kg/day group; 7 rats from 2 litters in
the 0.00015 mg/kg/day group; and 18 rats from 7 litters in the 0.0003 mg/kg/day group. Dissected
LVPMs were evaluated for the following electrophysiologic measurements: resting potential, action
potential, plateau, action potential duration, overshoot, end of early repolarization, and end of terminal
repolarization. Cardiac weight, lipid content, and morphometry, as well as left ventricular papillary
muscle histopathology were evaluated in pups from the 0.0003 mg/kg/day and control groups only.
HEXACHLOROCYCLOHEXANE (HCH) A-32
APPENDIX A
The study authors indicated that the high-dose offspring were less sensitive to anesthesia and more
sensitive to noise than other groups, but details of these assessments and findings were not provided.
Body weights of pups were significantly decreased by 21% in the 0.0003 mg/kg/day group, compared to
controls; no significant body weight changes were observed in other groups.
Morphometry analysis showed that hearts from pups in the 0.0003 mg/kg/day group had a 9% increase in
heart width (relative to controls), but no significant change in length, with a corresponding 9% decrease in
length-to-width ratio. Heart weights and total lipid content were not significantly different in the
0.0003 mg/kg/day group compared to control. At 0.0003 mg/kg/day, offspring heart morphology was
described as more round and “cherry like.” The study authors reported that hearts of treated offspring
showed hypertrophied areas with thinning of the left ventricular wall and few developed papillary
muscles. Histopathological examination in 0.0003 mg/kg/day offspring showed that the heart tissue
muscle bundles and layers were unorganized and dissociated, with large hemorrhagic interspaces and
dispersion of cell nuclei, destruction of fibroblasts, and dispersion and disorganization of collagen
bundles, compared to control heart muscle. Incidences of changes were not reported, and these
parameters were not assessed in pups from the 0.5 and 0.00015 mg/kg/day groups.
Electrophysiology changes were evident in LVPMs from animals exposed to 0.00015 mg/kg/day and
0.0003 mg/kg/day γ-HCH. Action potential durations were unchanged at
0.000076 mg/kg/day, but the
plateau was shortened moderately at 0.00015 mg/kg/day, and significantly shortened at
0.0003 mg/kg/day. At 0.0003 mg/kg/day, the slow repolarizing phase was also significantly shortened.
The effects at the high dose (0.0003 mg/kg/day) represent a serious LOAEL for cardiac effects
(histopathology and electrophysiology changes) and significant body weight decrements (21% decrease)
in the developing rat. The only effect at the middle dose (0.00015 mg/kg/day) was shortened action
potential duration at the initial plateau phase (measured at 0 millivolts); similar results were not observed
in the early repolarization or terminal repolarization phases (measured at 40 and 10 millivolts,
respectively). However, at the high dose (0.0003 mg/kg/day), there were effects in all three phases,
suggesting a dose-response relationship. There was no assessment of cardiac morphometry or
histopathology in offspring from the middle dose group. The electrophysiology changes observed at
0.00015 mg/kg/day are considered to represent a minimal LOAEL. The lowest dose
(0.000076 mg/kg/day) was not associated with electrophysiology changes and is considered to be a
NOAEL.
Selection of the Point of Departure for the MRL: The data on electrophysiology changes in the study by
Sauviat et al. (2005) are not amenable to BMD modeling, as the authors did not report variability
measures. Thus, the NOAEL of 0.000076 mg/kg/day was selected as the POD for MRL derivation.
Adjustment for Intermittent Exposure: Not applicable.
Uncertainty Factor: The NOAEL of 0.000076 mg/kg/day was divided by a total uncertainty factor (UF)
of 100:
• 10 for extrapolation from animals to humans
• 10 for human variability
MRL = NOAEL ÷ (UF)
0.000076 mg/kg/day ÷ (10 x 10) ≈ 0.0000008 mg/kg/day (8x10
-7
mg/kg/day)
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Sauviat et al.
(2007) conducted a related study examining whether the cardiac effects seen after maternal exposure
HEXACHLOROCYCLOHEXANE (HCH) A-33
APPENDIX A
could be induced by paternal exposure to γ-HCH in drinking water. In this study, male rats were exposed
to a concentration of 2 μg/L for an unspecified “chronic” duration prior to mating with untreated females.
The lack of information on exposure duration in the males precluded estimation of doses. In offspring
sacrificed at 6 weeks of age, there were no effects on pup heart weight or shape or electrophysiology, but
there were histopathology changes in the hearts similar to those reported by Sauviat et al. (2005) at the
same drinking water concentration.
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) A-34
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: γ-HCH
CAS Number: 58-89-9
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration oral MRL for γ-HCH.
Rationale for Not Deriving an MRL: No adequate exposure-response data were available for humans.
Chronic-duration oral studies of γ-HCH that evaluated noncancer endpoints include a 2-year dog study
(Rivett et al. 1978), 18-month and 2-year studies in rats (Ali and Shakoori 1998; Fitzhugh et al. 1950);
and 78–80-week studies in mice (EPA 2000a; Herbst et al. 1975; Weisse and Herbst 1977). Table A-7
summarizes effect levels from these chronic studies. As the table shows, all of the effect levels are much
higher than the POD (0.000076 mg/kg/day) used for derivation of the intermediate oral MRL for γ-HCH.
Thus, the available oral chronic data were not considered adequate for MRL derivation.
Table A-7. Summary of NOAELs and LOAELs from Candidate Chronic-Duration
Studies in Laboratory Animals Orally Exposed to γ-Hexachlorocyclohexane
Species
Exposure
scenario
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Dog
(Beagle)
4 M, 4 F
104 weeks
(F)
2.92
ND
No body weight, hepatic,
hematological, or ocular effects
Rivett et al.
1978
Rat (Wistar)
10 M, 10 F
107 weeks
(F)
4
7 M
Increased liver weight (35%); very
slight microscopic liver damage;
very slight microscopic kidney
damage
Fitzhugh et
al. 1950
Rat
(Sprague-
Dawley)
3–5 NS
18 month
(F)
ND
9
Increased cell, nucleus, and
nucleolus size; extensive
cytoplasmolysis; slight
cytoplasmic degeneration;
increasing nuclear distortion
Ali and
Shakoori
1998
Mouse
(CD-1)
50 M, 50 F
78 weeks
(F)
5.2 M
20.5 M
Centrilobular hepatocyte
hypertrophy
EPA 2000a
Mouse
(NMRI)
50 M, 50 F
80 weeks
(F)
8.2 M
ND
No body weight or liver effects
Herbst et al.
1975; Weisse
and Herbst
1977
(F) = feed; F = female(s); LOAEL = lowest-observed-adverse-effect level; M = male(s); ND = not determined;
NOAEL = no-observed-adverse-effect level; NS = not specified
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-35
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Number:
319-86-8
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration inhalation MRL for
δ-HCH.
Rationale for Not Deriving an MRL: No acute-duration inhalation studies of δ-HCH in humans or
animals were located, precluding derivation of an acute-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-36
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Number:
319-86-8
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration inhalation MRL
for δ-HCH.
Rationale for Not Deriving an MRL: No intermediate-duration inhalation studies of δ-HCH in humans
or animals were located, precluding derivation of an intermediate-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-37
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Number: 319-86-8
Date: March 2024
Profile Status: Final
Route: Inhalation
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration inhalation MRL for
δ-HCH.
Rationale for Not Deriving an MRL: No chronic-duration inhalation studies of δ-HCH in humans or
animals were located, precluding derivation of a chronic-duration inhalation MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-38
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Number:
319-86-8
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration oral MRL for δ-HCH.
Rationale for Not Deriving an MRL: No acute-duration oral studies of δ-HCH in humans or animals
were located, precluding derivation of an acute-duration oral MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-39
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Numbers: 319-86-8
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration oral MRL for
δ-HCH.
Rationale for Not Deriving an MRL: No intermediate-duration oral studies of δ-HCH in humans were
located. Two intermediate-duration oral studies of δ-HCH administered in feed were identified: a
48-week study in rats (Ito et al. 1975) and a 24-week study in mice (Ito et al. 1973). Both studies were
focused on the evaluation of liver cancer, and only the liver was evaluated (organ weight and
histopathology). Due to the lack of data pertaining to other potential target organs, these data are not
considered adequate for derivation of an intermediate-duration oral MRL.
Agency Contacts (Chemical Managers): Malcolm Williams
HEXACHLOROCYCLOHEXANE (HCH) A-40
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: δ-HCH
CAS Number: 319-86-8
Date: March 2024
Profile Status: Final
Route: Oral
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration oral MRL for δ-HCH.
Rationale for Not Deriving an MRL: No chronic-duration oral studies of δ-HCH in humans or animals
were located, precluding derivation of a chronic-duration oral MRL.
Agency Contacts (Chemical Managers): Malcolm Williams

HEXACHLOROCYCLOHEXANE (HCH) B-1
APPENDIX B. LITERATURE SEARCH FRAMEWORK FOR HCH
The objective of the toxicological profile is to evaluate the potential for human exposure and the potential
health hazards associated with inhalation, oral, or dermal/ocular exposure to HCH.
B.1 LITERATURE SEARCH AND SCREEN
A literature search and screen were conducted to identify studies examining health effects, toxicokinetics,
mechanisms of action, susceptible populations, biomarkers, chemical interactions, physical and chemical
properties, production, use, environmental fate, environmental releases, and environmental and biological
monitoring data for HCH. ATSDR primarily focused on peer-reviewed articles without publication date
or language restrictions. Foreign language studies are reviewed based on available English-language
abstracts and/or tables (or summaries in regulatory assessments, such as International Agency for
Research on Cancer [IARC] documents). If the study appears critical for hazard identification or MRL
derivation, translation into English is requested. Non-peer-reviewed studies that were considered relevant
to the assessment of the health effects of HCH have undergone peer review by at least three ATSDR-
selected experts who have been screened for conflict of interest. The inclusion criteria used to identify
relevant studies examining the health effects of HCH are presented in Table B-1.
Table B-1. Inclusion Criteria for the Literature Search and Screen
Health Effects
Species
Human
Laboratory mammals
Route of exposure
Inhalation
Oral
Dermal (or ocular)
Parenteral (these studies will be considered supporting data)
Health outcome
Death
Systemic effects
Body weight effects
Respiratory effects
Cardiovascular effects
Gastrointestinal effects
Hematological effects
Musculoskeletal effects
Hepatic effects
Renal effects
Dermal effects
Ocular effects
Endocrine effects
Immunological effects
Neurological effects
Reproductive effects

HEXACHLOROCYCLOHEXANE (HCH) B-2
APPENDIX B
Table B-1. Inclusion Criteria for the Literature Search and Screen
Developmental effects
Other noncancer effects
Cancer
Toxicokinetics
Absorption
Distribution
Metabolism
Excretion
PBPK models
Biomarkers
Biomarkers of exposure
Biomarkers of effect
Interactions with other chemicals
Potential for human exposure
Releases to the environment
Air
Water
Soil
Environmental fate
Transport and partitioning
Transformation and degradation
Environmental monitoring
Air
Water
Sediment and soil
Other media
Biomonitoring
General populations
Occupation populations
B.1.1 Literature Search
The current literature search was intended to update the 2005 draft toxicological profile for HCH released
for public comment in January 2023; thus, the literature search was restricted to studies published
between January 2020 and June 2023. The following main databases were searched in June 2023:
• PubMed
• National Technical Reports Library (NTRL)
• Scientific and Technical Information Network’s TOXCENTER
The search strategy used the chemical names, Chemical Abstracts Service (CAS) numbers,
synonyms, Medical Subject Headings (MeSH) headings, and keywords for HCH. The query strings
used for the literature search are presented in Table B-2.

HEXACHLOROCYCLOHEXANE (HCH) B-3
APPENDIX B
The search was augmented by searching the Toxic Substances Control Act Test Submissions (TSCATS),
NTP website, and National Institute of Health Research Portfolio Online Reporting Tools Expenditures
and Results (NIH RePORTER) databases using the queries presented in Table B-3. Additional databases
were searched in the creation of various tables and figures, such as the TRI Explorer, the Substance
Priority List (SPL) resource page, and other items as needed. Regulations applicable to HCH were
identified by searching international and U.S. agency websites and documents.
Review articles were identified and used for the purpose of providing background information and
identifying additional references. ATSDR also identified reports from the grey literature, which included
unpublished research reports, technical reports from government agencies, conference proceedings and
abstracts, and theses and dissertations.
Table B-2. Database Query Strings
Database
search date
Query string
PubMed
06/2023
((319-84-6[rn] OR 319-85-7[rn] OR YM80ODM9PD[rn] OR 319-86-8[rn] OR 58-89-9[rn] OR
59NEE7PCAB[rn] OR 608-73-1[rn] OR "Hexachlorocyclohexane"[mh]) AND
(("Hexachlorocyclohexane/toxicity"[mh] OR "Hexachlorocyclohexane/adverse effects"[mh]
OR "Hexachlorocyclohexane/poisoning"[mh] OR
"Hexachlorocyclohexane/pharmacokinetics"[mh] OR "Hexachlorocyclohexane/blood"[mh]
OR "Hexachlorocyclohexane/cerebrospinal fluid"[mh] OR
"Hexachlorocyclohexane/urine"[mh] OR "Hexachlorocyclohexane/antagonists and
inhibitors"[mh] OR ("Hexachlorocyclohexane/metabolism"[mh] AND ("humans"[mh] OR
"animals"[mh]))) OR ("Hexachlorocyclohexane"[mh] AND ("environmental exposure"[mh]
OR ci[sh])) OR ("Hexachlorocyclohexane"[mh] AND toxicokinetics[mh:noexp]) OR
("Hexachlorocyclohexane"[mh] AND ("endocrine system"[mh] OR "hormones, hormone
substitutes, and hormone antagonists"[mh] OR "endocrine disruptors"[mh])) OR
("Hexachlorocyclohexane"[mh] AND ("computational biology"[mh] OR "medical
informatics"[mh] OR genomics[mh] OR genome[mh] OR proteomics[m
h] OR proteome[mh]
OR metabolomics[mh] OR metabolome[mh] OR genes[mh] OR "gene expression"[mh] OR
phenotype[mh] OR genetics[mh] OR genotype[mh] OR transcriptome[mh] OR ("systems
biology"[mh] AND ("environmental exposure"[mh] OR "epidemiological monitoring"
[mh] OR
analysis[sh])) OR "transcription, genetic "[mh] OR "reverse transcription"[mh] OR
"transcriptional activation"[mh] OR "transcription factors"[mh] OR ("biosynthesis"[sh] AND
(RNA[mh] OR DNA[mh])) OR "RNA, messenger"[mh] OR "RNA, transfer"[mh] OR "peptide
biosynthesis"[mh] OR "protein biosynthesis"[mh] OR "reverse transcriptase polymerase
chain reaction"[mh] OR "base sequence"[mh] OR "trans-activators"[mh] OR "gene
expression profiling"[mh])) OR ("Hexachlorocyclohexane"[mh] AND (("Neoplasms"[mh] OR
"Carcinogens"[mh] OR "Lymphoproliferative disorders"[mh] OR "Myeloproliferative
disorders"[mh] OR "Toxicity Tests"[mh] OR ((cancer*[tiab] OR carcinogen*[tiab]) AND
(risk*[tiab] OR health[tiab]) AND assessment*[tiab]) OR "Mutagens"[mh] OR "Mutagenicity
Tests"[mh] OR "Chromosome Aberrations"[mh] OR "DNA Damage"[mh] OR "DNA
Repair"[mh] OR "DNA Replication/drug effects"[mh] OR "DNA/drug effects"[mh] OR
"DNA/metabolism"[mh] OR "Genomic Instability"[mh] OR "Salmonella typhimurium/drug
effects"[mh] OR "Salmonella typhimurium/genetics"[mh] OR "Sister Chromatid
Exchange"[mh] OR strand-break*[tiab]))) OR Hexachlorocyclohexane/pharmacology[majr])
AND (2020/10/01:3000[mhda] OR 2020:3000[dp])) OR ((("gamma-Lindane"[tw] OR
"1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "alpha-Benzene hexachloride"[tw] OR "alpha-
Hexachlorocyclohexane"[tw] OR "beta-Benzene hexachloride"[tw] OR "beta-
hexachlorocyclohexane"[tw] OR "delta-Benzene hexachloride"[tw] OR "delta-
Benzenehexachloride"[tw] OR "delta-Hexachlorocyclohexane"[tw] OR "gamma-Benzene

HEXACHLOROCYCLOHEXANE (HCH) B-4
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
hexachloride"[tw] OR "gamma-Hexachlorocyclohexane"[tw] OR "1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "A-Hexachlorocyclohexane"[tw] OR "alpha-Benzene
hexachloride"[tw] OR "alpha-Benzenehexachloride"[tw] OR "alpha-
Hexachlorocyclohexane"[tw] OR "alpha-Lindane"[tw] OR "Aalindan"[tw] OR "Aficide"[tw]
OR "Agrocide"[tw] OR "Agronexit"[tw] OR "Ameisentod"[tw] OR "Aparasin"[tw] OR
"Aphtiria"[tw] OR "Aplidal"[tw] OR "Arbitex"[tw] OR "B-Hexachlorocyclohexane"[tw] OR
"Benzene hexachloride"[tw] OR "Benzenehexachloride"[tw] OR "beta-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "beta-Hexachloro-cyclohexane"[tw] OR "beta-
Hexachlorocyclohexane"[tw] OR "beta-Lindane"[tw] OR "Ben-Hex"[tw] OR "Benhexol"[tw]
OR "Bexol"[tw] OR "Celanex"[tw] OR "Chloresene"[tw] OR "Codechine"[tw] OR
"Cyclohexane, 1,2,3,4,5,6-hexachloro-"[tw] OR "delta-Benzene hexachloride"[tw] OR
"delta-Benzenehexachloride"[tw] OR "delta-Hexachlorocyclohexane"[tw] OR "delta-
Lindane"[tw] OR "Devoran"[tw] OR "Entomoxan"[tw] OR "Forst-Nexen"[tw] OR
"Gallogama"[tw] OR "Gamacarbatox"[tw] OR "Gamacid"[tw] OR "Gamacide"[tw] OR
"Gamaphex"[tw] OR "Gamene"[tw] OR "Gamiso"[tw] OR "gamma Benzene
hexachloride"[tw] OR "gamma-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "gamma-
Benzenehexachloride"[tw] OR "gamma-Benzohexachloride"[tw] OR "gamma-
Hexachlorcyclohexanum"[tw] OR "gamma-Hexachloro-cyclohexane"[tw] OR "gamma-
Hexachlorobenzene"[tw] OR "gamma-Hexachlorocyclohexane"[tw] OR "Gammalin"[tw] OR
"Gammaterr"[tw] OR "Gammexane"[tw] OR "Geobilan"[tw] OR "Gexane"[tw] OR
"HEXACHLORCYCLOHEXANE"[tw] OR "Hexachloro-cyclohexane"[tw] OR
"Hexachlorocyclohexane"[tw] OR "hexachlorocyclohexanes"[tw] OR "Hexachlor"[tw] OR
"Hexachlorcyclohexan"[tw] OR "Heclotox"[tw] OR "Hexachloran"[tw] OR
"Hexachlorane"[tw] OR "Hexaverm"[tw] OR "Hexcidum"[tw] OR "Hexicide"[tw] OR
"Hexyclan"[tw] OR "Hilbeech"[tw] OR "Hortex"[tw] OR "Hungaria L 7"[tw] OR "Hungaria L-
7"[tw] OR "Jacutin"[tw] OR "Kokotine"[tw] OR "Kwell"[tw] OR "Lindane"[tw] OR
"Lasochron"[tw] OR "Lendine"[tw] OR "Lentox"[tw] OR "Lidenal"[tw] OR "Lindafor"[tw] OR
"Lindagam"[tw] OR "Lindagrain"[tw] OR "Lindagranox"[tw] OR "Lindan"[tw] OR
"Lindanum"[tw] OR "Lindapoudre"[tw] OR "Lindatox"[tw] OR "Lindosep"[tw] OR "Lintox"[tw]
OR "Linvur"[tw] OR "Lorexane"[tw] OR "Mszycol"[tw] OR "Neo-Scabicidol"[tw] OR "Nexol
E"[tw] OR "Nexol-E"[tw] OR "Nicochloran"[tw] OR "Novigam"[tw] OR "Omnitox"[tw] OR
"Ovadziak"[tw] OR "Owadziak"[tw] OR "Pedraczak"[tw] OR "Pflanzol"[tw] OR
"Quellada"[tw] OR "Technical HCH"[tw] OR "technical grade HCH"[tw] OR "Scabene"[tw]
OR "Silvanol"[tw] OR "Spritz-Rapidin"[tw] OR "Spritzlindane"[tw] OR "Spruehpflanzol"[tw]
OR "Streunex"[tw] OR "α-Benzohexachloride"[tw] OR "α-Hexachlorocyclohexane"[tw] OR
"α-Lindane"[tw] OR "β-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "β-Benzene
hexachloride"[tw] OR "β-Hexachlorocyclohexane"[tw] OR "β-Lindane"[tw] OR "γ-
1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "γ-Benzene hexachloride"[tw] OR "γ-
Benzohexachloride"[tw] OR "γ-Hexachlorobenzene"[tw] OR "γ-
Hexachlorocyclohexane"[tw]
OR "γ-Lindane"[tw] OR "δ-Benzene hexachloride"[tw] OR "δ-Hexachlorocyclohexane"[tw]
OR "δ-Lindane"[tw] OR "alpha BHC"[tw] OR "alpha-BHC"[tw] OR "alpha-HCH"[tw] OR
"beta BHC"[tw] OR "beta-BHC"[tw] OR "beta-HCH"[tw] OR "delta BHC"[tw] OR "delta-
BHC"[tw] OR "delta-HCH"[tw] OR "Gamma-BHC"[tw] OR "gamma-HCH"[tw] OR "Nexit
Stark"[tw] OR "Nexit-stark"[tw] OR "α-BHC"[tw] OR "α-HCH"[tw] OR "β-666"[tw] OR "β-
BHC"[tw] OR "β-HCH"[tw] OR "γ-666"[tw] OR "γ-BHC"[tw] OR "γ-HCH"[tw] OR "δ-
BHC"[tw]
OR "δ-HCH"[tw] OR "total BHC"[tw] OR "α-666"[tw] OR "δ-666"[tw] OR "BHC-gamma"[tw]
OR "α-Benzenehexachloride"[tw] OR "Detox 25"[tw] OR "Dol Granule"[tw] OR "ENT
7,796"[tw] OR "TAP 85"[tw] OR "Ameisenmittel merck"[tw] OR "Arcotal S"[tw] OR "Bentox
10"[tw] OR "Benzene-1,2,3,4,5,6-hexachloride"[tw] OR "beta-Hexachlorobenzene"[tw] OR
"Detmol Extract"[tw] OR "Fenoform forte"[tw] OR "Gamma-mean 400"[tw] OR "Geolin G

HEXACHLOROCYCLOHEXANE (HCH) B-5
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
3"[tw] OR "Hungaria L7"[tw] OR "Mglawik L"[tw] OR "Milbol 49"[tw] OR "Nexen FB"[tw] OR
"sang-gamma"[tw] OR "sang gamma"[tw] OR "Submar"[tw] OR "Verindal Ultra"[tw] OR
"HCH, technical grade"[tw] OR "beta-Hexachlorobenzene"[tw] OR "β-
Hexachlorobenzene"[tw]) NOT medline[sb]) AND (2020/10/01:3000[crdt] OR
2020/10/01:3000[edat] OR 2020:3000[dp])) OR ("Hexachlorocyclohexane"[mh] AND
2022/04/01:3000[mhda])
"A-HCCH"[tw] OR "BHC alpha"[tw] OR "BHC beta"[tw] OR "BHC delta"[tw] OR "BHC-
alpha"[tw] OR "BHC-beta"[tw] OR "BHC-delta"[tw] OR "D-BHC"[tw] OR "D-HCCH"[tw] OR
"Dolmix"[tw] OR "ENT 9,232"[tw] OR "HCH (mixed isomers)"[tw] OR "Tri-
6 Dust No. 30"[tw]
OR "(1.alpha.,2.alpha.,3.beta.,4.alpha.,5.alpha.,6.beta.)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR
"(1.alpha.,2.alpha.,3.beta.,4.alpha.,5.beta.,6.beta.)1,2,3,4,5,6-Hexachlorocyclohexane"[tw]
OR "(1.alpha.,2.beta.,3.alpha.,4.beta.,5.alpha.,6.beta.)-1,2,3,4,5,6-hexachloro-
cyclohexane"[tw] OR "(1alpha,2alpha,3alpha,4beta,5alpha,6beta)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1alpha,2alpha,3beta,4alpha,5beta,6beta)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1alpha,2beta,3alpha,4beta,5alpha,6beta)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1r,2r,3r,4r,5r,6r)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw]
OR "(1R,2R,3R,4R,5S,6S)-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR
"(1R,2S,3r,4R,5S,6r)-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "(1R,2S,3r,4R,5S,6s)-
1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "alpha-hexachlorcyclohexane"[tw] OR
"1,2,3,4,5,6-G-HEXACHLOROCYCLOHEXANE"[tw] OR "1,2,3,4,5,6-
Hexachloro(1a,2a,3a,4b,5a,6b)cyclohexane"[tw] OR "1,2,3,4,5,6-
Hexachloro(1a,2b,3a,4b,5a,6b)cyclohexane"[tw] OR "1,2,3,4,5,6-Hexachloro-
(1.alpha.,2.alpha.,3.alpha.,4.beta.,5.alpha.,6.beta.) cyclohexane"[tw] OR "1-alpha,2-
alpha,3-alpha,4-beta,5-alpha,6-beta-Hexachlorocyclohexane"[tw] OR "1-alpha,2-beta,3-
alpha,4-beta,5-alpha,6-beta-Hexachlorocyclohexane"[tw] OR "1a,2a,3b,4a,5b,6b-
Hexachlorocyclohexane"[tw] OR "1α,2α,3β,4α,5α,6β)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1α,2α,3α,4β,5α,6.β)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1α,2α,3α,4β,5α,6β)-1,2,3,4,5,6-
Hexachlorcyclohexan"[tw] OR "(1α,2α,3α,4β,5α,6β)-1,2,3,4,5,6-
hexachlorocyclohexane"[tw] OR "(1α,2α,3α,4β,5α,6β)-1,2,3,4,5,6-
hexaclorociclohexano"[tw] OR "(1α,2α,3β,4α,5α,6β)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1α,2α,3β,4α,5β,6β)-1,2,3,4,5,6-
Hexachlorcyclohexan"[tw] OR "(1α,2α,3β,4α,5β,6β)-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "(1α,2α,3β,4α,5β,6β)-1,2,3,4,5,6-
hexaclorociclohexano"[tw] OR "(1α,2β,3α,4β,5α,6β)-1,2,3,4,5,6-Hexachlorcyclohexan"[tw]
OR "(1α,2β,3α,4β,5α,6β)-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR
"(1α,2β,3α,4β,5α,6β)-1,2,3,4,5,6-hexaclorociclohexano"[tw] OR "A-Benzene
hexachloride"[tw] OR "ALPHA-1,2,3,4,5,6-HEXACHLORCYCLOHEXAN"[tw] OR "alpha-
1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "alpha-Hexachloran"[tw] OR "alpha-
Hexachlorane"[tw] OR "B-Benzene hexachloride"[tw] OR "Bercema-Spritz-Lindan 50"[tw]
OR "Benzanex"[tw] OR "BETA-1,2,3,4,5,6-HEXACHLORCYCLOHEXAN"[tw] OR "beta-
Hexachloran"[tw] OR "delta-(Aeeeee)-1,2,3,4,5,6-hexachlorocyclohexane"[tw] OR
"Gamaxene"[tw] OR "Gamoline"[tw] OR "Gamtox"[tw] OR "Grammexene"[tw] OR
"Gyben"[tw] OR "Hexablanc"[tw] OR "Hexachlorine cyclohexane"[tw] OR
"Hexachlorzyklohexan"[tw] OR "Hexamul"[tw] OR "Hexapoudre"[tw] OR "Isatox"[tw] OR
"Kanodane"[tw] OR "Lidano"[tw] OR "Prodactif"[tw] OR "Scabecid"[tw] OR "trans-alpha-
Benzenehexachloride"[tw] OR "Trives-T"[tw] OR "BHC, d-"[tw] OR "BHC, total"[tw] OR
"Cyclohexane,1,2,3,4,5,6-hexachloro-(1.alpha.,2.alpha.,3.beta.,4.alpha.,5.alpha.,6.beta.)-
]"[tw] OR "Ciclohexano, 1,2,3,4,5,6-hexacloro-"[tw] OR "Cyclohexane, beta-1,2,3,4,5,6-

HEXACHLOROCYCLOHEXANE (HCH) B-6
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
hexachloro-"[tw] OR "Cyclohexane, delta-1,2,3,4,5,6-hexachloro-"[tw] OR "Cyclohexane,
l,2,3,4,5,6-hexachloro-, (1alpha,2alpha,3beta,4alpha,5beta,6beta)-"[tw] OR "1,2,3,4,5,6-
Benzenehexachloride"[tw] OR "1,2,3,4,5,6-Hexachlorohexane"[tw] OR "D-Benzene
hexachloride"[tw] OR "D-Hexachlorocyclohexane"[tw] OR "delta-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw] OR "α-1,2,3,4,5,6-Hexachlorocyclohexane"[tw] OR "α-
Hexachloran"[tw] OR "α-Hexachlorane"[tw] OR "α-Hexachlorcyclohexane"[tw] OR "β-
Hexachloran"[tw] OR "γ-Hexachloran"[tw] OR "γ-Hexachlorane"[tw] OR "δ-1,2,3,4,5,6-
Hexachlorocyclohexane"[tw]
NTRL
06/2023
Date limit 2020-2023
Search Titles OR Keywords;
"Hexachlorocyclohexane" OR "Benzene hexachloride" OR "Lindane" OR "Hexachlorane"
OR "Benzenehexachloride"
Toxcenter
06/2023
FILE 'TOXCENTER' ENTERED AT 16:26:48 ON 12 MAY 2023
L1 35805 SEA 319-84-6 OR 319-85-7 OR 319-86-8 OR 58-89-9 OR 608-73-1
L2 35767 SEA L1 NOT TSCATS/FS
L3 35033 SEA L2 NOT PATENT/DT
L4 1214 SEA L3 AND ED>=20201001
ACTIVATE TOXQUERY/Q
---------
L7 QUE (CHRONIC OR IMMUNOTOX? OR NEUROTOX? OR TOXICOKIN? OR
BIOMARKER? OR NEUROLOG?)
L8 QUE (PHARMACOKIN? OR SUBCHRONIC OR PBPK OR
EPIDEMIOLOGY/ST,CT,
IT)
L9 QUE (ACUTE OR SUBACUTE OR LD50# OR LD(W)50 OR LC50# OR
LC(W)50)
L10 QUE (TOXICITY OR ADVERSE OR POISONING)/ST,CT,IT
L11 QUE (INHAL? OR PULMON? OR NASAL? OR LUNG? OR RESPIR?)
L12 QUE ((OCCUPATION? OR WORKPLACE? OR WORKER?) AND EXPOS?)
L13 QUE (ORAL OR ORALLY OR INGEST? OR GAVAGE? OR DIET OR DIETS
OR
DIETARY OR DRINKING(W)WATER?)
L14 QUE (MAXIMUM AND CONCENTRATION? AND (ALLOWABLE OR
PERMISSIBLE))
L15 QUE (ABORT? OR ABNORMALIT? OR EMBRYO? OR CLEFT? OR FETUS?)
L16 QUE (FOETUS? OR FETAL? OR FOETAL? OR FERTIL? OR MALFORM?
OR
OVUM?)
L17 QUE (OVA OR OVARY OR PLACENTA? OR PREGNAN? OR PRENATAL?)
L18 QUE (PERINATAL? OR POSTNATAL? OR REPRODUC? OR STERIL? OR
TERATOGEN?)
L19 QUE (SPERM OR SPERMAC? OR SPERMAG? OR SPERMATI? OR
SPERMAS? OR
SPERMATOB? OR SPERMATOC? OR SPERMATOG?)
L20 QUE (SPERMATOI? OR SPERMATOL? OR SPERMATOR? OR
SPERMATOX? OR

HEXACHLOROCYCLOHEXANE (HCH) B-7
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
SPERMATOZ? OR SPERMATU? OR SPERMI? OR SPERMO?)
L21 QUE (NEONAT? OR NEWBORN? OR DEVELOPMENT OR
DEVELOPMENTAL?)
L22 QUE (ENDOCRIN? AND DISRUPT?)
L23 QUE (ZYGOTE? OR CHILD OR CHILDREN OR ADOLESCEN? OR
INFANT?)
L24 QUE (WEAN? OR OFFSPRING OR AGE(W)FACTOR?)
L25 QUE (DERMAL? OR DERMIS OR SKIN OR EPIDERM? OR CUTANEOUS?)
L26 QUE (CARCINOG? OR COCARCINOG? OR CANCER? OR PRECANCER?
OR
NEOPLAS?)
L27 QUE (TUMOR? OR TUMOUR? OR ONCOGEN? OR LYMPHOMA? OR
CARCINOM?)
L28 QUE (GENETOX? OR GENOTOX? OR MUTAGEN? OR
GENETIC(W)TOXIC?)
L29 QUE (NEPHROTOX? OR HEPATOTOX?)
L30 QUE (ENDOCRIN? OR ESTROGEN? OR ANDROGEN? OR HORMON?)
L31 QUE (OCCUPATION? OR WORKER? OR WORKPLACE? OR EPIDEM?)
L32 QUE L7 OR L8 OR L9 OR L10 OR L11 OR L12 OR L13 OR L14 OR L15
OR L16 OR L17 OR L18 OR L19 OR L20 OR L21 OR L22 OR L23 OR L24
OR L25 OR L26 OR L27 OR L28 OR L29 OR L30 OR L31
---------
L42 643 SEA L4 AND L32
L44 71 SEA L42 AND MEDLINE/FS
L45 125 SEA L42 AND BIOSIS/FS
L46 447 SEA L42 AND CAPLUS/FS
L47 0 SEA L42 NOT (MEDLINE/FS OR BIOSIS/FS OR CAPLUS/FS)
L48 564 DUP REM L44 L45 L46 (79 DUPLICATES REMOVED)
L*** DEL 71 S L42 AND MEDLINE/FS
L*** DEL 71 S L42 AND MEDLINE/FS
L49 71 SEA L48
L*** DEL 125 S L42 AND BIOSIS/FS
L*** DEL 125 S L42 AND BIOSIS/FS
L50 113 SEA L48
L*** DEL 447 S L42 AND CAPLUS/FS
L*** DEL 447 S L42 AND CAPLUS/FS
L51 380 SEA L48
L52 493 SEA (L49 OR L50 OR L51) NOT MEDLINE/FS
D SCAN L52
Table B-3. Strategies to Augment the Literature Search
Source
Query and number screened when available
TSCATS via
ChemView
06/2023
Compounds searched: 319-84-6, 319-85-7, 319-86-8, 58-89-9, 608-73-1

HEXACHLOROCYCLOHEXANE (HCH) B-8
APPENDIX B
Table B-3. Strategies to Augment the Literature Search
Source
Query and number screened when available
NTP
06/2023
Date limit 2020-2023
"58-89-9" "Hexachlorocyclohexane" "Lindane" "Benzene hexachloride"
"319-84-6" "319-85-7" "319-86-8" "608-73-1"
"Hexachlorane" "Benzenehexachloride"
Regulations.gov
06/2023
"Lindane"
Hexachlorocyclohexane
"Benzene hexachloride"
"Hexachlorane"
"Benzenehexachloride"
"319-84-6"
"319-85-7"
"319-86-8"
"58-89-9"
"608-73-1"
NIH RePORTER
07/2023
Search Criteria: Fiscal Year: Active Projects; Text Search: lindane OR
Hexachlorocyclohexane OR Hexachlorocyclohexanes OR "Benzene
hexachloride" OR
Hexachlorane OR Benzenehexachloride (advanced); Limit to: Project Title, Project
Terms, Project Abstracts
Search Criteria: Fiscal Year: Active Projects; Text Search: "gamma-Lindane" OR
"1,2,3,4,5,6-Hexachlorocyclohexane" OR "alpha-Benzene hexachloride" OR "alpha-
Hexachlorocyclohexane" OR "beta-Benzene hexachloride" OR "beta-
hexachlorocyclohexane" OR "delta-Benzene hexachloride" OR "delta-
Benzenehexachloride" OR "delta-Hexachlorocyclohexane" OR "gamma-Benzene
hexachloride" OR "gamma-Hexachlorocyclohexane" OR "1,2,3,4,5,6-
Hexachlorocyclohexane" OR "A-Hexachlorocyclohexane" OR "alpha-Benzene
hexachloride" OR "alpha-Benzenehexachloride" OR "alpha-Hexachlorocyclohexane"
OR "alpha-Lindane" OR "Aalindan" OR "Aficide" OR "Agrocide" OR "Agronexit" OR
"Ameisentod" OR "Aparasin" OR "Aphtiria" OR "Aplidal" OR "Arbitex" OR "B-
Hexachlorocyclohexane" OR "Benzene hexachloride" OR "Benzenehexachloride" OR
"beta-1,2,3,4,5,6-Hexachlorocyclohexane" OR "beta-Hexachloro-cyclohexane" OR
"beta-Hexachlorocyclohexane" OR "beta-Lindane" OR "Ben-Hex" OR "Benhexol" OR
"Bexol" OR "Celanex" OR "Chloresene" OR "Codechine" OR "Cyclohexane,
1,2,3,4,5,6-hexachloro-" OR "delta-Benzene hexachloride" OR "delta-
Benzenehexachloride" OR "delta-Hexachlorocyclohexane" OR "delta-Lindane" OR
"Devoran" OR "Entomoxan" OR "Forst-Nexen" OR "Gallogama" OR "Gamacarbatox"
OR "Gamacid" OR "Gamacide" OR "Gamaphex" OR "Gamene" OR "Gamiso" OR
"gamma Benzene hexachloride" OR "gamma-1,2,3,4,5,6-Hexachlorocyclohexane" OR
"gamma-Benzenehexachloride" OR "gamma-Benzohexachloride" OR "gamma-
Hexachlorcyclohexanum" OR "gamma-Hexachloro-cyclohexane" OR "gamma-
Hexachlorobenzene" OR "gamma-Hexachlorocyclohexane" OR "Gammalin" OR
"Gammaterr" OR "Gammexane" OR "Geobilan" OR "Gexane" OR
"HEXACHLORCYCLOHEXANE" OR "Hexachloro-cyclohexane" OR
"Hexachlorocyclohexane" OR "hexachlorocyclohexanes" OR "Hexachlor" OR
"Hexachlorcyclohexan" OR "Heclotox" OR "Hexachloran" OR "Hexachlorane" OR
"Hexaverm" OR "Hexcidum" OR "Hexicide" OR "Hexyclan" OR "Hilbeech" OR "Hortex"
OR "Hungaria L 7" OR "Hungaria L-7" OR "Jacutin" OR "Kokotine" OR "Kwell" OR
"Lindane" OR "Lasochron" OR "Lendine" OR "Lentox" OR "Lidenal" OR "Lindafor" OR

HEXACHLOROCYCLOHEXANE (HCH) B-9
APPENDIX B
Table B-3. Strategies to Augment the Literature Search
Source
Query and number screened when available
"Lindagam" OR "Lindagrain" OR "Lindagranox" OR "Lindan" OR "Lindanum" OR
"Lindapoudre" OR "Lindatox" OR "Lindosep" OR "Lintox" OR "Linvur" OR "Lorexane"
OR "Mszycol" OR "Neo-Scabicidol" OR "Nexol E" OR "Nexol-E" OR "Nicochloran" OR
"Novigam" OR "Omnitox" OR "Ovadziak" OR "Owadziak" OR "Pedraczak" OR
"Pflanzol" OR "Quellada" OR "Technical HCH" OR "technical grade HCH" OR
"Scabene" OR "Silvanol" OR "Spritz-Rapidin" OR "Spritzlindane" OR "Spruehpflanzol"
OR "Streunex" (advanced); Limit to: Project Title, Project Terms, Project Abstracts
Search Criteria: Fiscal Year: Active Projects; Text Search: "α-Benzohexachloride" OR
"α-Hexachlorocyclohexane" OR "α-Lindane" OR "β-1,2,3,4,5,6-
Hexachlorocyclohexane" OR "β-Benzene hexachloride" OR "β-
Hexachlorocyclohexane" OR "β-Lindane" OR "γ-1,2,3,4,5,6-Hexachlorocyclohexane"
OR "γ-Benzene hexachloride" OR "γ-Benzohexachloride" OR "γ-Hexachlorobenzene"
OR "γ-Hexachlorocyclohexane" OR "γ-Lindane" OR "δ-Benzene hexachloride" OR "δ-
Hexachlorocyclohexane" OR "δ-Lindane" OR "alpha BHC" OR "alpha-BHC" OR
"alpha-HCH" OR "beta BHC" OR "beta-BHC" OR "beta-HCH" OR "delta BHC" OR
"delta-BHC" OR "delta-HCH" OR "Gamma-BHC" OR "gamma-HCH" OR "Nexit Stark"
OR "Nexit-stark" OR "α-BHC" OR "α-HCH" OR "β-666" OR "β-BHC" OR "β-HCH" OR
"γ-666" OR "γ-BHC" OR "γ-HCH" OR "δ-BHC" OR "δ-HCH" OR "total BHC" OR "α-
666" OR "δ-666" OR "BHC-gamma" OR "α-Benzenehexachloride" OR "Detox 25" OR
"Dol Granule" OR "ENT 7,796" OR "TAP 85" OR "Ameisenmittel merck" OR "Arcotal
S" OR "Bentox 10" OR "Benzene-1,2,3,4,5,6-hexachloride" OR "beta-
Hexachlorobenzene" OR "Detmol Extract" OR "Fenoform forte" OR "Gamma-mean
400" OR "Geolin G 3" OR "Hungaria L7" OR "Mglawik L" OR "Milbol 49" OR "Nexen
FB" OR "sang-gamma" OR "sang gamma" OR "Submar" OR "Verindal Ultra" OR
"HCH, technical grade" OR "beta-Hexachlorobenzene" OR "β-Hexachlorobenzene"
(advanced); Limit to: Project Title, Project Terms, Project Abstracts
Other
Identified throughout the assessment process
The 2023 results were:
• Number of records identified from PubMed, NTRL, and TOXCENTER (after duplicate
removal): 673
• Number of records identified from other strategies: 18
• Total number of records to undergo literature screening: 691
B
.1.2 Literature Screening
A two-step process was used to screen the literature search to identify relevant studies on HCH:
• Title and abstract screen
• Full text screen
Title and Abstract Screen. Within the reference library, titles and abstracts were screened manually for
relevance. Studies that were considered relevant (see Table B-1 for inclusion criteria) were moved to the
second step of the literature screening process. Studies were excluded when the title and abstract clearly
indicated that the study was not relevant to the toxicological profile.
• Number of titles and abstracts screened: 691
• Number of studies considered relevant and moved to the next step: 129
HEXACHLOROCYCLOHEXANE (HCH) B-10
APPENDIX B
Full Text Screen. The second step in the literature screening process was a full text review of individual
studies considered relevant in the title and abstract screen step. Each study was reviewed to determine
whether it was relevant for inclusion in the toxicological profile.
• Number of studies undergoing full text review: 129
• Number of studies cited in the pre-public draft of the toxicological profile: 809
• Total number of studies cited in the profile: 860
A summary of the results of the literature search and screening is presented in Figure B-1.

HEXACHLOROCYCLOHEXANE (HCH) B-11
APPENDIX B
Figure B-1. June 2023 Literature Search Results and Screen for HCH

HEXACHLOROCYCLOHEXANE (HCH) C-1
APPENDIX C. FRAMEWORK FOR ATSDR’S SYSTEMATIC REVIEW OF
HEALTH EFFECTS DATA FOR HCH
To increase the transparency of ATSDR’s process of identifying, evaluating, synthesizing, and
interpreting the scientific evidence on the health effects associated with exposure to HCH, ATSDR
utilized a slight modification of NTP’s Office of Health Assessment and Translation (OHAT) systematic
review methodology (NTP 2013, 2015; Rooney et al. 2014). ATSDR’s framework is an eight-step
process for systematic review with the goal of identifying the potential health hazards of exposure to
HCH:
• Step 1. Problem Formulation
• Step 2. Literature Search and Screen for Health Effects Studies
• Step 3. Extract Data from Health Effects Studies
• Step 4. Identify Potential Health Effect Outcomes of Concern
• Step 5. Assess the Risk of Bias for Individual Studies
• Step 6. Rate the Confidence in the Body of Evidence for Each Relevant Outcome
• Step 7. Translate Confidence Rating into Level of Evidence of Health Effects
• Step 8. Integrate Evidence to Develop Hazard Identification Conclusions
The systematic review for this profile is divided into four sections:
1. Steps 1, 2, and 3 for α-, β-, and γ-HCH (Sections C.1, C.2, and C.3)
2. Steps 4, 5, 6, 7, and 8 for α-HCH (Sections C.4, C.5, C.6, C.7, and C.8)
3. Steps 4, 5, 6, 7, and 8 for β-HCH (Sections C.9, C.10, C.11, C.12, and C.13)
4. Steps 4, 5, 6, 7, and 8 for γ-HCH (Sections C.14, C.15, C.16, C.17, and C.18)
C.
1 PROBLEM FORMULATION
The objective of the toxicological profile and this systematic review was to identify the potential health
hazards associated with inhalation, oral, or dermal/ocular exposure to HCH. The inclusion criteria used to
identify relevant studies examining the health effects of HCH are presented in Table C-1.
Data from human and laboratory animal studies were considered relevant for addressing this objective.
Human studies were divided into two broad categories: observational epidemiology studies and
controlled exposure studies. The observational epidemiology studies were further divided: cohort studies
(retrospective and prospective studies), population studies (with individual data or aggregate data), and
case-control studies.
Table C-1. Inclusion Criteria for Identifying Health Effects Studies
Species
Human
Laboratory mammals
Route of exposure
Inhalation
Oral
Dermal (or ocular)
Parenteral (these studies will be considered supporting data)

HEXACHLOROCYCLOHEXANE (HCH) C-2
APPENDIX C
Table C-1. Inclusion Criteria for Identifying Health Effects Studies
Health outcome
Death
Systemic effects
Body weight effects
Respiratory effects
Cardiovascular effects
Gastrointestinal effects
Hematological effects
Musculoskeletal effects
Hepatic effects
Renal effects
Dermal effects
Ocular effects
Endocrine effects
Immunological effects
Neurological effects
Reproductive effects
Developmental effects
Other noncancer effects
Cancer
C.2 LITERATURE SEARCH AND SCREEN FOR HEALTH EFFECTS STUDIES
A literature search and screen were conducted to identify studies examining the health effects of HCH.
The literature search framework for the toxicological profile is discussed in detail in Appendix B.
C.2.1 Literature Search
As noted in Appendix B, the current literature search was intended to update the draft toxicological
profile for HCH released for public comment in January 2023. See Appendix B for the databases
searched and the search strategy.
A total of 691 records relevant to all sections of the toxicological profile were identified (after
duplicate removal).
C.2.2 Literature Screening
As described in Appendix B, a two-step process was used to screen the literature search to identify
relevant studies examining the health effects of HCH.
Title and Abstract Screen. In the Title and Abstract Screen step, 691 records were reviewed;
45 documents were considered to meet the health effects inclusion criteria in Table C-1 and were moved
to the next step in the process.

HEXACHLOROCYCLOHEXANE (HCH) C-3
APPENDIX C
Full Text Screen. In the second step in the literature screening process for the systematic review, a full
text review of 299 health effect documents (documents identified in the update literature search and
documents cited in older versions of the profile) was performed. From those 299 documents
(446 studies), 64 documents (70 studies) were included in the qualitative review.
The epidemiological database for HCH is extensive. To facilitate the selection and inclusion of human
studies of greater utility in assessing the hazards of HCH, only studies meeting the criteria below were
included in the Toxicological Profile.
• Exposure was assessed for individuals, either using a biomarker or through detailed individual
history (i.e., ecological study designs were excluded);
• The study presented an effect estimate specific to one or more HCH isomers;
• The statistical analysis of the association was multivariate, with consideration of at least one
potential covariate. Studies limited to bivariate analyses (i.e., Pearson or Spearman correlation
coefficients) were not included, nor were studies in which the analysis was limited to a
comparison between blood concentrations in cases and controls;
• The health outcomes evaluated in the study were not mechanistic in nature (e.g., oxidative stress,
genotoxicity);
• Case reports and case series were included if there was clear evidence of exposure to one or more
HCH isomers.
C.3 EXTRACT DATA FROM HEALTH EFFECTS STUDIES
Relevant data extracted from the individual studies selected for inclusion in the systematic review were
collected in customized data forms. A summary of the type of data extracted from each study is presented
in Table C-2. For references that included more than one experiment or species, data extraction records
were created for each experiment or species.
Table C-2. Data Extracted From Individual Studies
Citation
Chemical form
Route of exposure (e.g., inhalation, oral, dermal)
Specific route (e.g., gavage in oil, drinking water)
Species
Strain
Exposure duration category (e.g., acute, intermediate, chronic)
Exposure duration
Frequency of exposure (e.g., 6 hours/day, 5 days/week)
Exposure length
Number of animals or subjects per sex per group
Dose/exposure levels
Parameters monitored
Description of the study design and method
Summary of calculations used to estimate doses (if applicable)
Summary of the study results
Reviewer’s comments on the study
Outcome summary (one entry for each examined outcome)

HEXACHLOROCYCLOHEXANE (HCH) C-4
APPENDIX C
Table C-2. Data Extracted From Individual Studies
No-observed-adverse-effect level (NOAEL) value
Lowest-observed-adverse-effect level (LOAEL) value
Effect observed at the LOAEL value
A summary of the extracted data for each study is presented in the Supplemental Documents for HCH
isomers and overviews of the results of the inhalation, oral, and dermal exposure studies are presented in
Sections 2.2–2.18 of the profile and in the Levels Significant Exposures tables in Section 2.1 of the
profile (Tables 2-1 through 2-2).
There was only a single study of δ-HCH, and inadequate data for derivation of an MRL, so a systematic
review of the data for this isomer was not undertaken.
C.4 IDENTIFY POTENTIAL HEALTH EFFECT OUTCOMES OF CONCERN—α-HCH
Overviews of the potential health effect outcomes for α-HCH identified in human and animal studies are
presented in Tables C-3 and C-4, respectively. There was a small number of human studies examining a
handful of endpoints; the largest number of studies was devoted to developmental endpoints. The human
studies used measures of α-HCH in blood or tissues to assess exposure, so the route is unknown; for the
purpose of enumerations, these studies are considered to reflect oral exposure (e.g., through contaminated
food). There were no inhalation or dermal animal studies of α-HCH, and very few oral studies. The
available animal studies primarily examined liver effects and cancer. The most sensitive effect in animal
studies were hepatic. Studies examining hepatic effects were carried through to Steps 4–8 of the
systematic review. There were 70 studies (published in 64 documents) examining these potential
outcomes carried through to Steps 4–8 of the systematic review. There were no human studies examining
hepatic effects of α-HCH.

HEXACHLOROCYCLOHEXANE (HCH) C-5
APPENDIX C
Table C-3. Overview of the Health Outcomes for α-Hexachlorocyclohexane Evaluated In Human Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
Neurological
Reproductive
Developmental
Other Noncancer
Can
cer
Inhalation studies
Cohort
Case control
Population
Case series
Oral studies
Cohort
Case control
2
1
3
4
2
5
1
1
2
3
3
Population
2
4
1
3
3
1
1
1
2
Case series
Dermal studies
Cohort
Case control
Population
Case series
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10

HEXACHLOROCYCLOHEXANE (HCH) C-6
APPENDIX C
Table C-4. Overview of the Health Outcomes for α-Hexachlorocyclohexane Evaluated in Experimental Animal
Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
a
Neurological
a
Reproductive
a
Developmental
Other Noncancer
Can
cer
Inhalation studies
Acute-duration
Intermediate-duration
Chronic-duration
Oral studies
Acute-duration
1
4
1
1
1
Intermediate-duration
1
10
2
6
7
6
Chronic-duration
1
2
1
2
1
2
1
2
Dermal studies
Acute-duration
Intermediate-duration
Chronic-duration
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10
a
Number of studies examining endpoint includes studies evaluating histopathology, but not evaluating function.

HEXACHLOROCYCLOHEXANE (HCH) C-7
APPENDIX C
C.5 ASSESS THE RISK OF BIAS FOR INDIVIDUAL STUDIES—α-HCH
C
.5.1 Risk of Bias Assessment—α-HCH
The risk of bias of individual studies was assessed using OHAT’s Risk of Bias Tool (NTP 2015). The
risk of bias questions for observational epidemiology studies, human-controlled exposure studies, and
animal experimental studies are presented in Tables C-5, C-6, and C-7, respectively. Each risk of bias
question was answered on a four-point scale:
• Definitely low risk of bias (++)
• Probably low risk of bias (+)
• Probably high risk of bias (-)
• Definitely high risk of bias (– –)
In general, “definitely low risk of bias” or “definitely high risk of bias” were used if the question could be
answered with information explicitly stated in the study report. If the response to the question could be
inferred, then “probably low risk of bias” or “probably high risk of bias” responses were typically used.
Table C-5. Risk of Bias Questionnaire for Observational Epidemiology Studies
Selection bias
Were the comparison groups appropriate?
Confounding bias
Did the study design or analysis account for important confounding and modifying variables?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?
Selective reporting bias
Were all measured outcomes reported?
Table C-6. Risk of Bias Questionnaire for Human-Controlled Exposure Studies
Selection bias
Was administered dose or exposure level adequately randomized?
Was the allocation to study groups adequately concealed?
Performance bias
Were the research personnel and human subjects blinded to the study group during the study?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?

HEXACHLOROCYCLOHEXANE (HCH) C-8
APPENDIX C
Table C-6. Risk of Bias Questionnaire for Human-Controlled Exposure Studies
Selective reporting bias
Were all measured outcomes reported?
Table C-7. Risk of Bias Questionnaire for Experimental Animal Studies
Selection bias
Was administered dose or exposure level adequately randomized?
Was the allocation to study groups adequately concealed?
Performance bias
Were experimental conditions identical across study groups?
Were the research personnel blinded to the study group during the study?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?
Selective reporting bias
Were all measured outcomes reported?
After the risk of bias questionnaires were completed for the health effects studies, the studies were
assigned to one of three risk of bias tiers based on the responses to the key questions listed below and the
responses to the remaining questions.
• Is there confidence in the exposure characterization? (only relevant for observational studies)
• Is there confidence in the outcome assessment?
• Does the study design or analysis account for important confounding and modifying variables?
(only relevant for observational studies)
First Tier. Studies placed in the first tier received ratings of “definitely low” or “probably low” risk of
bias on the key questions AND received a rating of “definitely low” or “probably low” risk of bias on the
responses to at least 50% of the other applicable questions.
Second Tier. A study was placed in the second tier if it did not meet the criteria for the first or third tiers.
Third Tier. Studies placed in the third tier received ratings of “definitely high” or “probably high” risk of
bias for the key questions AND received a rating of “definitely high” or “probably high” risk of bias on
the response to at least 50% of the other applicable questions.
The results of the risk of bias assessment for the α-HCH health effects studies (animal experimental
studies) are presented in Table C-8. There were no observational epidemiology or human controlled
experimental studies of α-HCH.

HEXACHLOROCYCLOHEXANE (HCH) C-9
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for α-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental
conditions
identical across study groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Outcome: Hepatic effects
Oral intermediate exposure
Sumida et al. 2007 (rat;
28 days)
– – + + – + + ++ NA First
Ito et al. 1973 (mouse;
24 weeks)
– – + + + ++ + ++ NA First
Ito et al. 1975 (rat; 24 or
48 weeks)
– – + + – ++ + ++ NA First
Nagasaki et al. 1975 (rat;
24 weeks)
– – + + – – +
++
NA Second
Nagasaki et al. 1975
(mouse; 24 weeks)
– – + + – – +
++
NA Second
Nagasaki et al. 1975
(hamster; 24 weeks)
– – + + – – +
++
NA Second
Tryphonas and Iverson
1983 (mouse; 50 weeks)
– – + + ++ + + ++ NA First

HEXACHLOROCYCLOHEXANE (HCH) C-10
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for α-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental
conditions
identical across study groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Oral chronic exposure
Fitzhugh et al. 1950 (rat;
72 weeks)
– – + + ++ + + ++ NA First
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; na = not applicable
*Key question used to assign risk of bias tier
HEXACHLOROCYCLOHEXANE (HCH) C-11
APPENDIX C
C.6 RATE THE CONFIDENCE IN THE BODY OF EVIDENCE FOR EACH RELEVANT
OUTCOME—α-HCH
Confidence in the body of human and animal evidence were evaluated independently for each potential
outcome. ATSDR did not evaluate the confidence in the body of evidence for carcinogenicity; rather, the
Agency defaulted to the cancer weight-of-evidence assessment of other agencies including HHS, EPA,
and IARC. The confidence in the body of evidence for an association or no association between exposure
to HCH and a particular outcome was based on the strengths and weaknesses of individual studies. Four
descriptors were used to describe the confidence in the body of evidence for effects or when no effect was
found:
• High confidence: the true effect is highly likely to be reflected in the apparent relationship
• Moderate confidence: the true effect may be reflected in the apparent relationship
• Low confidence: the true effect may be different from the apparent relationship
• Very low confidence: the true effect is highly likely to be different from the apparent
relationship
Confidence in the body of evidence for a particular outcome was rated for each type of study: case-
control, case series, cohort, population, human-controlled exposure, and experimental animal. In the
absence of data to the contrary, data for a particular outcome were collapsed across animal species, routes
of exposure, and exposure durations. If species (or strain), route, or exposure duration differences were
noted, then the data were treated as separate outcomes.
In the case of α-HCH, the only available type of study was experimental animal.
C.6.1 Initial Confidence Rating—α-HCH
In ATSDR’s modification to the OHAT approach, the body of evidence for an association (or no
association) between exposure to α-HCH and a particular outcome was given an initial confidence rating
based on the key features of the individual studies examining that outcome. The presence of these key
features of study design was determined for individual studies using four “yes or no” questions, which
were customized for epidemiology, human controlled exposure, or experimental animal study designs.
Separate questionnaires were completed for each outcome assessed in a study. The key features for
observational epidemiology (cohort, population, and case-control) studies, human controlled exposure
studies, and experimental animal studies are presented in Tables C-9, C-10, and C-11, respectively. The
initial confidence in the study was determined based on the number of key features present in the study
design:
• High Initial Confidence: Studies in which the responses to the four questions were “yes”.
• Moderate Initial Confidence: Studies in which the responses to only three of the questions
were “yes”.
• Low Initial Confidence: Studies in which the responses to only two of the questions were “yes”.
• Very Low Initial Confidence: Studies in which the response to one or none of the questions
was “yes”.

HEXACHLOROCYCLOHEXANE (HCH) C-12
APPENDIX C
Table C-9. Key Features of Study Design for Observational Epidemiology
Studies
Exposure was experimentally controlled
Exposure occurred prior to the outcome
Outcome was assessed on individual level rather than at the population level
A comparison group was used
Table C-10. Key Features of Study Design for Human Controlled Exposure
Studies
A comparison group was used or the subjects served as their own control
A sufficient number of subjects were tested
Appropriate methods were used to measure outcomes (i.e., clinically-confirmed outcome versus self-
reported)
Appropriate statistical analyses were performed and reported or the data were reported in such a way to
allow independent statistical analysis
Table C-11. Key Features of Study Design for Experimental Animal Studies
A concurrent control group was used
A sufficient number of animals per group were tested
Appropriate parameters were used to assess a potential adverse effect
Appropriate statistical analyses were performed and reported or the data were reported in such a way to
allow independent statistical analysis
The presence or absence of the key features and the initial confidence levels for studies of hepatic effects
observed in the animal experimental studies are presented in Table C-12.
Table C-12. Presence of Key Features of Study Design for
α-Hexachlorocyclohexane—Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number of
animals per group
Appropriate parameters
to assess potential
effect
Adequate data for
statistical analysis
Initial
study
confidenc
e
Outcome: Hepatic effects
Oral intermediate exposure
Sumida et al. 2007 (rat; 28 days)
Yes
No
Yes
Yes
Moderate
Ito et al. 1973 (mouse; 24 weeks)
Yes
Yes
Yes
Yes
High

HEXACHLOROCYCLOHEXANE (HCH) C-13
APPENDIX C
Table C-12. Presence of Key Features of Study Design for
α-Hexachlorocyclohexane—Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number of
animals per group
Appropriate parameters
to assess potential
effect
Adequate data for
statistical analysis
Initial
study
confidenc
e
Ito et al. 1975 (rat; 24 or 48 weeks)
Yes
Yes
Yes
No
Moderate
Nagasaki et al. 1975 (rat; 24 weeks)
Yes
No
Yes
No
Low
Nagasaki et al. 1975 (mouse;
24 weeks)
Yes Yes Yes No Moderate
Nagasaki et al. 1975 (hamster;
24 weeks)
Yes No Yes No Low
Tryphonas and Iverson 1983 (mouse;
50 weeks)
Yes Yes Yes Yes High
Oral chronic exposure
Fitzhugh et al. 1950 (rat; 72 weeks)
Yes
No
Yes
Yes
Moderate
A summary of the initial confidence ratings for each outcome is presented in Table C-13. If individual
studies for a particular outcome and study type had different study quality ratings, then the highest
confidence rating for the group of studies was used to determine the initial confidence rating for the body
of evidence; any exceptions were noted in Table C-13.
Table C-13. Initial Confidence Rating for α-HCH Health Effects Studies
Initial study
confidence
Initial confidence
rating
Outcome: Hepatic effects
Oral intermediate exposure
Animal studies
Sumida et al. 2007 (rat; 28 days)
Moderate
High
Ito et al. 1973 (mouse; 24 weeks)
High
Ito et al. 1975 (rat; 24 or 48 weeks)
Moderate
Nagasaki et al. 1975 (rat; 24 weeks)
Low
Nagasaki et al. 1975 (mouse; 24 weeks)
Moderate
Nagasaki et al. 1975 (hamster; 24 weeks)
Low
Tryphonas and Iverson 1983 (mouse; 50 weeks)
High
Oral chronic exposure
Animal studies
Fitzhugh et al. 1950
Moderate
Moderate
HEXACHLOROCYCLOHEXANE (HCH) C-14
APPENDIX C
C
.6.2 Adjustment of the Confidence Rating—α-HCH
The initial confidence rating was then downgraded or upgraded depending on whether there were
substantial issues that would decrease or increase confidence in the body of evidence. The nine properties
of the body of evidence that were considered are listed below. The summaries of the assessment of the
confidence in the body of evidence for hepatic effects are presented in Table C-14. If the confidence
ratings for a particular outcome were based on more than one type of human study, then the highest
confidence rating was used for subsequent analyses. An overview of the confidence in the body of
evidence for all health effects associated with α-HCH exposure is presented in Table C-15.
Five properties of the body of evidence were considered to determine whether the confidence rating
should be downgraded:
• Risk of bias. Evaluation of whether there is substantial risk of bias across most of the studies
examining the outcome. This evaluation used the risk of bias tier groupings for individual studies
examining a particular outcome. Below are the criteria used to determine whether the initial
confidence in the body of evidence for each outcome should be downgraded for risk of bias:
o No downgrade if most studies are in the risk of bias first tier
o Downgrade one confidence level if most studies are in the risk of bias second tier
o Downgrade two confidence levels if most studies are in the risk of bias third tier
• U
nexplained inconsistency. Evaluation of whether there is inconsistency or large variability in
the magnitude or direction of estimates of effect across studies that cannot be explained. Below
are the criteria used to determine whether the initial confidence in the body of evidence for each
outcome should be downgraded for unexplained inconsistency:
o No downgrade if there is little inconsistency across studies or if only one study evaluated
the outcome
o Downgrade one confidence level if there is variability across studies in the magnitude or
direction of the effect
o Downgrade two confidence levels if there is substantial variability across studies in the
magnitude or direct of the effect
• Indirectness. Evaluation of four factors that can affect the applicability, generalizability, and
relevance of the studies:
o Relevance of the animal model to human health—unless otherwise indicated, studies in
rats, mice, and other mammalian species are considered relevant to humans
o Directness of the endpoints to the primary health outcome—examples of secondary
outcomes or nonspecific outcomes include organ weight in the absence of histopathology
or clinical chemistry findings in the absence of target tissue effects
o Nature of the exposure in human studies and route of administration in animal studies—
inhalation, oral, and dermal exposure routes are considered relevant unless there are
compelling data to the contrary
o Duration of treatment in animal studies and length of time between exposure and
outcome assessment in animal and prospective human studies—this should be considered
on an outcome-specific basis
Below are the criteria used to determine whether the initial confidence in the body of evidence for
each outcome should be downgraded for indirectness:
o No downgrade if none of the factors are considered indirect
o Downgrade one confidence level if one of the factors is considered indirect
HEXACHLOROCYCLOHEXANE (HCH) C-15
APPENDIX C
o Downgrade two confidence levels if two or more of the factors are considered indirect
• Imprecision. Evaluation of the narrowness of the effect size estimates and whether the studies
have adequate statistical power. Data are considered imprecise when the ratio of the upper to
lower 95% CIs for most studies is ≥10 for tests of ratio measures (e.g., odds ratios) and ≥100 for
absolute measures (e.g., percent control response). Adequate statistical power is determined if
the study can detect a potentially biologically meaningful difference between groups (20%
change from control response for categorical data or risk ratio of 1.5 for continuous data). Below
are the criteria used to determine whether the initial confidence in the body of evidence for each
outcome should be downgraded for imprecision:
o No downgrade if there are no serious imprecisions
o Downgrade one confidence level for serious imprecisions
o Downgrade two confidence levels for very serious imprecisions
• Publication bias. Evaluation of the concern that studies with statistically significant results are
more likely to be published than studies without statistically significant results.
o Downgrade one level of confidence for cases where there is serious concern with
publication bias
Four properties of the body of evidence were considered to determine whether the confidence rating
should be upgraded:
• Large magnitude of effect. Evaluation of whether the magnitude of effect is sufficiently large
so that it is unlikely to have occurred as a result of bias from potential confounding factors.
o Upgrade one confidence level if there is evidence of a large magnitude of effect in a few
studies, provided that the studies have an overall low risk of bias and there is no serious
unexplained inconsistency among the studies of similar dose or exposure levels;
confidence can also be upgraded if there is one study examining the outcome, provided
that the study has an overall low risk of bias
• Dose response. Evaluation of the dose-response relationships measured within a study and
across studies. Below are the criteria used to determine whether the initial confidence in the body
of evidence for each outcome should be upgraded:
o Upgrade one confidence level for evidence of a monotonic dose-response gradient
o Upgrade one confidence level for evidence of a non-monotonic dose-response gradient
where there is prior knowledge that supports a non-monotonic dose-response and a non-
monotonic dose-response gradient is observed across studies
• Plausible confounding or other residual biases. This factor primarily applies to human studies
and is an evaluation of unmeasured determinants of an outcome such as residual bias towards the
null (e.g., “healthy worker” effect) or residual bias suggesting a spurious effect (e.g., recall bias).
Below is the criterion used to determine whether the initial confidence in the body of evidence for
each outcome should be upgraded:
o Upgrade one confidence level for evidence that residual confounding or bias would
underestimate an apparent association or treatment effect (i.e., bias toward the null) or
suggest a spurious effect when results suggest no effect
• Consistency in the body of evidence. Evaluation of consistency across animal models and
species, consistency across independent studies of different human populations and exposure

HEXACHLOROCYCLOHEXANE (HCH) C-16
APPENDIX C
scenarios, and consistency across human study types. Below is the criterion used to determine
whether the initial confidence in the body of evidence for each outcome should be upgraded:
o Upgrade one confidence level if there is a high degree of consistency in the database
Table C-14. Adjustments to the Initial Confidence in the Body of Evidence
Initial confidence
Adjustments to the initial confidence
rating
Final
confidence
Hepatic effects:
Human studies
NA
NA
NA
Animal studies
High
+1 consistency, +1 magnitude
High
Table C-15. Confidence in the Body of Evidence for α-Hexachlorocyclohexane
Outcome
Confidence in body of evidence
Human studies
Animal studies
Hepatic
NA
High
C.
7 TRANSLATE CONFIDENCE RATING INTO LEVEL OF EVIDENCE OF HEALTH
EFFECTS—α-HCH
In the seventh step of the systematic review of the health effects data for HCH, the confidence in the body
of evidence for specific outcomes was translated to a level of evidence rating. The level of evidence
rating reflected the confidence in the body of evidence and the direction of the effect (i.e., toxicity or no
toxicity); route-specific differences were noted. The level of evidence for health effects was rated on a
five-point scale:
• High level of evidence: High confidence in the body of evidence for an association between
exposure to the substance and the health outcome
• Moderate level of evidence: Moderate confidence in the body of evidence for an association
between exposure to the substance and the health outcome
• Low level of evidence: Low confidence in the body of evidence for an association between
exposure to the substance and the health outcome
• Evidence of no health effect: High confidence in the body of evidence that exposure to the
substance is not associated with the health outcome
• Inadequate evidence: Low or moderate confidence in the body of evidence that exposure to the
substance is not associated with the health outcome OR very low confidence in the body of
evidence for an association between exposure to the substance and the health outcome
A summary of the level of evidence of health effects for HCH is presented in Table C-16.

HEXACHLOROCYCLOHEXANE (HCH) C-17
APPENDIX C
Table C-16. Level of Evidence of Health Effects for α-Hexachlorocyclohexane
Outcome
Confidence in body
of evidence
Direction of health
effect
Level of evidence for
health effect
Human studies
NA
Animal studies
Hepatic
High
Health Effect
High
C.
8 INTEGRATE EVIDENCE TO DEVELOP HAZARD IDENTIFICATION CONCLUSIONS—
α-HCH
The final step involved the integration of the evidence streams for the human studies and animal studies
to allow for a determination of hazard identification conclusions. For health effects, there were four
hazard identification conclusion categories:
• Known to be a hazard to humans
• Presumed to be a hazard to humans
• Suspected to be a hazard to humans
• Not classifiable as to the hazard to humans
The initial hazard identification was based on the highest level of evidence in the human studies and the
level of evidence in the animal studies; if there were no data for one evidence stream (human or animal),
then the hazard identification was based on the one data stream (equivalent to treating the missing
evidence stream as having low level of evidence). The hazard identification scheme is presented in
Figure C-1 and described below:
• Known: A health effect in this category would have:
o High level of evidence for health effects in human studies AND a high, moderate, or low
level of evidence in animal studies.
• Presumed: A health effect in this category would have:
o Moderate level of evidence in human studies AND high or moderate level of evidence in
animal studies OR
o Low level of evidence in human studies AND high level of evidence in animal studies
• Suspected: A health effect in this category would have:
o Moderate level of evidence in human studies AND low level of evidence in animal
studies OR
o Low level of evidence in human studies AND moderate level of evidence in animal
studies
• Not classifiable: A health effect in this category would have:
o Low level of evidence in human studies AND low level of evidence in animal studies

HEXACHLOROCYCLOHEXANE (HCH) C-18
APPENDIX C
Figure C-1. Hazard Identification Scheme
Other relevant data such as mechanistic or mode-of-action data were considered to raise or lower the level
of the hazard identification conclusion by providing information that supported or opposed biological
plausibility.
Two hazard identification conclusion categories were used when the data indicated that there may be no
health effect in humans:
• Not identified to be a hazard in humans
• Inadequate to determine hazard to humans
If the human level of evidence conclusion of no health effect was supported by the animal evidence of no
health effect, then the hazard identification conclusion category of “not identified” was used. If the
human or animal level of evidence was considered inadequate, then a hazard identification conclusion
category of “inadequate” was used. As with the hazard identification for health effects, the impact of
other relevant data was also considered for no health effect data.
The hazard identification conclusions for α-HCH are listed below and summarized in Table C-17.

HEXACHLOROCYCLOHEXANE (HCH) C-19
APPENDIX C
Presumed Health Effects
• Hepatic
o No information on hepatic effects in humans exposed to α-HCH.
o High evidence level in animals including increased liver weight and histopathological lesions
after oral exposure to α-HCH (Fitzhugh et al. 1950; Ito et al. 1973, 1975; Nagasaki et al.
1975; Sumida et al. 2007; Tryphonas and Iverson 1983).
o Plausible mechanism based on increased oxidative stress markers in livers of animals
exposed to low oral doses in vivo (Barros et al. 1991).
Table C-17. Hazard Identification Conclusions for α-Hexachlorocyclohexane
Outcome
Hazard identification
Hepatic effects
Presumed health effect
C.9 IDENTIFY POTENTIAL HEALTH EFFECT OUTCOMES OF CONCERN—β-HCH
Overviews of the potential health effect outcomes for β-HCH identified in human and animal studies are
presented in Tables C-18 and C-19, respectively. Human studies examined a wide range of outcomes,
with more studies of endocrine (thyroid hormone levels) developmental outcomes, other noncancer
endpoints (diabetes and metabolic perturbations) and cancer than other outcomes. The human studies
used measures of β-HCH in blood or tissues to assess exposure, so the route is unknown; for the purpose
of enumerations, these studies are considered to reflect oral exposure (e.g., through contaminated food).
Animal studies are limited to oral exposures, and the endpoints examined were limited. The animal data
show that the liver and nervous system are sensitive effects of exposure to β-HCH; studies examining
these potential outcomes were carried through to Steps 4–8 of the systematic review. There were
15 studies (published in 15 documents) examining these potential outcomes carried through to Steps 4–8
of the systematic review.

HEXACHLOROCYCLOHEXANE (HCH) C-20
APPENDIX C
Table C-18. Overview of the Health Outcomes for β-Hexachlorocyclohexane Evaluated in Human Studies
Body
weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
Neurological
Reproductive
Developmental
Other Noncancer
Can
cer
Inhalation studies
Cohort
Case control
Population
Case series
Oral studies
Cohort
1
1
1
2
7
2
3
1
1
2
4
1
Case cohort
1
1
Case control
2
1
1
5
4
9
7
25
1
1
4
4
4
5
11
Nested case control
1
1
1
Population
2
1
3
2
2
9
2
2
5
13
11
1
2
1
1
6
1
1
2
8
5
1
Case series
Dermal studies
Cohort
Case control
Population
Case series
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10

HEXACHLOROCYCLOHEXANE (HCH) C-21
APPENDIX C
Table C-19. Overview of the Health Outcomes for β-Hexachlorocyclohexane Evaluated in Experimental Animal
Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
a
Neurological
a
Reproductive
a
Developmental
Other Noncancer
Can
cer
Inhalation studies
Acute-duration
Intermediate-duration
Chronic-duration
Oral studies
Acute-duration
1
2
3
1
1
2
1
2
1
2
1
Intermediate-duration
2
1
4
1
3
2
3
1
1
1
4
1
3
2
3
Chronic-duration
1
1
1
1
1
1
1
1
Dermal studies
Acute-duration
Intermediate-duration
Chronic-duration
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10
a
Number of studies examining endpoint includes study evaluating histopathology, but not evaluating function.
HEXACHLOROCYCLOHEXANE (HCH) C-22
APPENDIX C
C.10 ASSESS THE RISK OF BIAS FOR INDIVIDUAL STUDIES—β-HCH
C.10.1 Risk of Bias Assessment—β-HCH
The risk of bias of individual studies was assessed using OHAT’s Risk of Bias Tool (NTP 2015). The
risk of bias questions for observational epidemiology studies, human-controlled exposure studies, and
animal experimental studies were presented above in Tables C-5, C-6, and C-7, respectively. As
described in Section C.5.1, each risk of bias question was answered on a four-point scale and studies were
assigned to one of three risk of bias tiers.
The results of the risk of bias assessment for the different types of β-HCH health effects studies
(observational epidemiology and animal experimental studies) are presented in Tables C-20 and C-21,
respectively.

HEXACHLOROCYCLOHEXANE (HCH) C-23
APPENDIX C
Table C-20. Summary of Risk of Bias Assessment for β-Hexachlorocyclohexane—Observational
Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding
bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the comparison groups
appropriate?
Did the study design or
analysis account for important
confounding
and modifying variables?*
Were outcome data complete
without attrition or exclusion
from analysis?
Is there confidence in the
exposure characterization?*
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Outcome: Neurological effects
Cohort
Medehouenou et al. 2019
++
++
+
++
++
++
First
Case-control
Petersen et al. 2008
+
–
++
++
+
++
Second
Richardson et al. 2009, 2011
–
+
++
++
++
++
First
Singh et al. 2012, 2013, 2014
+
+
++
++
++
++
First
Xu et al. 2022
+
+
++
++
++
++
First
Zhang et al. 2021
+
+
++
++
++
++
First
Cross-sectional
Kim et al. 2015
++
++
+
++
++
++
First
Steenland et al. 2014
–
+
+
++
++
++
First

HEXACHLOROCYCLOHEXANE (HCH) C-24
APPENDIX C
Table C-20. Summary of Risk of Bias Assessment for β-Hexachlorocyclohexane—Observational
Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding
bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the comparison groups
appropriate?
Did the study design or
analysis account for important
confounding
and modifying variables?*
Were outcome data complete
without attrition or exclusion
from analysis?
Is there confidence in the
exposure characterization?*
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Outcome: Hepatic effects
Cross-sectional
Arrebola et al. 2014
++
++
–
++
++
++
First
Freire et al. 2015
++
++
–
++
++
++
First
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; NA = not applicable
*Key question used to assign risk of bias tier

HEXACHLOROCYCLOHEXANE (HCH) C-25
APPENDIX C
Table C-21. Summary of Risk of Bias Assessment for β-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data
complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Outcome: Hepatic effects
Oral intermediate exposure
Van Velsen et al. 1986
(rat; 13 weeks)
– – + + ++ ++ + ++
NA
First
Hanada et al. 1973
(mouse; 32 weeks)
– – + + + – + ++
NA
First
Ito et al. 1973 (mouse;
24 weeks)
– – + + + ++ + ++
NA
First
Ito et al. 1975 (rat; 24–
48 weeks)
– – + + – ++ + ++
NA
First
Oral chronic exposure
Fitzhugh et al. 1950 (rat;
107 weeks)
– – + + ++ + + ++
NA
First

HEXACHLOROCYCLOHEXANE (HCH) C-26
APPENDIX C
Table C-21. Summary of Risk of Bias Assessment for β-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data
complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Outcome: Neurological effects
Oral acute exposure
Van Velsen et al. 1986
(rat; 2 weeks)
– – + + ++ ++ + ++
NA
First
Cornacoff et al. 1988
(mouse; 1 week)
– – + + – ++ + ++
NA
First
Oral intermediate exposure
Muller et al. 1981 (rat;
30 days)
– – + + – – + ++
NA
Second
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; NA = not applicable
*Key question used to assign risk of bias tier

HEXACHLOROCYCLOHEXANE (HCH) C-27
APPENDIX C
C.11 RATE THE CONFIDENCE IN THE BODY OF EVIDENCE FOR EACH RELEVANT
OUTCOME—β-HCH
As discussed in greater detail in Section C.6, confidences in the bodies of human and animal evidence
were evaluated independently for each potential outcome for each type of study: case-control, case series,
cohort, population, human-controlled exposure, and experimental animal. In the absence of data to the
contrary, data for a particular outcome were collapsed across animal species, routes of exposure, and
exposure durations. If species (or strain), route, or exposure duration differences were noted, then the
data were treated as separate outcomes.
C.11.1 Initial Confidence Rating—β-HCH
As discussed in greater detail in Section C.6.1, the body of evidence for an association (or no association)
between exposure to β-HCH and a particular outcome was given an initial confidence rating based on the
key features of the individual studies examining that outcome. Refer to Tables C-9, C-10, and C-11,
respectively, for the key features for observational epidemiology (cohort, population, and case-control)
studies, human controlled exposure studies, and experimental animal studies.
The presence or absence of the key features and the initial confidence levels for studies examining
neurological and hepatic effects observed in the observational epidemiology and animal experimental
studies are presented in Tables C-22 and C-23, respectively.
Table C-22. Presence of Key Features of Study Design for
β-Hexachlorocyclohexane—Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure
prior to
outcome
Outcomes
assessed
on an
individual
level
Comparis
on group
Initial
study
confidence
Outcome: Neurological effects
Cohort
Medehouenou et al. 2019
No
Yes
Yes
Yes
Moderate
Case-control
Petersen et al. 2008
No
No
Yes
Yes
Low
Richardson et al. 2009, 2011
No
No
Yes
Yes
Low
Singh et al. 2012, 2013, 2014
No
No
Yes
Yes
Low
Xu et al. 2022
No
No
Yes
Yes
Low
Zhang et al. 2021
No
No
Yes
Yes
Low
Cross-sectional
Kim et al. 2015
No
No
Yes
Yes
Low
Steenland et al. 2014
No
No
Yes
Yes
Low
Outcome: Hepatic effects
Cross-sectional
Arrebola et al. 2014
No
No
Yes
Yes
Low
Freire et al. 2015
No
No
Yes
Yes
Low

HEXACHLOROCYCLOHEXANE (HCH) C-28
APPENDIX C
Table C-23. Presence of Key Features of Study Design for
β-Hexachlorocyclohexane—Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number of
animals per group
Appropriate parameters
to assess potential
effect
Adequate data for
statistical analysis
Initial
study
confidenc
e
Outcome: Hepatic effects
Oral intermediate exposure
Van Velsen et al. 1986 (rat; 13 weeks)
Yes
Yes
Yes
Yes
High
Hanada et al. 1973 (mouse; 32 weeks)
Yes
Yes
Yes
No
Moderate
Ito et al. 1973 (mouse; 24 weeks)
Yes
Yes
Yes
Yes
High
Ito et al. 1975 (rat; 48 weeks)
Yes
Yes
Yes
No
Moderate
Oral chronic exposure
Fitzhugh et al. 1950 (rat; 107 weeks)
Yes
No
Yes
Yes
Moderate
Outcome: Neurological effects
Oral acute exposure
Van Velsen et al. 1986 (rat; 2 weeks)
Yes
Yes
Yes
No
Moderate
Cornacoff et al. 1988 (mouse; 1 week)
Yes
Yes
Yes
No
Moderate
Oral intermediate exposure
Muller et al. 1981 (rat; 30 days)
Yes
Yes
Yes
Yes
High
A summary of the initial confidence ratings for each outcome is presented in Table C-24. If individual
studies for a particular outcome and study type had different study quality ratings, then the highest
confidence rating for the group of studies was used to determine the initial confidence rating for the body
of evidence; any exceptions were noted in Table C-24.
Table C-24. Initial Confidence Rating for
β-Hexachlorocyclohexane Health
Effects Studies
Initial study
confidence
Initial confidence
rating
Outcome: Neurological effects
Oral acute exposure
Animal studies
Van Velsen et al. 1986
Moderate
Moderate
Cornacoff et al. 1988
Moderate
Oral intermediate exposure
Animal studies
Muller et al. 1981
High
High
HEXACHLOROCYCLOHEXANE (HCH) C-29
APPENDIX C
Table C-24. Initial Confidence Rating for
β-Hexachlorocyclohexane Health
Effects Studies
Initial study
confidence
Initial confidence
rating
Oral chronic exposure
Human studies
Medehouenou et al. 2019
Moderate
Moderate
Singh et al. 2012, 2013, 2014
Low
Petersen et al. 2008
Low
Richardson et al. 2009, 2011
Low
Kim et al. 2015
Low
Steenland et al. 2014
Low
Xu et al. 2022
Low
Zhang et al. 2021
Low
Outcome: Hepatic effects
Oral intermediate exposure
Animal studies
Van Velsen et al. 1986 (rat; 13 weeks)
High
Hanada et al. 1973 (mouse; 32 weeks)
Moderate
Ito et al. 1973 (mouse; 24 weeks)
High
High
Ito et al. 1975 (rat; 48 weeks)
Moderate
Oral chronic exposure
Human studies
Arrebola et al. 2014
Low
Low
Freire et al. 2015
Low
Animal studies
Fitzhugh et al. 1950
Moderate
Moderate
C
.11.2 Adjustment of the Confidence Rating—β-HCH
The initial confidence rating was then downgraded or upgraded depending on whether there were
substantial issues that would decrease or increase confidence in the body of evidence. The five properties
of the body of evidence that were considered to determine whether the confidence rating should be
downgraded and the four properties of the body of evidence that were considered to determine whether
the confidence rating should be upgraded are described above in Section C.6.2. The summaries of the
assessment of the confidence in the body of evidence for neurological and hepatic effects are presented in
Table C-25. If the confidence ratings for a particular outcome were based on more than one type of
human study, then the highest confidence rating was used for subsequent analyses. An overview of the
confidence in the body of evidence for all health effects associated with β-HCH exposure is presented in
Table C-26.

HEXACHLOROCYCLOHEXANE (HCH) C-30
APPENDIX C
Table C-25. Adjustments to the Initial Confidence in the Body of Evidence
Initial confidence
Adjustments to the initial confidence
rating
Final
confidence
Neurological effects
Human studies
Moderate
+1 consistency, -1 indirectness
Moderate
Animal studies
High
-1 indirectness, +1 dose-response,
+1 consistency
High
Hepatic effects
Human studies
Low
-1 indirectness
Very low
Animal studies
High
+1 dose-response, +1 consistency
High
Table C-26. Confidence in the Body of Evidence for β-Hexachlorocyclohexane
Outcome
Confidence in body of evidence
Human studies
Animal studies
Neurological
Moderate
High
Hepatic
Very Low
High
C
.12 TRANSLATE CONFIDENCE RATING INTO LEVEL OF EVIDENCE OF HEALTH
EFFECTS—β-HCH
As described in Section C.7, the confidence in the body of evidence for specific outcomes was translated
to a level of evidence rating. The level of evidence rating reflected the confidence in the body of
evidence and the direction of the effect (i.e., toxicity or no toxicity); route-specific differences were
noted.
A summary of the level of evidence of health effects for β-HCH is presented in Table C-27.
Table C-27. Level of Evidence of Health Effects for β-Hexachlorocyclohexane
Outcome
Confidence in body
of evidence
Direction of health
effect
Level of evidence for
health effect
Human studies
Neurological
Moderate
Health effect
Moderate
Hepatic
Very Low
No health effect
Inadequate
Animal studies
Neurological
High
Health effect
High
Hepatic
High
Health effect
High

HEXACHLOROCYCLOHEXANE (HCH) C-31
APPENDIX C
C.13 INTEGRATE EVIDENCE TO DEVELOP HAZARD IDENTIFICATION CONCLUSIONS–
β-HCH
The final step involved the integration of the evidence streams for the human studies and animal studies
to allow for a determination of hazard identification conclusions. Refer to Section C.8 for the four hazard
identification conclusion categories for health effects, the hazard characterization scheme (see
Figure C-1), and the hazard identification conclusion categories.
The hazard identification conclusions for β-HCH are listed below and summarized in Table C-28.
Presumed Health Effects
• Neurological
o Moderate level of evidence in humans based on case-control studies reporting associations
between serum β-HCH and risk of Parkinson and Alzheimer diseases (Petersen et al. 2008;
Richardson et al. 2009, 2011; Singh et al. 2012, 2013, 2014; Xu et al. 2022) and a cross-
sectional study showing an association between risk of cognitive deficits and β-HCH in blood
(Kim et al. 2015).
o High level of evidence in animals exposed orally based on clinical signs of neurotoxicity in
rats and mice after acute durations (Cornacoff et al. 1988; Van Velsen et al. 1986) and
reduced nerve conduction velocity in rats exposed for an intermediate duration (Muller et al.
1981). Clinical signs showed dose-related increase in severity.
o Supported by evidence for neurological effects of γ-HCH in humans and animals (see Section
2.15).
• Hepatic
o Very low level of evidence in humans based on two cross-sectional studies reporting no
association between serum or adipose levels of β-HCH and hepatic clinical chemistry
endpoints except for increased serum bilirubin in females (Arrebola et al. 2014; Freire et al.
2015).
o High level of evidence in animals based on liver weight and histopathology changes in rats
and mice exposed by dietary administration for intermediate and chronic durations (Fitzhugh
et al. 1950; Hanada et al. 1973; Ito et al. 1973, 1975; Van Velsen et al. 1986).
Table C-28. Hazard Identification Conclusions for – β-HCHs
Outcome
Hazard identification
Neurological
Presumed health effect
Hepatic
Presumed health effect
C.14
IDENTIFY POTENTIAL HEALTH EFFECT OUTCOMES OF CONCERN—γ-HCH
Overviews of the potential health effect outcomes for γ-HCH identified in human and animal studies are
presented in Tables C-29 and C-30, respectively. Most of the human studies evaluated developmental,
reproductive, renal, endocrine, or cancer endpoints. Most of human studies of noncancer endpoints used
measures of γ-HCH in blood or tissues to assess exposure, so the route is unknown; for the purpose of
enumerations, these studies are considered to reflect oral exposure (e.g., through contaminated food).
Studies of occupational exposure via pesticide application are considered to reflect primarily inhalation
exposure. Most of the animal studies used oral administration, and the available studies examined
comprehensive noncancer and cancer endpoints. The effects seen at the lowest doses in the animal
studies were developmental and immune system effects. Studies examining these potential outcomes
HEXACHLOROCYCLOHEXANE (HCH) C-32
APPENDIX C
were carried through to Steps 4–8 of the systematic review. There were 41 studies (published in
35 documents) examining these potential outcomes carried through to Steps 4–8 of the systematic review.

HEXACHLOROCYCLOHEXANE (HCH) C-33
APPENDIX C
Table C-29. Overview of the Health Outcomes for γ-Hexachlorocyclohexane Evaluated In Human Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
Neurological
Reproductive
Developmental
Other Noncancer
Caner
Inhalation studies
Cohort
1
1
1
1
Case control
Population
Case series
Oral studies
Cohort
2
1
2
1
3
3
2
1
1
1
Case cohort
Case control
2
1
2
2
6
3
20
1
1
1
1
4
1
8
Population
1
2
4
2
4
2
2
1
1
1
2
1
1
Case series
Meta analysis
1
Dermal studies
Cohort
Case control
Population
Case series
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10

HEXACHLOROCYCLOHEXANE (HCH) C-34
APPENDIX C
Table C-30. Overview of the Health Outcomes for γ-Hexachlorocyclohexane Evaluated in Experimental Animal
Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
a
Neurological
a
Reproductive
a
Developmental
Other Noncancer
Caner
Inhalation studies
Acute-duration
1
1
2
2
Intermediate-duration
2
2
1
1
1
1
Chronic-duration
Oral studies
Acute-duration
12
2
2
2
2
18
4
2
3
19
11
22
2
2
11
2
3
17
5
16
Intermediate-duration
7
2
4
3
19
10
2
8
13
16
12
2
2
1
3
15
7
2
7
12
13
9
2
1
Chronic-duration
3
1
3
2
1
1
4
1
3
1
1
1
2
Dermal studies
Acute-duration
2
1
1
1
2
0
1
1
2
Intermediate-duration
1
1
1
1
2
1
1
0
1
1
1
1
1
Chronic-duration
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10
a
Number of studies examining endpoint includes study evaluating histopathology, but not evaluating function.
HEXACHLOROCYCLOHEXANE (HCH) C-35
APPENDIX C
C.15 ASSESS THE RISK OF BIAS FOR INDIVIDUAL STUDIES—γ-HCH
C.15.1 Risk of Bias Assessment—γ-HCH
The risk of bias of individual studies was assessed using OHAT’s Risk of Bias Tool (NTP 2015). The
risk of bias questions for observational epidemiology studies, human-controlled exposure studies, and
animal experimental studies were presented above in Tables C-5, C-6, and C-7, respectively. As
described in Section C.5.1, each risk of bias question was answered on a four-point scale and studies were
assigned to one of three risk of bias tiers.
The results of the risk of bias assessment for the different types of γ-HCH health effects studies
(observational epidemiology and animal experimental studies) are presented in Tables C-31 and C-32,
respectively.

HEXACHLOROCYCLOHEXANE (HCH) C-36
APPENDIX C
Table C-31. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Observational Epidemiology
Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding
bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting bias
Risk of bias tier
Were the comparison groups
appropriate?
Did the study design or
analysis account for important
confounding
and modifying variables?*
Were outcome data complete
without attrition or exclusion
from analysis?
Is there confidence in the
exposure characterization?*
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Outcome: Developmental effects
Cohort
Fenster et al. 2006
++
+
–
+
++
++
First
Yang et al. 2021a
++
+
+
++
++
++
First
Garcia- Villarino et al. 2022
++
++
+
++
++
++
First
Case-control
Fernandez et al. 2007
++
++
+
++
++
++
First
Mustafa et al. 2013
+
+
+
++
+
++
First
Sharma et al. 2012
+
+
+
++
++
++
First
Siddiqui et al. 2003
+
+
+
++
+
++
First
Yang et al. 2021b
++
+
+
++
++
++
First
Yin et al. 2021
++
+
+
++
++
++
First
Cross-sectional
Fang et al. 2019a, 2019b
++
+
++
++
+
++
First
Freire et al. 2011
++
+
–
++
–
++
Second

HEXACHLOROCYCLOHEXANE (HCH) C-37
APPENDIX C
Table C-31. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Observational Epidemiology
Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding
bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting bias
Risk of bias tier
Were the comparison groups
appropriate?
Did the study design or
analysis account for important
confounding
and modifying variables?*
Were outcome data complete
without attrition or exclusion
from analysis?
Is there confidence in the
exposure characterization?*
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Outcome: Immunological effects
Cohort
Landgren et al. 2009
++
++
++
+
++
++
First
Case-control
Meng et al. 2016
+
–
++
+
++
++
Second
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; NA = not applicable
*Key question used to assign risk of bias tier

HEXACHLOROCYCLOHEXANE (HCH) C-38
APPENDIX C
Table C-32. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study
groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Outcome: Developmental effects
Oral acute exposure
Dalsenter et al. 1997a
(rat; once)
– – + + ++ – + ++ NA First
Dalsenter et al. 1997b
(rat; once)
– – + + – + + ++ NA First
Dalsenter et al. 1997b
(rat; LDs 8–14)
– – + + – + + ++ NA First
Johri et al. 2008 (rat;
once)
– – + + – – + ++ NA Second
Khera et al. 1979 (rat;
GDs 6–15)
– – + + + + + ++ NA First
Palmer et al. 1978 (rat;
GDs 6–15)
+ + + + + – + ++ NA First
Rivera et al. 1991 (rat;
once)
+ + + + – + + ++ NA First
Rivera et al. 1998 (rat;
once)
+ + + + – – + ++ NA First

HEXACHLOROCYCLOHEXANE (HCH) C-39
APPENDIX C
Table C-32. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study
groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Rivera et al. 1998 (rat;
PNDs 8–14)
– – + + – – – ++ NA Third
Serrano et al. 1990 (rat;
PNDs 8–10)
+ + + + – – + ++ NA First
Di Consiglio et al. 2009
(mouse; GDs 9–16)
+ + + + + – + ++ NA First
Hassoun and Stohs
1996a (mouse, once)
– – + + ++ – + ++ NA First
La Sala et al. 2009
(mouse; 3 days)
+ + + + – – + ++ NA First
Maranghi et al. 2007
(mouse, GDs 91–6)
+ + + + ++ – + ++ NA First
Traina et al. 2003 (mouse;
GDs 9–16)
+ + + + ++ – + ++ NA First
Palmer et al. 1978 (rabbit;
GDs 6–18)
+ + + + + – + ++ NA First
Oral intermediate exposure
Breton et al. 2005 (rat;
~21 weeks (2-generation,
premating–PND 98)
– – + + – – + ++ NA First

HEXACHLOROCYCLOHEXANE (HCH) C-40
APPENDIX C
Table C-32. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study
groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
EPA 1991a (rat; 2-
generation, 70 days prior
to mating until sacrifice)
+ + + + + + ++ ++
NA
First
EPA 1999c (rat; GD 6–
LD 10)
– – + + ++ ++ + ++ NA First
Johri et al. 2007 (rat; GDs
521)
+ + + + – – + ++ NA First
Johri et al. 2008 (rat; GDs
5–21 and PND 45)
+ + + + – – + ++ NA First
Matsuura et al. 2005 (rat;
~10 weeks (2-generation;
premating–PND 21))
++ + + + + ++ + ++ NA First
Sauviat et al. 2005 (rat;
~13 weeks)
– – + + – – + ++ NA Second
Srinivasan et al. 1991 (rat;
GDs 0–21, LDs 1–28)
– – + + + – + ++ NA First

HEXACHLOROCYCLOHEXANE (HCH) C-41
APPENDIX C
Table C-32. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study
groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Srivastava et al. 2019 (rat,
GDs 5–21)
– – + + – – – ++ NA Third
Seiler et al. 1994 (rabbit;
12–15 weeks, 3
days/week)
– – + + – + + ++ NA First
Outcome: Immunological effects
Oral acute exposure
Mediratta et al. 2008 (rat;
14 days)
+ + + + ++ – + ++
NA
First
Hong and Boorman 1993
(mouse; 10 days)
+ + + + – – + ++
NA
First
Hong and Boorman 1993
(mouse; 3 days)
+ + + + – – + ++
NA
First
Oral intermediate exposure
Koner et al. 1998 (rat;
8 weeks)
+ + + + – ++ + ++ NA First

HEXACHLOROCYCLOHEXANE (HCH) C-42
APPENDIX C
Table C-32. Summary of Risk of Bias Assessment for γ-Hexachlorocyclohexane—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusion
bias
Detection bias
Selective
reporting
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately concealed?
Were experimental conditions
identical across study
groups?
Were the research personnel
blinded to the study group during
the study?
Were outcome data complete
without attrition or exclusion from
analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured outcomes
reported?
Did the study design or analysis
account for important
confounding and modifying
variables?
Risk of bias tier
Mediratta et al. 2008 (rat;
21 days)
+ + + + ++ – + ++ NA First
Meera et al. 1992 (mouse;
24 weeks)
– – + + – + + ++ NA First
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; NA = not applicable
*Key question used to assign risk of bias tier

HEXACHLOROCYCLOHEXANE (HCH) C-43
APPENDIX C
C.16 RATE THE CONFIDENCE IN THE BODY OF EVIDENCE FOR EACH RELEVANT
OUTCOME—γ-HCH
As discussed in greater detail in Section C.6, confidences in the bodies of human and animal evidence
were evaluated independently for each potential outcome for each type of study: case-control, case series,
cohort, population, human-controlled exposure, and experimental animal. In the absence of data to the
contrary, data for a particular outcome were collapsed across animal species, routes of exposure, and
exposure durations. If species (or strain), route, or exposure duration differences were noted, then the
data were treated as separate outcomes.
C.16.1 Initial Confidence Rating—γ-HCH
As discussed in greater detail in Section C.6.1, the body of evidence for an association (or no association)
between exposure to γ-HCH and a particular outcome was given an initial confidence rating based on the
key features of the individual studies examining that outcome. Refer to Tables C-9, C-10, and C-11,
respectively, for the key features for observational epidemiology (cohort, population, and case-control)
studies, human controlled exposure studies, and experimental animal studies.
The presence or absence of the key features and the initial confidence levels for studies examining
developmental and immune system effects in the observational epidemiology and animal experimental
studies are presented in Tables C-33 and C-34, respectively.
Table C-33. Presence of Key Features of Study Design for
γ-Hexachlorocyclohexane—Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure
prior to
outcome
Outcomes
assessed
on an
individual
l l
Comparis
on group
Initial
study
confidence
Outcome: Developmental effects
Cohort
Fenster et al. 2006
No
Yes
Yes
Yes
Moderate
Yang et al. 2021a
No
Yes
Yes
Yes
Moderate
Garcia- Villarino et al. 2022
No
Yes
Yes
Yes
Moderate
Case-control
Fernandez et al. 2007
No
No
Yes
Yes
Low
Mustafa et al. 2013
No
No
Yes
Yes
Low
Sharma et al. 2012
No
No
Yes
Yes
Low
Siddiqui et al. 2003
No
No
Yes
Yes
Low
Yang et al. 2021b
No
No
Yes
Yes
Low
Yin et al. 2021
No
No
Yes
Yes
Low
Cross-sectional
Fang et al. 2019a, 2019b
No
No
Yes
Yes
Low

HEXACHLOROCYCLOHEXANE (HCH) C-44
APPENDIX C
Table C-33. Presence of Key Features of Study Design for
γ-Hexachlorocyclohexane—Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure
prior to
outcome
Outcomes
assessed
on an
individual
l l
Comparis
on group
Initial
study
confidence
Outcome: Immunological effects
Cohort
Landgren et al. 2009
No
Yes
Yes
Yes
Moderate
Case-control
Meng et al. 2016
No
No
Yes
Yes
Low
Cross-sectional
Wang et al. 2021a
No
No
Yes
Yes
Low
Table C-34. Presence of Key Features of Study Design for
γ-Hexachlorocyclohexane—Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number of
animals per group
Appropriate parameters
to assess potential
effect
Adequate data for
statistical analysis
Initial
study
confidenc
e
Outcome: Developmental effects
Oral acute exposure
Dalsenter et al. 1997a (rat; once)
Yes
Yes
Yes
Yes
High
Dalsenter et al. 1997b (rat; once)
Yes
Yes
Yes
Yes
High
Dalsenter et al. 1997b (rat; LDs 8–14)
Yes
Yes
Yes
Yes
High
Johri et al. 2008 (rat; once)
Yes
Yes
Yes
Yes
High
Khera et al. 1979 (rat; GDs 6–15)
Yes
Yes
Yes
Yes
High
Palmer et al. 1978 (rat; GDs 6–15)
Yes
Yes
Yes
Yes
High
Rivera et al. 1991 (rat; once)
Yes
No
Yes
Yes
Moderate
Rivera et al. 1998 (rat; once)
Yes
No
Yes
Yes
Moderate
Rivera et al. 1998 (rat; PNDs 8–14)
Yes
No
Yes
Yes
Moderate
Serrano et al. 1990 (rat; PNDs 8–10)
Yes
Yes
Yes
No
Moderate
Di Consiglio et al. 2009 (mouse; GDs
9–16)
Yes No Yes Yes Moderate
Hassoun and Stohs 1996a (mouse,
once)
Yes Yes Yes Yes High
La Sala et al. 2009 (mouse; 3 days)
Yes
No
Yes
Yes
Moderate

HEXACHLOROCYCLOHEXANE (HCH) C-45
APPENDIX C
Table C-34. Presence of Key Features of Study Design for
γ-Hexachlorocyclohexane—Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number of
animals per group
Appropriate parameters
to assess potential
effect
Adequate data for
statistical analysis
Initial
study
confidenc
e
Maranghi et al. 2007 (mouse, GDs 91–
6)
Yes Yes Yes Yes High
Traina et al. 2003 (mouse; GDs 9–16)
Yes
Yes
Yes
Yes
High
Palmer et al. 1978 (rabbit; GDs 6–18)
Yes
Yes
Yes
Yes
High
Oral intermediate exposure
Breton et al. 2005 (rat; ~21 weeks,
2-generation, premating–PND 98)
Yes No Yes Yes Moderate
EPA 1991a (rat; 2-generation, 70 days
prior to mating until sacrifice)
Yes Yes Yes Yes High
EPA 1999c (rat; GD 6–LD 10)
Yes
Yes
Yes
Yes
High
Johri et al. 2007 (rat; GDs 5–21)
Yes
Yes
Yes
Yes
High
Johri et al. 2008 (rat; GDs 5–21 and
PND 45)
Yes Yes Yes Yes High
Matsuura et al. 2005 (rat; ~10 weeks,
2-generation; premating–PND 21)
Yes Yes Yes Yes High
Sauviat et al. 2005 (rat; ~13 weeks)
Yes
No
Yes
Yes
Moderate
Srinivasan et al. 1991 (rat; GDs 0–21,
LDs 1–28)
Yes No Yes Yes Moderate
Srivastava et al. 2019 (rat, GDs 5–21)
Yes
Yes
Yes
Yes
High
Seiler et al. 1994 (rabbit; 12–15 weeks,
3 days/week)
Yes No Yes Yes Moderate
Outcome: Immunological effects
Oral acute exposure
Mediratta et al. 2008 (rat; 14 days)
Yes
Yes
Yes
Yes
High
Hong and Boorman 1993 (mouse;
10 days)
Yes Yes Yes Yes High
Hong and Boorman 1993 (mouse;
3 days)
Yes Yes Yes Yes High
Oral intermediate exposure
Koner et al. 1998 (rat; 8 weeks)
Yes
No
Yes
Yes
Moderate
Mediratta et al. 2008 (rat; 21 days)
Yes
No
Yes
Yes
Moderate
Meera et al. 1992 (mouse; 24 weeks)
Yes
Yes
Yes
Yes
High

HEXACHLOROCYCLOHEXANE (HCH) C-46
APPENDIX C
A summary of the initial confidence ratings for each outcome is presented in Table C-35. If individual
studies for a particular outcome and study type had different study quality ratings, then the highest
confidence rating for the group of studies was used to determine the initial confidence rating for the body
of evidence; any exceptions were noted in Table C-35.
Table C-35. Initial Confidence Rating for γ-Hexachlorocyclohexane Health
Effects Studies
Initial study
confidence
Initial confidence
rating
Outcome: Developmental effects
Oral acute exposure
Animal studies
Dalsenter et al. 1997a
High
High
Dalsenter et al. 1997b
High
Dalsenter et al. 1997b
High
Johri et al. 2008
High
Khera et al. 1979
High
Palmer et al. 1978
High
Rivera et al. 1991
Moderate
Rivera et al. 1998
Moderate
Rivera et al. 1998
Moderate
Serrano et al. 1990
Moderate
Di Consiglio et al. 2009
Moderate
Hassoun and Stohs 1996a
High
Hassoun and Stohs 1996a
Moderate
La Sala et al. 2009
High
Maranghi et al. 2007
High
Traina et al. 2003
High
Palmer et al. 1978
High
Oral intermediate exposure
Animal studies
Breton et al. 2005
Moderate
High
EPA 1991a
High
EPA 1999c
High
Johri et al. 2007
High
Johri et al. 2008
High
Matsuura et al. 2005
High
Sauviat et al. 2005
Moderate
Srinivasan et al. 1991
Moderate
Srivastava et al. 2019
High
Seiler et al. 1994
Moderate
Oral chronic exposure
Human studies
Fenster et al. 2006
Moderate
Moderate

HEXACHLOROCYCLOHEXANE (HCH) C-47
APPENDIX C
Table C-35. Initial Confidence Rating for γ-Hexachlorocyclohexane Health
Effects Studies
Initial study
confidence
Initial confidence
rating
Yang et al. 2021a
Moderate
Garcia-Villarino et al. 2022
Moderate
Fernandez et al. 2007
Low
Mustafa et al. 2013
Low
Sharma et al. 2012
Low
Siddiqui et al. 2003
Low
Fang et al. 2019a, 2019b
Low
Yang et al. 2021b
Low
Yin et al. 2021
Low
Outcome: Immunological effects
Oral acute exposure
Animal studies
Mediratta et al. 2008
High
Hong and Boorman 1993
High
High
Hong and Boorman 1993
High
Oral intermediate exposure
Animal studies
Koner et al. 1998
Moderate
High
Mediratta et al. 2008
Moderate
Meera et al. 1992
High
Oral chronic exposure
Human studies
Landgren et al. 2009
Moderate
Moderate
Meng et al. 2016
Low
C
.16.2 Adjustment of the Confidence Rating—γ-HCH
The initial confidence rating was then downgraded or upgraded depending on whether there were
substantial issues that would decrease or increase confidence in the body of evidence. The five properties
of the body of evidence that were considered to determine whether the confidence rating should be
downgraded and the four properties of the body of evidence that were considered to determine whether
the confidence rating should be upgraded are described above in Section C.6.2. The summaries of the
assessment of the confidence in the body of evidence for developmental and immune system effects are
presented in Table C-36. If the confidence ratings for a particular outcome were based on more than one
type of human study, then the highest confidence rating was used for subsequent analyses. An overview
of the confidence in the body of evidence for all health effects associated with γ-HCH exposure is
presented in Table C-37.

HEXACHLOROCYCLOHEXANE (HCH) C-48
APPENDIX C
Table C-36. Adjustments to the Initial Confidence in the Body of Evidence
Initial confidence
Adjustments to the initial
confidence rating
Final
confidence
Developmental effects
Human studies
Moderate
-1 imprecision
Low
Animal studies
High
+1 consistency
High
Immunological effects
Human Studies
Moderate
-1 risk of bias
Low
Animal Studies
High
+1 consistency
High
Table C-37. Confidence in the Body of Evidence for γ-Hexachlorocyclohexane
Outcome
Confidence in body of evidence
Human studies
Animal studies
Developmental
Low
High
Immune
Low
High
C
.17 TRANSLATE CONFIDENCE RATING INTO LEVEL OF EVIDENCE OF HEALTH
EFFECTS—γ-HCH
As described in Section C.7, the confidence in the body of evidence for specific outcomes was translated
to a level of evidence rating. The level of evidence rating reflected the confidence in the body of
evidence and the direction of the effect (i.e., toxicity or no toxicity); route-specific differences were
noted.
A summary of the level of evidence of health effects for γ-HCH is presented in Table C-38.
Table C-38. Level of Evidence of Health Effects for γ-Hexachlorocyclohexane
Outcome
Confidence in body
of evidence
Direction of health
effect
Level of evidence for
health effect
Human studies
Developmental
Low
Health effect
Low
Immunological
Low
Health effect
Low
Animal studies
Developmental
High
Health effect
High
Immunological
High
Health effect
High

HEXACHLOROCYCLOHEXANE (HCH) C-49
APPENDIX C
C.18 INTEGRATE EVIDENCE TO DEVELOP HAZARD IDENTIFICATION CONCLUSIONS—
γ-HCH
The final step involved the integration of the evidence streams for the human studies and animal studies
to allow for a determination of hazard identification conclusions. Refer to Section C.8 for the four hazard
identification conclusion categories for health effects, the hazard characterization scheme (see
Figure C-1), and the hazard identification conclusion categories.
The hazard identification conclusions for γ-HCH are listed below and summarized in Table C-39.
Presumed Health Effects
• Developmental
o Low level of evidence in humans based on associations between γ-HCH in maternal or fetal
blood (or tissue) and intrauterine growth retardation/fetal growth retardation in small case-
control studies in India (Sharma et al. 2012; Siddiqui et al. 2003), decreased gestational age
and increased preterm birth in a cross-sectional study in China (Fang et al. 2019a, 2019b) and
a case-control study in India (Mustafa et al. 2013), and cryptorchidism or hypospadias in a
nested case-control study in Spain (Fernandez et al. 2007).
o High level of evidence in animals based on studies in a variety of species exposed to γ-HCH
for acute or intermediate durations during gestation or postnatal development demonstrating
effects on a wide range of developmental endpoints, including birth outcomes (Beard et al.
1997; EPA 1991a, 1999c; Hassoun and Stohs 1996a; Matsuura et al. 2005; Sauviat et al.
2005) and development of the male and female reproductive tracts (Agrahari et al. 2019;
Dalsenter et al. 1997a, 1997b; Di Consiglio et al. 2009; La Sala et al. 2009; Maranghi et al.
2007; Matsuura et al. 2005; Traina et al. 2003), central nervous system (Albertson et al. 1985;
Breton et al. 2005; EPA 1999c; Johri et al. 2007, 2008; Rivera et al. 1991, 1998; Srivastava et
al. 2019), heart (Sauviat et al. 2005), liver (Srinivasan et al. 1991), and thymus and spleen
(Hassoun et al. 1996; Matsuura et al. 2005).
• Immunological
o Low level of evidence in humans based on association between asthma and plasma levels of
γ-HCH in children (Meng et al. 2016) and no evidence for increased prevalence of
monoclonal gammopathy of undetermined significance in cohort of male pesticide
applicators followed for 9 years (Landgren et al. 2009).
o High level of evidence in animals based on
acute- and intermediate-duration studies of
γ-HCH administered orally to rats, mice, rabbits, and sheep showing suppression of the
immune system (Banerjee et al. 1996; Desi et al. 1978; Dewan et al. 1980; Khurana et al.
1999; Koner et al. 1998; Mediratta et al. 2008; Meera et al. 1992) and effects on thymus,
spleen, and lymph node weights or histology (Hong and Boorman 1993; Meera et al. 1992).
Table C-39. Hazard Identification Conclusions for γ-Hexachlorocyclohexane
Outcome
Hazard identification
Developmental
Presumed health effect
Immunological
Presumed health effect
HEXACHLOROCYCLOHEXANE (HCH) D-1
APPENDIX D. USER'S GUIDE
Chapter 1. Relevance to Public Health
This chapter provides an overview of U.S. exposures, a summary of health effects based on evaluations of
existing toxicologic, epidemiologic, and toxicokinetic information, and an overview of the minimal risk
levels. This is designed to present interpretive, weight-of-evidence discussions for human health
endpoints by addressing the following questions:
1. What effects are known to occur in humans?
2. What effects observed in animals are likely to be of concern to humans?
3. What exposure conditions are likely to be of concern to humans, especially around hazardous
waste sites?
Minimal Risk Levels (MRLs)
Where sufficient toxicologic information is available, ATSDR derives MRLs for inhalation and oral
routes of entry at each duration of exposure (acute, intermediate, and chronic). These MRLs are not
meant to support regulatory action, but to acquaint health professionals with exposure levels at which
adverse health effects are not expected to occur in humans.
MRLs should help physicians and public health officials determine the safety of a community living near
a hazardous substance emission, given the concentration of a contaminant in air or the estimated daily
dose in water. MRLs are based largely on toxicological studies in animals and on reports of human
occupational exposure.
MRL users should be familiar with the toxicologic information on which the number is based.
Section 1.2, Summary of Health Effects, contains basic information known about the substance. Other
sections, such as Section 3.2 Children and Other Populations that are Unusually Susceptible and
Section 3.4 Interactions with Other Substances, provide important supplemental information.
MRL users should also understand the MRL derivation methodology. MRLs are derived using a
modified version of the risk assessment methodology that the Environmental Protection Agency (EPA)
provides (Barnes and Dourson 1988) to determine reference doses (RfDs) for lifetime exposure.
To derive an MRL, ATSDR generally selects the most sensitive endpoint which, in its best judgement,
represents the most sensitive human health effect for a given exposure route and duration. ATSDR
cannot make this judgement or derive an MRL unless information (quantitative or qualitative) is available
for all potential systemic, neurological, and developmental effects. If this information and reliable
quantitative data on the chosen endpoint are available, ATSDR derives an MRL using the most sensitive
species (when information from multiple species is available) with the highest no-observed-adverse-effect
level (NOAEL) that does not exceed any adverse effect levels. When a NOAEL is not available, a
lowest-observed-adverse-effect level (LOAEL) can be used to derive an MRL, and an uncertainty factor
of 10 must be employed. Additional uncertainty factors of 10 must be used both for human variability to
protect sensitive subpopulations (people who are most susceptible to the health effects caused by the
substance) and for interspecies variability (extrapolation from animals to humans). In deriving an MRL,
these individual uncertainty factors are multiplied together. The product is then divided into the
inhalation concentration or oral dosage selected from the study. Uncertainty factors used in developing a

HEXACHLOROCYCLOHEXANE (HCH) D-2
APPENDIX D
substance-specific MRL are provided in the footnotes of the levels of significant exposure (LSE) tables
that are provided in Chapter 2. Detailed discussions of the MRLs are presented in Appendix A.
Chapter 2. Health Effects
Tables and Figures for Levels of Significant Exposure (LSE)
Tables and figures are used to summarize health effects and illustrate graphically levels of exposure
associated with those effects. These levels cover health effects observed at increasing dose
concentrations and durations, differences in response by species and MRLs to humans for noncancer
endpoints. The LSE tables and figures can be used for a quick review of the health effects and to locate
data for a specific exposure scenario. The LSE tables and figures should always be used in conjunction
with the text. All entries in these tables and figures represent studies that provide reliable, quantitative
estimates of NOAELs, LOAELs, or Cancer Effect Levels (CELs).
The legends presented below demonstrate the application of these tables and figures. Representative
examples of LSE tables and figures follow. The numbers in the left column of the legends correspond to
the numbers in the example table and figure.
TABLE LEGEND
See Sample LSE Table (page D-5)
(1) Route of exposure. One of the first considerations when reviewing the toxicity of a substance
using these tables and figures should be the relevant and appropriate route of exposure.
Typically, when sufficient data exist, three LSE tables and two LSE figures are presented in the
document. The three LSE tables present data on the three principal routes of exposure
(i.e., inhalation, oral, and dermal). LSE figures are limited to the inhalation and oral routes. Not
all substances will have data on each route of exposure and will not, therefore, have all five of the
tables and figures. Profiles with more than one chemical may have more LSE tables and figures.
(2) Exposure period. Three exposure periods—acute (<15 days), intermediate (15–364 days), and
chronic (≥365 days)—are presented within each relevant route of exposure. In this example, two
oral studies of chronic-duration exposure are reported. For quick reference to health effects
occurring from a known length of exposure, locate the applicable exposure period within the LSE
table and figure.
(3) Figure key. Each key number in the LSE table links study information to one or more data points
using the same key number in the corresponding LSE figure. In this example, the study
represented by key number 51 identified NOAELs and less serious LOAELs (also see the three
"51R" data points in sample LSE Figure 2-X).
(4) Species (strain) No./group. The test species (and strain), whether animal or human, are identified
in this column. The column also contains information on the number of subjects and sex per
group. Chapter 1, Relevance to Public Health, covers the relevance of animal data to human
toxicity and Section 3.1, Toxicokinetics, contains any available information on comparative
toxicokinetics. Although NOAELs and LOAELs are species specific, the levels are extrapolated
to equivalent human doses to derive an MRL.
(5) Exposure parameters/doses. The duration of the study and exposure regimens are provided in
these columns. This permits comparison of NOAELs and LOAELs from different studies. In
this case (key number 51), rats were orally exposed to “Chemical X” via feed for 2 years. For a

HEXACHLOROCYCLOHEXANE (HCH) D-3
APPENDIX D
more complete review of the dosing regimen, refer to the appropriate sections of the text or the
original reference paper (i.e., Aida et al. 1992).
(6) Parameters monitored. This column lists the parameters used to assess health effects. Parameters
monitored could include serum (blood) chemistry (BC), biochemical changes (BI), body weight
(BW), clinical signs (CS), developmental toxicity (DX), food intake (FI), gross necropsy (GN),
hematology (HE), histopathology (HP), immune function (IX), lethality (LE), neurological
function (NX), organ function (OF), ophthalmology (OP), organ weight (OW), reproductive
function (RX), urinalysis (UR), and water intake (WI).
(7) Endpoint. This column lists the endpoint examined. The major categories of health endpoints
included in LSE tables and figures are death, body weight, respiratory, cardiovascular,
gastrointestinal, hematological, musculoskeletal, hepatic, renal, dermal, ocular, endocrine,
immunological, neurological, reproductive, developmental, other noncancer, and cancer. "Other
noncancer" refers to any effect (e.g., alterations in blood glucose levels) not covered in these
systems. In the example of key number 51, three endpoints (body weight, hematological, and
hepatic) were investigated.
(8) NOAEL. A NOAEL is the highest exposure level at which no adverse effects were seen in the
organ system studied. The body weight effect reported in key number 51 is a NOAEL at
25.5 mg/kg/day. NOAELs are not reported for cancer and death; with the exception of these two
endpoints, this field is left blank if no NOAEL was identified in the study.
(9) LOAEL. A LOAEL is the lowest dose used in the study that caused an adverse health effect.
LOAELs have been classified into "Less Serious" and "Serious" effects. These distinctions help
readers identify the levels of exposure at which adverse health effects first appear and the
gradation of effects with increasing dose. A brief description of the specific endpoint used to
quantify the adverse effect accompanies the LOAEL. Key number 51 reports a less serious
LOAEL of 6.1 mg/kg/day for the hepatic system, which was used to derive a chronic exposure,
oral MRL of 0.008 mg/kg/day (see footnote "c"). MRLs are not derived from serious LOAELs.
A cancer effect level (CEL) is the lowest exposure level associated with the onset of
carcinogenesis in experimental or epidemiologic studies. CELs are always considered serious
effects. The LSE tables and figures do not contain NOAELs for cancer, but the text may report
doses not causing measurable cancer increases. If no LOAEL/CEL values were identified in the
study, this field is left blank.
(10) Reference. The complete reference citation is provided in Chapter 8 of the profile.
(11) Footnotes. Explanations of abbreviations or reference notes for data in the LSE tables are found
in the footnotes. For example, footnote "c" indicates that the LOAEL of 6.1 mg/kg/day in key
number 51 was used to derive an oral MRL of 0.008 mg/kg/day.
FIGURE LEGEND
See Sample LSE Figure (page D-6)
LSE figures graphically illustrate the data presented in the corresponding LSE tables. Figures help the
reader quickly compare health effects according to exposure concentrations for particular exposure
periods.
(12) Exposure period. The same exposure periods appear as in the LSE table. In this example, health
effects observed within the chronic exposure period are illustrated.

HEXACHLOROCYCLOHEXANE (HCH) D-4
APPENDIX D
(13) Endpoint. These are the categories of health effects for which reliable quantitative data exist.
The same health effect endpoints appear in the LSE table.
(14) Levels of exposure. Concentrations or doses for each health effect in the LSE tables are
graphically displayed in the LSE figures. Exposure concentration or dose is measured on the log
scale "y" axis. Inhalation exposure is reported in mg/m
3
or ppm and oral exposure is reported in
mg/kg/day.
(15) LOAEL. In this example, the half-shaded circle that is designated 51R identifies a LOAEL
critical endpoint in the rat upon which a chronic-duration oral exposure MRL is based. The key
number 51 corresponds to the entry in the LSE table. The dashed descending arrow indicates the
extrapolation from the exposure level of 6.1 mg/kg/day (see entry 51 in the sample LSE table) to
the MRL of 0.008 mg/kg/day (see footnote "c" in the sample LSE table).
(16) CEL. Key number 59R is one of studies for which CELs were derived. The diamond symbol
refers to a CEL for the test species (rat). The number 59 corresponds to the entry in the LSE
table.
(17) Key to LSE figure. The key provides the abbreviations and symbols used in the figure.

HEXACHLOROCYCLOHEXANE (HCH) D-5
APPENDIX D

HEXACHLOROCYCLOHEXANE (HCH) D-6
APPENDIX D

HEXACHLOROCYCLOHEXANE (HCH) E-1
APPENDIX E. QUICK REFERENCE FOR HEALTH CARE PROVIDERS
Toxicological Profiles are a unique compilation of toxicological information on a given hazardous
substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation
of available toxicologic and epidemiologic information on a substance. Health care providers treating
patients potentially exposed to hazardous substances may find the following information helpful for fast
answers to often-asked questions.
Primary Chapters/Sections of Interest
Chapter 1: Relevance to Public Health: The Relevance to Public Health Section provides an overview
of exposure and health effects and evaluates, interprets, and assesses the significance of toxicity
data to human health. A table listing minimal risk levels (MRLs) is also included in this chapter.
Chapter 2: Health Effects: Specific health effects identified in both human and animal studies are
reported by type of health effect (e.g., death, hepatic, renal, immune, reproductive), route of
exposure (e.g., inhalation, oral, dermal), and length of exposure (e.g., acute, intermediate, and
chronic).
NOTE: Not all health effects reported in this section are necessarily observed in the clinical
setting.
Pediatrics:
Section 3.2 Children and Other Populations that are Unusually Susceptible
Section 3.3 Biomarkers of Exposure and Effect
ATSDR Information Center
Phone: 1-800-CDC-INFO (800-232-4636) or 1-888-232-6348 (TTY)
Internet: http://www.atsdr.cdc.gov
ATSDR develops educational and informational materials for health care providers categorized by
hazardous substance, clinical condition, and/or by susceptible population. The following additional
materials are available online:
Clinical Briefs and Overview discuss health effects and approaches to patient management in a
brief/factsheet style. They are narrated PowerPoint presentations with Continuing Education
credit available (see https://www.atsdr.cdc.gov/emes/health_professionals/clinician-briefs-
overviews.html).
Managing Hazardous Materials Incidents is a set of recommendations for on-scene (prehospital) and
hospital medical management of patients exposed during a hazardous materials incident (see
https://www.atsdr.cdc.gov/MHMI/index.html).
Fact Sheets (ToxFAQs™) provide answers to frequently asked questions about toxic substances (see
https://www.atsdr.cdc.gov/toxfaqs/Index.asp).

HEXACHLOROCYCLOHEXANE (HCH) E-2
APPENDIX E
Other Agencies and Organizations
The National Center for Environmental Health (NCEH) focuses on preventing or controlling disease,
injury, and disability related to the interactions between people and their environment outside the
workplace. Contact: NCEH, Mailstop F-29, 4770 Buford Highway, NE, Atlanta, GA
30341-3724 • Phone: 770-488-7000 • FAX: 770-488-7015 • Web Page:
https://www.cdc.gov/nceh/.
The National Institute for Occupational Safety and Health (NIOSH) conducts research on occupational
diseases and injuries, responds to requests for assistance by investigating problems of health and
safety in the workplace, recommends standards to the Occupational Safety and Health
Administration (OSHA) and the Mine Safety and Health Administration (MSHA), and trains
professionals in occupational safety and health. Contact: NIOSH, 395 E Street, S.W., Suite 9200,
Patriots Plaza Building, Washington, DC 20201 • Phone: 202-245-0625 or 1-800-CDC-INFO
(800-232-4636) • Web Page: https://www.cdc.gov/niosh/.
The National Institute of Environmental Health Sciences (NIEHS) is the principal federal agency for
biomedical research on the effects of chemical, physical, and biologic environmental agents on
human health and well-being. Contact: NIEHS, PO Box 12233, 104 T.W. Alexander Drive,
Research Triangle Park, NC 27709 • Phone: 919-541-3212 • Web Page:
https://www.niehs.nih.gov/.
Clinical Resources (Publicly Available Information)
The Association of Occupational and Environmental Clinics (AOEC) has developed a network of clinics
in the United States to provide expertise in occupational and environmental issues. Contact:
AOEC, 1010 Vermont Avenue, NW, #513, Washington, DC 20005 • Phone: 202-347-4976
The American College of Occupational and Environmental Medicine (ACOEM) is an association of
physicians and other health care providers specializing in the field of occupational and
environmental medicine. Contact: ACOEM, 25 Northwest Point Boulevard, Suite 700, Elk
Grove Village, IL 60007-1030 • Phone: 847-818-1800 • FAX: 847-818-9266 • Web Page:
http://www.acoem.org/.
The American College of Medical Toxicology (ACMT) is a nonprofit association of physicians with
recognized expertise in medical toxicology. Contact: ACMT, 10645 North Tatum Boulevard,
Suite 200-111, Phoenix AZ 85028 • Phone: 844-226-8333 • FAX: 844-226-8333 • Web Page:
http://www.acmt.net.
The Pediatric Environmental Health Specialty Units (PEHSUs) is an interconnected system of specialists
who respond to questions from public health professionals, clinicians, policy makers, and the
public about the impact of environmental factors on the health of children and reproductive-aged
adults. Contact information for regional centers can be found at http://pehsu.net/findhelp.html.
The American Association of Poison Control Centers (AAPCC) provide support on the prevention and
treatment of poison exposures. Contact: AAPCC, 515 King Street, Suite 510, Alexandria VA
22314 • Phone: 701-894-1858 • Poison Help Line: 1-800-222-1222 • Web Page:
http://www.aapcc.org/.
HEXACHLOROCYCLOHEXANE (HCH) F-1
APPENDIX F. GLOSSARY
Absorption—The process by which a substance crosses biological membranes and enters systemic
circulation. Absorption can also refer to the taking up of liquids by solids, or of gases by solids or liquids.
Acute Exposure—Exposure to a chemical for a duration of ≤14 days, as specified in the Toxicological
Profiles.
Adsorption—The adhesion in an extremely thin layer of molecules (as of gases, solutes, or liquids) to the
surfaces of solid bodies or liquids with which they are in contact.
Adsorption Coefficient (K
oc
)—The ratio of the amount of a chemical adsorbed per unit weight of
organic carbon in the soil or sediment to the concentration of the chemical in solution at equilibrium.
Adsorption Ratio (Kd)—The amount of a chemical adsorbed by sediment or soil (i.e., the solid phase)
divided by the amount of chemical in the solution phase, which is in equilibrium with the solid phase, at a
fixed solid/solution ratio. It is generally expressed in micrograms of chemical sorbed per gram of soil or
sediment.
Benchmark Dose (BMD) or Benchmark Concentration (BMC)—is the dose/concentration
corresponding to a specific response level estimate using a statistical dose-response model applied to
either experimental toxicology or epidemiology data. For example, a BMD
10
would be the dose
corresponding to a 10% benchmark response (BMR). The BMD is determined by modeling the dose-
response curve in the region of the dose-response relationship where biologically observable data are
feasible. The BMDL or BMCL is the 95% lower confidence limit on the BMD or BMC.
Bioconcentration Factor (BCF)—The quotient of the concentration of a chemical in aquatic organisms
at a specific time or during a discrete time period of exposure divided by the concentration in the
surrounding water at the same time or during the same period.
Biomarkers—Indicators signaling events in biologic systems or samples, typically classified as markers
of exposure, effect, and susceptibility.
Cancer Effect Level (CEL)—The lowest dose of a chemical in a study, or group of studies, that
produces significant increases in the incidence of cancer (or malignant tumors) between the exposed
population and its appropriate control.
Carcinogen—A chemical capable of inducing cancer.
Case-Control Study—A type of epidemiological study that examines the relationship between a
particular outcome (disease or condition) and a variety of potential causative agents (such as toxic
chemicals). In a case-control study, a group of people with a specified and well-defined outcome is
identified and compared to a similar group of people without the outcome.
Case Report—A report that describes a single individual with a particular disease or exposure. These
reports may suggest some potential topics for scientific research, but are not actual research studies.
Case Series—Reports that describe the experience of a small number of individuals with the same
disease or exposure. These reports may suggest potential topics for scientific research, but are not actual
research studies.
HEXACHLOROCYCLOHEXANE (HCH) F-2
APPENDIX F
Ceiling Value—A concentration that must not be exceeded.
Chronic Exposure—Exposure to a chemical for ≥365 days, as specified in the Toxicological Profiles.
Clastogen—A substance that causes breaks in chromosomes resulting in addition, deletion, or
rearrangement of parts of the chromosome.
Cohort Study—A type of epidemiological study of a specific group or groups of people who have had a
common insult (e.g., exposure to an agent suspected of causing disease or a common disease) and are
followed forward from exposure to outcome, and who are disease-free at start of follow-up. Often, at
least one exposed group is compared to one unexposed group, while in other cohorts, exposure is a
continuous variable and analyses are directed towards analyzing an exposure-response coefficient.
Cross-sectional Study—A type of epidemiological study of a group or groups of people that examines
the relationship between exposure and outcome to a chemical or to chemicals at a specific point in time.
Data Needs—Substance-specific informational needs that, if met, would reduce the uncertainties of
human health risk assessment.
Developmental Toxicity—The occurrence of adverse effects on the developing organism that may result
from exposure to a chemical prior to conception (either parent), during prenatal development, or
postnatally to the time of sexual maturation. Adverse developmental effects may be detected at any point
in the life span of the organism.
Dose-Response Relationship—The quantitative relationship between the amount of exposure to a
toxicant and the incidence of the response or amount of the response.
Embryotoxicity and Fetotoxicity—Any toxic effect on the conceptus as a result of prenatal exposure to
a chemical; the distinguishing feature between the two terms is the stage of development during which the
effect occurs. Effects include malformations and variations, altered growth, and in utero death.
Epidemiology—The investigation of factors that determine the frequency and distribution of disease or
other health-related conditions within a defined human population during a specified period.
Excretion—The process by which metabolic waste products are removed from the body.
Genotoxicity—A specific adverse effect on the genome of living cells that, upon the duplication of
affected cells, can be expressed as a mutagenic, clastogenic, or carcinogenic event because of specific
alteration of the molecular structure of the genome.
Half-life—A measure of rate for the time required to eliminate one-half of a quantity of a chemical from
the body or environmental media.
Health Advisory—An estimate of acceptable drinking water levels for a chemical substance derived by
EPA and based on health effects information. A health advisory is not a legally enforceable federal
standard, but serves as technical guidance to assist federal, state, and local officials.
Immediately Dangerous to Life or Health (IDLH)—A condition that poses a threat of life or health, or
conditions that pose an immediate threat of severe exposure to contaminants that are likely to have
adverse cumulative or delayed effects on health.
HEXACHLOROCYCLOHEXANE (HCH) F-3
APPENDIX F
Immunotoxicity—Adverse effect on the functioning of the immune system that may result from
exposure to chemical substances.
Incidence—The ratio of new cases of individuals in a population who develop a specified condition to
the total number of individuals in that population who could have developed that condition in a specified
time period.
Intermediate Exposure—Exposure to a chemical for a duration of 15–364 days, as specified in the
Toxicological Profiles.
In Vitro—Isolated from the living organism and artificially maintained, as in a test tube.
In Vivo—Occurring within the living organism.
Lethal Concentration
(LO)
(LC
LO
)—The lowest concentration of a chemical in air that has been reported
to have caused death in humans or animals.
Lethal Concentration
(50)
(LC
50
)—A calculated concentration of a chemical in air to which exposure for
a specific length of time is expected to cause death in 50% of a defined experimental animal population.
Lethal Dose
(LO)
(LD
Lo
)—The lowest dose of a chemical introduced by a route other than inhalation that
has been reported to have caused death in humans or animals.
Lethal Dose
(50)
(LD
50
)—The dose of a chemical that has been calculated to cause death in 50% of a
defined experimental animal population.
Lethal Time
(50)
(LT
50
)—A calculated period of time within which a specific concentration of a chemical
is expected to cause death in 50% of a defined experimental animal population.
Lowest-Observed-Adverse-Effect Level (LOAEL)—The lowest exposure level of chemical in a study,
or group of studies, that produces statistically or biologically significant increases in frequency or severity
of adverse effects between the exposed population and its appropriate control.
Lymphoreticular Effects—Represent morphological effects involving lymphatic tissues such as the
lymph nodes, spleen, and thymus.
Malformations—Permanent structural changes that may adversely affect survival, development, or
function.
Metabolism—Process in which chemical substances are biotransformed in the body that could result in
less toxic and/or readily excreted compounds or produce a biologically active intermediate.
Minimal LOAEL—Indicates a minimal adverse effect or a reduced capacity of an organ or system to
absorb additional toxic stress that does not necessarily lead to the inability of the organ or system to
function normally.
Minimal Risk Level (MRL)—An estimate of daily human exposure to a hazardous substance that is
likely to be without an appreciable risk of adverse noncancer health effects over a specified route and
duration of exposure.
HEXACHLOROCYCLOHEXANE (HCH) F-4
APPENDIX F
Modifying Factor (MF)—A value (greater than zero) that is applied to the derivation of a Minimal Risk
Level (MRL) to reflect additional concerns about the database that are not covered by the uncertainty
factors. The default value for a MF is 1.
Morbidity—The state of being diseased; the morbidity rate is the incidence or prevalence of a disease in
a specific population.
Mortality—Death; the mortality rate is a measure of the number of deaths in a population during a
specified interval of time.
Mutagen—A substance that causes mutations, which are changes in the DNA sequence of a cell’s DNA.
Mutations can lead to birth defects, miscarriages, or cancer.
Necropsy—The gross examination of the organs and tissues of a dead body to determine the cause of
death or pathological conditions.
Neurotoxicity—The occurrence of adverse effects on the nervous system following exposure to a
hazardous substance.
No-Observed-Adverse-Effect Level (NOAEL)—The dose of a chemical at which there were no
statistically or biologically significant increases in frequency or severity of adverse effects seen between
the exposed population and its appropriate control. Although effects may be produced at this dose, they
are not considered to be adverse.
Octanol-Water Partition Coefficient (K
ow
)—The equilibrium ratio of the concentrations of a chemical
in n-octanol and water, in dilute solution.
Odds Ratio (OR)—A means of measuring the association between an exposure (such as toxic substances
and a disease or condition) that represents the best estimate of relative risk (risk as a ratio of the incidence
among subjects exposed to a particular risk factor divided by the incidence among subjects who were not
exposed to the risk factor). An odds ratio that is greater than 1 is considered to indicate greater risk of
disease in the exposed group compared to the unexposed group.
Permissible Exposure Limit (PEL)—An Occupational Safety and Health Administration (OSHA)
regulatory limit on the amount or concentration of a substance not to be exceeded in workplace air
averaged over any 8-hour work shift of a 40-hour workweek.
Pesticide—General classification of chemicals specifically developed and produced for use in the control
of agricultural and public health pests (insects or other organisms harmful to cultivated plants or animals).
Pharmacokinetics—The dynamic behavior of a material in the body, used to predict the fate
(disposition) of an exogenous substance in an organism. Utilizing computational techniques, it provides
the means of studying the absorption, distribution, metabolism, and excretion of chemicals by the body.
Pharmacokinetic Model—A set of equations that can be used to describe the time course of a parent
chemical or metabolite in an animal system. There are two types of pharmacokinetic models: data-based
and physiologically-based. A data-based model divides the animal system into a series of compartments,
which, in general, do not represent real, identifiable anatomic regions of the body, whereas the
physiologically-based model compartments represent real anatomic regions of the body.
HEXACHLOROCYCLOHEXANE (HCH) F-5
APPENDIX F
Physiologically Based Pharmacodynamic (PBPD) Model—A type of physiologically based dose-
response model that quantitatively describes the relationship between target tissue dose and toxic
endpoints. These models advance the importance of physiologically based models in that they clearly
describe the biological effect (response) produced by the system following exposure to an exogenous
substance.
Physiologically Based Pharmacokinetic (PBPK) Model—A type of physiologically based dose-
response model that is comprised of a series of compartments representing organs or tissue groups with
realistic weights and blood flows. These models require a variety of physiological information, including
tissue volumes, blood flow rates to tissues, cardiac output, alveolar ventilation rates, and possibly
membrane permeabilities. The models also utilize biochemical information, such as blood:air partition
coefficients, and metabolic parameters. PBPK models are also called biologically based tissue dosimetry
models.
Prevalence—The number of cases of a disease or condition in a population at one point in time.
Prospective Study—A type of cohort study in which a group is followed over time and the pertinent
observations are made on events occurring after the start of the study.
Recommended Exposure Limit (REL)—A National Institute for Occupational Safety and Health
(NIOSH) time-weighted average (TWA) concentration for up to a 10-hour workday during a 40-hour
workweek.
Reference Concentration (RfC)—An estimate (with uncertainty spanning perhaps an order of
magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups)
that is likely to be without an appreciable risk of deleterious noncancer health effects during a lifetime.
The inhalation RfC is expressed in units of mg/m
3
or ppm.
Reference Dose (RfD)—An estimate (with uncertainty spanning perhaps an order of magnitude) of the
daily oral exposure of the human population to a potential hazard that is likely to be without risk of
deleterious noncancer health effects during a lifetime. The oral RfD is expressed in units of mg/kg/day.
Reportable Quantity (RQ)—The quantity of a hazardous substance that is considered reportable under
the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). RQs are
(1) ≥1 pound or (2) for selected substances, an amount established by regulation either under CERCLA or
under Section 311 of the Clean Water Act. Quantities are measured over a 24-hour period.
Reproductive Toxicity—The occurrence of adverse effects on the reproductive system that may result
from exposure to a hazardous substance. The toxicity may be directed to the reproductive organs and/or
the related endocrine system. The manifestation of such toxicity may be noted as alterations in sexual
behavior, fertility, pregnancy outcomes, or modifications in other functions that are dependent on the
integrity of this system.
Retrospective Study—A type of cohort study based on a group of persons known to have been exposed
at some time in the past. Data are collected from routinely recorded events, up to the time the study is
undertaken. Retrospective studies are limited to causal factors that can be ascertained from existing
records and/or examining survivors of the cohort.
Risk—The possibility or chance that some adverse effect will result from a given exposure to a hazardous
substance.
HEXACHLOROCYCLOHEXANE (HCH) F-6
APPENDIX F
Risk Factor—An aspect of personal behavior or lifestyle, an environmental exposure, existing health
condition, or an inborn or inherited characteristic that is associated with an increased occurrence of
disease or other health-related event or condition.
Risk Ratio/Relative Risk—The ratio of the risk among persons with specific risk factors compared to the
risk among persons without risk factors. A risk ratio that is greater than 1 indicates greater risk of disease
in the exposed group compared to the unexposed group.
Serious LOAEL—A dose that evokes failure in a biological system and can lead to morbidity or
mortality.
Short-Term Exposure Limit (STEL)—A STEL is a 15-minute TWA exposure that should not be
exceeded at any time during a workday.
Standardized Mortality Ratio (SMR)—A ratio of the observed number of deaths and the expected
number of deaths in a specific standard population.
Target Organ Toxicity—This term covers a broad range of adverse effects on target organs or
physiological systems (e.g., renal, cardiovascular) extending from those arising through a single limited
exposure to those assumed over a lifetime of exposure to a chemical.
Teratogen—A chemical that causes structural defects that affect the development of an organism.
Threshold Limit Value (TLV)—An American Conference of Governmental Industrial Hygienists
(ACGIH) concentration of a substance to which it is believed that nearly all workers may be repeatedly
exposed, day after day, for a working lifetime without adverse effect. The TLV may be expressed as a
Time-Weighted Average (TLV-TWA), as a Short-Term Exposure Limit (TLV-STEL), or as a ceiling
limit (TLV-C).
Time-Weighted Average (TWA)—An average exposure within a given time period.
Toxicokinetic—The absorption, distribution, metabolism, and elimination of toxic compounds in the
living organism.
Toxics Release Inventory (TRI)—The TRI is an EPA program that tracks toxic chemical releases and
pollution prevention activities reported by industrial and federal facilities.
Uncertainty Factor (UF)—A factor used in operationally deriving the Minimal Risk Level (MRL),
Reference Dose (RfD), or Reference Concentration (RfC) from experimental data. UFs are intended to
account for (1) the variation in sensitivity among the members of the human population, (2) the
uncertainty in extrapolating animal data to the case of human, (3) the uncertainty in extrapolating from
data obtained in a study that is of less than lifetime exposure, and (4) the uncertainty in using lowest-
observed-adverse-effect level (LOAEL) data rather than no-observed-adverse-effect level (NOAEL) data.
A default for each individual UF is 10; if complete certainty in data exists, a value of 1 can be used;
however, a reduced UF of 3 may be used on a case-by-case basis (3 being the approximate logarithmic
average of 10 and 1).
Xenobiotic—Any substance that is foreign to the biological system.
HEXACHLOROCYCLOHEXANE (HCH) G-1
APPENDIX G. ACRONYMS, ABBREVIATIONS, AND SYMBOLS
AAPCC American Association of Poison Control Centers
ACGIH American Conference of Governmental Industrial Hygienists
ACOEM American College of Occupational and Environmental Medicine
ACMT American College of Medical Toxicology
ADI acceptable daily intake
ADME absorption, distribution, metabolism, and excretion
AEGL Acute Exposure Guideline Level
AIC Akaike’s information criterion
AIHA American Industrial Hygiene Association
ALT alanine aminotransferase
AOEC Association of Occupational and Environmental Clinics
AP alkaline phosphatase
AST aspartate aminotransferase
atm atmosphere
ATSDR Agency for Toxic Substances and Disease Registry
AWQC Ambient Water Quality Criteria
BCF bioconcentration factor
BMD/C benchmark dose or benchmark concentration
BMD
X
dose that produces a X% change in response rate of an adverse effect
BMDL
X
95% lower confidence limit on the BMD
X
BMDS Benchmark Dose Software
BMR benchmark response
BUN blood urea nitrogen
C centigrade
CAA Clean Air Act
CAS Chemical Abstract Services
CDC Centers for Disease Control and Prevention
CEL cancer effect level
CERCLA Comprehensive Environmental Response, Compensation, and Liability Act
CFR Code of Federal Regulations
Ci curie
CI confidence interval
cm centimeter
CPSC Consumer Products Safety Commission
CWA Clean Water Act
DNA deoxyribonucleic acid
DOD Department of Defense
DOE Department of Energy
DWEL drinking water exposure level
EAFUS Everything Added to Food in the United States
ECG/EKG electrocardiogram
EEG electroencephalogram
EPA Environmental Protection Agency
ERPG emergency response planning guidelines
F Fahrenheit
F1 first-filial generation
FDA Food and Drug Administration
FIFRA Federal Insecticide, Fungicide, and Rodenticide Act
FR Federal Register
HEXACHLOROCYCLOHEXANE (HCH) G-2
APPENDIX G
FSH follicle stimulating hormone
g gram
GC gas chromatography
gd gestational day
GGT γ-glutamyl transferase
GRAS generally recognized as safe
HEC human equivalent concentration
HED human equivalent dose
HHS Department of Health and Human Services
HPLC high-performance liquid chromatography
HSDB Hazardous Substances Data Bank
IARC International Agency for Research on Cancer
IDLH immediately dangerous to life and health
IRIS Integrated Risk Information System
Kd adsorption ratio
kg kilogram
kkg kilokilogram; 1 kilokilogram is equivalent to 1,000 kilograms and 1 metric ton
K
oc
organic carbon partition coefficient
K
ow
octanol-water partition coefficient
L liter
LC liquid chromatography
LC
50
lethal concentration, 50% kill
LC
Lo
lethal concentration, low
LD
50
lethal dose, 50% kill
LD
Lo
lethal dose, low
LDH lactate dehydrogenase
LH luteinizing hormone
LOAEL lowest-observed-adverse-effect level
LSE Level of Significant Exposure
LT
50
lethal time, 50% kill
m meter
mCi millicurie
MCL maximum contaminant level
MCLG maximum contaminant level goal
MF modifying factor
mg milligram
mL milliliter
mm millimeter
mmHg millimeters of mercury
mmol millimole
MRL Minimal Risk Level
MS mass spectrometry
MSHA Mine Safety and Health Administration
Mt metric ton
NAAQS National Ambient Air Quality Standard
NAS National Academy of Science
NCEH National Center for Environmental Health
ND not detected
ng nanogram
NHANES National Health and Nutrition Examination Survey
NIEHS National Institute of Environmental Health Sciences
HEXACHLOROCYCLOHEXANE (HCH) G-3
APPENDIX G
NIOSH National Institute for Occupational Safety and Health
NLM National Library of Medicine
nm nanometer
nmol nanomole
NOAEL no-observed-adverse-effect level
NPL National Priorities List
NR not reported
NRC National Research Council
NS not specified
NTP National Toxicology Program
OR odds ratio
OSHA Occupational Safety and Health Administration
PAC Protective Action Criteria
PAH polycyclic aromatic hydrocarbon
PBPD physiologically based pharmacodynamic
PBPK physiologically based pharmacokinetic
PEHSU Pediatric Environmental Health Specialty Unit
PEL permissible exposure limit
PEL-C permissible exposure limit-ceiling value
pg picogram
PND postnatal day
POD point of departure
ppb parts per billion
ppbv parts per billion by volume
ppm parts per million
ppt parts per trillion
REL recommended exposure limit
REL-C recommended exposure limit-ceiling value
RfC reference concentration
RfD reference dose
RNA ribonucleic acid
SARA Superfund Amendments and Reauthorization Act
SCE sister chromatid exchange
SD standard deviation
SE standard error
SGOT serum glutamic oxaloacetic transaminase (same as aspartate aminotransferase or AST)
SGPT serum glutamic pyruvic transaminase (same as alanine aminotransferase or ALT)
SIC standard industrial classification
SLOAEL serious lowest-observed-adverse-effect level
SMR standardized mortality ratio
sRBC sheep red blood cell
STEL short term exposure limit
TLV threshold limit value
TLV-C threshold limit value-ceiling value
TRI Toxics Release Inventory
TSCA Toxic Substances Control Act
TWA time-weighted average
UF uncertainty factor
U.S. United States
USDA United States Department of Agriculture
USGS United States Geological Survey
HEXACHLOROCYCLOHEXANE (HCH) G-4
APPENDIX G
USNRC U.S. Nuclear Regulatory Commission
VOC volatile organic compound
WBC white blood cell
WHO World Health Organization
> greater than
≥ greater than or equal to
= equal to
< less than
≤ less than or equal to
% percent
α alpha
β beta
γ gamma
δ delta
μm micrometer
μg microgram
q
1
*
cancer slope factor
– negative
+ positive
(+) weakly positive result
(–) weakly negative result
