
IBC FAQs
1
APRIL 2022
FREQUENTLY ASKED QUESTIONS
University of Michigan Institutional Biosafety Committee
TABLE OF CONTENTS
IBC REGISTRATION: WHO NEEDS AN IBC APPLICATION, WHAT FOR, AND WHY?
1. What type of work requires IBC approval?
2. What is “exempt” work?
3. Do I need to register for PCR work?
4. I am not working with recombinant DNA. Do I still need an IBC application?
5. Do I need different IBC applications for different work?
6. Who can be the Principal Investigator on an IBC application?
7. What are my responsibilities as a Principal Investigator?
8. Can I use another investigator’s IBC application if we share a lab space?
9. The PI on our application is leaving UM, but the lab will still be active temporarily.
What do we do?
10. Do I need an IBC application for work involving potentially hazardous biological
substances that is performed in my colleague’s lab?
11. Do I need an IBC application if a Managed Core is doing work for me?
12. Do Managed Cores need IBC applications?
13. Who should be listed as the PI for a Managed Core IBC application?
14. I am an external investigator outside the University of Michigan. Do I need to
submit anything to the IBC in order to utilize the services of the Vector Core?
SUBMITTING AN IBC APPLICATION
15. What are the different types of submissions?
16. When should I submit an amendment?
17. When should I submit a renewal?
18. What is the deadline for submitting an IBC application?
19. How do I submit an IBC application?
20. Can I submit an IBC application for my PI?
3
3
3
4
4
4
5
6
6
6
6
6
7
7
7
7
8
8
8
8

IBC FAQs
2
APRIL 2022
21. I have been sharing an IBC application with another investigator, but now want
to have an application of my own. Can you duplicate our current IBC application
for me?
22. What is the process for submitting an IBC application for work done at BSL3 or
ABSL3?
23. What is the process for submitting a human gene transfer study?
24. Can we use a centralized IBC provider for our human gene therapy study?
25. Who can I contact with questions?
NAVIGATING THE IBC APPLICATION
26. Where can I find information about what research I currently have approval for?
27. What types of information can I find in my Approved Application Summary?
28. How do I withdraw an IBC application/amendment?
29. How do I update lab personnel without having to submit an amendment?
30. What if the name of the lab personnel I want to add to my application is not
available?
31. What if the building or room number that I want to add to my application is not
available?
EHS-RELATED QUESTIONS
32. What is the difference between Biosafety Level 1 and Biosafety Level 2?
33. Do I need to have a Biosafety Inspection before my IBC application is approved?
34. How do I resolve the corrective actions identified during my Biosafety
Inspections?
ANIMAL-RELATED QUESTIONS
35. What types of animal derived substances require an IBC application?
36. How does my IBC application relate to my IACUC animal protocol?
37. How do I add the administration of a biological substance to animals to my
application?
38. Do my animals require ABSL2 containment housing?
8
9
9
9
9
10
10
12
12
12
13
14
15
15
15
15
16
18

IBC FAQs
3
APRIL 2022
Any U-M investigator planning a research project that involves recombinant DNA (rDNA)
or synthetic nucleic acid (SNA) molecules must submit an application for Institutional
Biosafety Committee (IBC) review and approval before initiating the work. U-M
investigators must also register their work with other potentially hazardous biological
substances.
IBC approval is required for work with:
•
Recombinant DNA & SNA (including exempt work and human gene transfer)
•
Infectious agents (e.g., viruses, bacteria, parasites, fungi, prions)
•
Biological toxins
•
Human-derived materials
•
Animal-derived materials from:
◦
Non-human primates
◦
Ruminants
◦
Swine
◦
Chickens or other fowl
◦
Any wild vertebrate animals
•
Administration of the above substances to animals
•
Creation, purchase, or other acquisition of vertebrate or invertebrate transgenic
animals
•
federally regulated Select Agents, experiments with Dual Research of Concern
potential, and research requiring BSL3 containment
If you have any questions regarding whether you may need IBC approval, please email
IBC staff at IBCstaff@umich.edu.
What type of work requires IBC approval?
1
IBC REGISTRATION: WHO NEEDS AN IBC APPLICATION, WHAT FOR, AND WHY?
Section III-F of the NIH Guidelines set forth eight categories of research that are exempt
from the requirements of the NIH Guidelines; however, the University of Michigan still
requires that Principal Investigators register these categories of work with the IBC by
submitting an IBC application.
What is “exempt” work?
2
You need to register the work with the IBC if you are cloning the PCR product prior to
sequencing. The direct sequencing of PCR products does not need to be registered with
the IBC - as long as there is no cloning involved.
Do I need to register for PCR work?
3

IBC FAQs
4
APRIL 2022
Yes, if your work involves:
•
Infectious agents (e.g., viruses, bacteria, parasites, fungi, prions)
•
Biological toxins
•
Human-derived materials
•
Animal-derived materials from:
◦
Non-human primates
◦
Ruminants
◦
Swine
◦
Chickens or other fowl
◦
Any wild vertebrate animals
•
Administration of the above substances to animals
I am not working with recombinant DNA. Do I still need an IBC application?
4
No. PIs can only have one IBC application. This application covers all their laboratory’s
research with biohazards. The exceptions to this rule are BSL3 work and human gene
transfer studies. To learn more about these processes, please see “What is the process
for submitting an IBC application for work done at BSL3?” and “What is the process
for submitting a human gene transfer study?”.
Do I need different IBC applications for different work?
5
Who can be the Principal Investigator on an IBC application?
6
The principal investigator on an IBC application must be either tenure-track faculty
(Instructor through Professor), or research faculty (Research Investigator through
Research Professor). Exceptions to this requirement may be approved on a case-by-
case basis.
Another faculty member may be designated as a co-principal investigator on the
application. A co-principal investigator must also be either tenure-track or research
faculty and should be able to assume responsibility for the work covered by the
application if the principal investigator is unavailable. Co-principal investigators must
accept their role on the IBC application. Exceptions to this requirement may be
approved on a case-by-case basis.
The principal investigator may designate individuals with editing rights on their
application, however, the principal investigator is responsible for the content and must
be the one to submit the application in eRRM.

IBC FAQs
5
APRIL 2022
Principal investigators or project directors at U-M are responsible for:
•
Adhering to and promoting applicable biosafety procedures, including:
◦
Ensuring the use of proper microbiological practices and laboratory
techniques at the approved biosafety level;
◦
Ensuring that everyone working in your lab is aware of the potential
biosafety risks associated with the research they are undertaking and of
any protective measures (such as vaccines or special PPE) that can reduce
these risks;
◦
Ensuring that everyone working in the lab is aware of the potential
symptoms caused by exposure/infection;
◦
Providing all lab personnel listed in the IBC application with copies of the
procedures describing potential biohazards and appropriate precautions,
including an updated Biosafety Manual and Chemical Hygiene Plan;
◦
Providing all lab personnel listed in the IBC application with documented
formal and informal instruction and training in the practices and
techniques required to ensure safety and for dealing with accidental spills,
personnel contamination, and other laboratory accidents;
◦
If working at BSL2, downloading and compiling a BSL2 Biosafety Manual
for use by lab personnel;
◦
If working at BSL2, scheduling and completing an annual BSL2 laboratory
inspection with U-M Environment, Health & Safety (see the EHS Inspection
Checklist for details); and
◦
If working at BSL2, establishing policies and procedures to limit access to
BSL2 biohazardous materials to only those individuals who are listed on the
application, who have been advised on the potential hazards, and who
meet specific entry requirements (e.g., immunizations, training)
•
Fulfilling any additional PI/PD responsibilities as detailed in Section IV-B-7 of the
NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid
Molecules
•
Complying with the requirements pertaining to the possession, use, transfer, and
disposal of all regulated biologically hazardous materials in accordance with all
applicable federal, state, and local laws and regulations, and UM policies and
procedures
•
Being familiar with the CDC/NIH publication, "Biosafety in Microbiological and
Biomedical Laboratories,” including specific sections that apply to the PIs work
•
Abiding by the reporting requirements and submitting a report to the IBC,
Biological Safety Officer, and other appropriate authorities for activities that may
include, but are not limited to, the following:
◦
Any accident that results in exposure to infectious agents, biological toxins,
or recombinant DNA, or which presents a danger of environmental
contamination, or
◦
All spills of biohazardous materials outside of physical containment
equipment (e.g., outside of a biosafety cabinet, outside of centrifuge)
What are my responsibilities as a Principal Investigator?
7

IBC FAQs
6
APRIL 2022
Can I use another investigator’s IBC application if we share a lab space?
8
If Principal Investigators are working with the same materials and sharing staff, they may
also share an IBC application. In this case, one investigator should be named the
Principal Investigator on the IBC application and the other should be listed as the Co-PI.
The PI on our application is leaving UM, but the lab will still be active
temporarily. What do we do?
9
If the principal investigator leaves U-M for a position elsewhere or for retirement and
laboratory members are still working on projects covered by the IBC application, a
person must be designated who meets the requirements of principal investigator and
who is available to serve in an oversight capacity in the lab for the duration of time while
those projects are being completed. This change is made as an amendment to the IBC
application, and the committee will approve the change at a meeting.
Do I need an IBC application for work involving potentially hazardous
biological substances that is performed in my colleague’s lab?
10
If you are having work performed in another investigator's lab, you are responsible for
ensuring that it is occurring in a laboratory that is registered with the IBC for that type of
work and that it is conducted by individuals who have received training for the work
they are performing.
Do I need an IBC application if a Managed Core is doing work for me?
11
If a PI will be providing biological substances subject to IBC approval to a managed core,
or a managed core will be providing a PI with biological substances for use in their own
laboratory, then the PI must obtain IBC approval.
Do Managed Cores need IBC applications?
12
Yes, managed research cores that handle biological substances subject to IBC approval
must complete an IBC application regarding the work that occurs in the core with those
biological substances. For more information, see Policy on IBC Applications for Managed
Cores Serving Researchers at U-M.

IBC FAQs
7
APRIL 2022
Who should be listed as the PI for a Managed Core IBC application?
13
IBC applications for managed cores should be completed under the name of the staff
person who serves as the manager of the core.
The faculty member serving as director of the core should be listed as a Co-PI. This will
allow the faculty member to maintain a separate IBC application for their own research.
I am an external investigator outside the University of Michigan. Do I need to
submit anything to the IBC in order to utilize the services of the Vector Core?
14
No. External investigators are no longer required to submit a registration application for
this purpose.
What are the different types of submissions?
15
SUBMITTING AN IBC APPLICATION
The IBC application process includes submissions of:
•
An initial application to obtain IBC approval for a three-year period.
•
Amendment(s) to obtain approval for changes to the research.
•
Renewal(s) to extend IBC approval for ongoing work for another three-year
approval period.
When should I submit an amendment?
16
You must amend your approved IBC application in the eResearch Regulatory
Management (eRRM) system if you are adding or changing:
•
genes studied or host/vector systems used in your rDNA work
•
infectious agents or biological toxins
•
work with substances from humans or certain vertebrate animals
•
transgenic animal work or transgenic plant work
•
administration of biological substances to animals
•
administration of biological substances to plants
•
anything else that may have an impact on the biosafety level of the work being
performed
•
BSL2 laboratory space

IBC FAQs
8
APRIL 2022
When should I submit a renewal?
17
An approved IBC application is valid for three years. Amendments submitted within the
90-day period leading up to expiration are considered renewals. We recommend that
you submit your renewal at least 60 days before the expiration date. Amendments (i.e.,
applications submitted outside of the 90-day renewal window) have no impact on the
expiration date of a registration.
What is the deadline for submitting an IBC application?
18
The IBC meets monthly on the third Friday of each month. The deadline for submissions
to be considered at these meetings is generally the last Friday of the previous month.
Please visit our website for more information.
How do I submit an IBC application?
19
1. Log into the eResearch Regulatory Management (eRRM) system with your uniqname
and UMICH password.
2. Initiateyour application.
3. When finished and ready to submit,click"Submit"or"Submit Changes"from
theActivitiesmenu on the left-hand side of the page.
For more detailed instructions, please review this document from ITS.
Note: If you do not see the state of your application change to "IBC Staff Review," you
have not submitted your application.
Can I submit an IBC application for my PI?
20
No. Your PI is responsible for submitting the IBC Application. If your PI has given you edit
rights to the application, you may fill out various portions of the application, then move it
to the “Ready to Submit Inbox” to indicate to your PI that the application is ready for their
review.
21
Unfortunately, IBC applications cannot be duplicated at this time.
I have been sharing an IBC application with another investigator, but now
want to have an application of my own. Can you duplicate our current IBC
application for me?

IBC FAQs
9
APRIL 2022
What is the process for submitting an IBC application for work done at BSL3
or ABSL3?
22
Applications for research in the BSL3 Biocontainment Facility or the ABSL3
Biocontainment Facility are submitted through a process separate from that used for
BSL1 and BSL2 work. To learn more about how to submit an IBC application for BSL3 or
ABSL3 work, please contact IBC staff at IBCstaff@umich.edu.
What is the process for submitting a human gene transfer study?
23
The IBC serves as an Ancillary Committee to the IRB for these types of studies, so the way
you submit an application for a Human Gene Transfer clinical trial is through the IRB
application itself. There is a question in the IRB application that asks about whether it is a
gene transfer study. When answered yes, the gene transfer section of the application
opens for completion, and when the IRB application is submitted the IBC is notified. If
the IBC reviewers have any questions or comments about the application or the other
included materials, these are directed to the study team during this review process. The
total turnaround time from submission to IBC meeting varies depending on when the
application is submitted, but the average is around 30 days.
Can we use a centralized IBC provider for our human gene therapy study?
24
No, U-M does not employ a centralized IBC provider for human gene transfer studies.
Who can I contact with questions?
25
For general questions, please email IBC[email protected]. For questions about a specific
IBC Application, you can use IBC[email protected] or contact IBC staff directly:
Alicia Trombley
Phone:(734) 615-9637
Email:atrombl@umich.edu
Provides support to Principal Investigators with last names beginning: A - L
Jen Harley
Phone:(734) 936-1236
Email:jenhar@umich.edu
Provides support to Principal Investigators with last names beginning: M - Z

IBC FAQs
10
APRIL 2022
NAVIGATING THE IBC APPLICATION
Where can I find information about what research I currently have approval
for?
26
•
Open your application workspace and
confirm that the current state of your
application is Approved.
•
To view your entire approved application,
select View Application.
•
To view a summary of your approved work,
select Approved Application Summary.
What types of information can I find in my Approved Application Summary?
27
At the top of your Approved Application Summary, you will find your IBC Application
Number, Important Dates (Initial Approval Date, Last Amendment Date, and Expiration
Date), and Approval Notes from the IBC Committee (e.g., conditions of your approval).
(question continues on next page)
The NIH Guidelines specify the biosafety practices and containment principles for
constructing and handling: (1) recombinant nucleic acid molecules, (2) synthetic nucleic
acid molecules, including those that are chemically or otherwise modified but can base
pair with naturally occurring nucleic acid molecules, and (3) cells, organisms, and viruses
containing such molecules.

IBC FAQs
11
APRIL 2022
You can view which sections of the NIH Guidelines apply to your research by going to the
Recombinant DNA or Synthetic Nucleic Acids section of your Approved Application
Summary.
(question continues on next page)
As you scroll through your Approved Application Summary, you will see: (1) which
biological hazards you are approved to work with, and what biosafety levels they must be
handled at; (2) which animal species you are approved to work with, whether they are
transgenic or immunodeficient, and the required animal containment level; (3) which
biological hazards you are approved to administer to animals, the approved routes of
administration, and the required animal containment level.
For example: In this application, the PI is approved to work with human cadavers or body
parts at BSL2.
For example: In this application, the PI is approved to work with transgenic mice housed
at ABSL1.

IBC FAQs
12
APRIL 2022
For example: In this application, the PI is approved to administer human cadavers or
body parts to mice through an oral route of administration at ABSL2 for the duration of
the experiment.
How do I withdraw an IBC application/amendment?
28
To withdraw an IBC application or amendment, go to the
activities panel and select “Withdraw.” Please note that this
action is permanent. If you have any questions about
withdrawing an IBC application or amendment, please
contact IBC staff at IBCstaff@umich.edu.
How do I update lab personnel without having to submit an amendment?
29
To update lab personnel:
1. Log into eRRM and navigate to the main page of your
application.
2. Select “Edit Lab Personnel” under the “Activities”
header.
3. Update your lab personnel as needed.
What if the name of the lab personnel I want to add to my application is not
available?
30
If you cannot find the individual by searching for their name or uniqname, you may need
to create a new user account for them. Do this by selecting “Add” in the Lab Personnel
section, then clicking the “Create New User Account” link.
(question continues on next page)

IBC FAQs
13
APRIL 2022
What if the building or room number that I want to add to my application is
not available?
31
If you cannot find a certain building or room number, search for “Other” and select that
from the drop-down list.
Note: The IBC application uses the formal building name (for example, BSRB is Taubman
Biomedical Science Research Building).
IBC FAQs
14
APRIL 2022
EHS-RELATED QUESTIONS
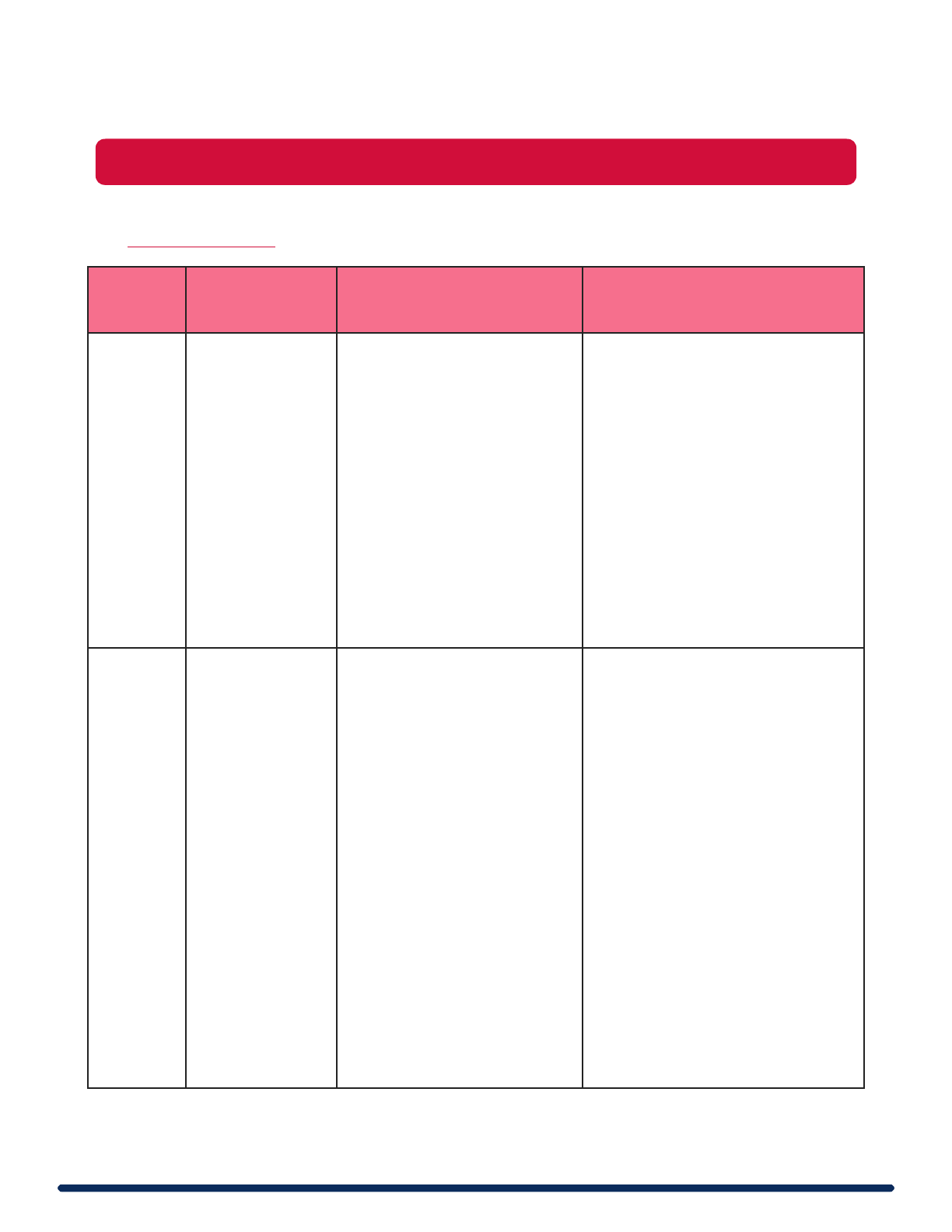
What is the difference between Biosafety Level 1 and Biosafety Level 2?
32
The table below gives a general comparison of BSL1 vs BSL2 requirements. Please see the
UM Biosafety Manual or contact EHS for additional information.
Biosafety
Level
Agents Used
Facility Requirements
Special Practices
BSL1
Non-infectious
agents
• Doors for access control
• Screens on windows
that open to the exterior
• Non-fabric chairs and
furniture easily cleanable
• Sink required
• Eyewash station readily
available in the lab
None
BSL2
Infectious
spread via
blood or
oral/fecal
transmission
Includes
human blood
and cell lines,
toxins, venom,
materials from
Non-human
primates
All BSL1 requirements,
plus:
• Door(s) should be self-
closing and lockable
• Vacuum lines protected
• Autoclave available or
approved alternative
decontamination
method
• Laboratories should be
under negative pressure
or must be neutral
pressure
All BSL1 requirements, plus:
• Controlled access
• Proficiency in SMPs &
techniques
• Medical surveillance as
appropriate and offered
available immunizations
• BSCs or other containment
device used for aerosol
generating procedures
• Lab equipment is routinely
decontaminated
• Method of decontamination
for lab waste
• Incidents/exposures to
infectious materials are
reported and evaluated

IBC FAQs
15
APRIL 2022
Please make sure you follow these directions for closing out Biosafety Inspections. If you
have any questions, please contact EHS at 734-647-1143 or
BioSafetyInspections@umich.edu.
Yes, if you are proposing work for BSL2 containment. In order for the IBC to issue
approval for work at BSL2 containment, you must have had a satisfactory biohazard
inspection by U-M Environment, Health & Safety (EHS) for that work within the past 12
months (including the resolution of any corrective action items). You can find a BSL2
inspection checklist from EHS here.
To schedule a Biosafety Inspection, please contact EHS at 734-647-1143 or
BioSafetyInspections@umich.edu.
Do I need to have a Biosafety Inspection before my IBC application is
approved?
33
How do I resolve the corrective actions identified during my Biosafety
Inspections?
34
ANIMAL-RELATED QUESTIONS
What types of animal derived substances require an IBC application?
35
IBC approval is required for work withanimal-derived tissues, fluids, or cells from:
•
Non-human primates
•
Ruminants
•
Swine
•
Chickens or other fowl
•
Any wild vertebrate animals
How does my IBC application relate to my IACUC animal protocol?
36
Before the IACUC approves the use of certain biohazards in animals, approval by the IBC
is required. Animal users must provide the required information needed to conduct the
risk assessment (e.g., the nature of the hazard and the route of administration) through a
submission to the IBC.
Failure to receive IBC approval may result in a delay in the approval of an IACUC
protocol.

IBC FAQs
16
APRIL 2022
1. Go to Section 6 (Research Involving Animals).
2. Select “Add” in question 6.1 to add the species you will be working with.
3. Fill out species detail questions, being sure to answer YES to question 5 (“Will you
administer any biological substances this species?”).
How do I add the administration of a biological substance to animals to my
application?
37
(question continues on next page)

IBC FAQs
17
APRIL 2022
4. If you plan on administering cells modified with rDNA/SNA to this species (e.g., rDNA-
modified human cells, rDNA-modified animal cells, rDNA-modified bacteria), answer
YES to question 6, then select the type(s) of cells you will be administering.
5. If you plan on directly administering any vectors, vectorless systems, or non-
recombinant biological substances to this species, answer YES to question 7, then
check the box(es) of each biological substance.
6. Select OK, then fill out this section in the same manner for any additional species you
plan on working with.
(question continues on next page)

IBC FAQs
18
APRIL 2022
7. If you do not see a box for a substance you plan to administer, revisit the section of
the application where you gave specific details about that biological substance.
Double check that you have indicated you will administer this biological substance to
animals. In the example below, the PI has indicated in Section 3-7 (Retrovirus Vectors)
that they will administer retrovirus vectors directly to animals.
8. Continue to Section 6-1 (Administration of Recombinant or Non-Recombinant
Biological Substances to Animals).
9. Select UPDATE in question 6-1.1 to give additional details about your planned
experiments.
Do my animals require ABSL2 containment housing?
38
Please consult this table to determine what animal biosafety level is appropriate for your
animals.
