
Unit 3 – The Periodic Table,
Electron Configuration,
&Periodic Trends
Chapters 4 & 5

Creation of the Periodic Table

Mendeleev’s Table
Dmitry Mendeleev
He created the first periodic
table based on the properties
of the elements


Create a Periodic Table Activity

Activity Compared to Real Table
Transition
metals
missing!


Electron Configuration

Electrons are found in energy
levels
Fill the lowest possible energy levels 1
st
and move outward as the energy levels fill
up
n=1 is lowest energy level
(closest to the nucleus)
Energy levels
called “rings” in
lower level
classes

Each Energy Level is Different…
1
st
Energy Level- can hold 2 electrons
E-
E-
Sphere
shape
1s
2
1
st
energy
level
# of
electrons
This is how you
write out the
location of the
electrons
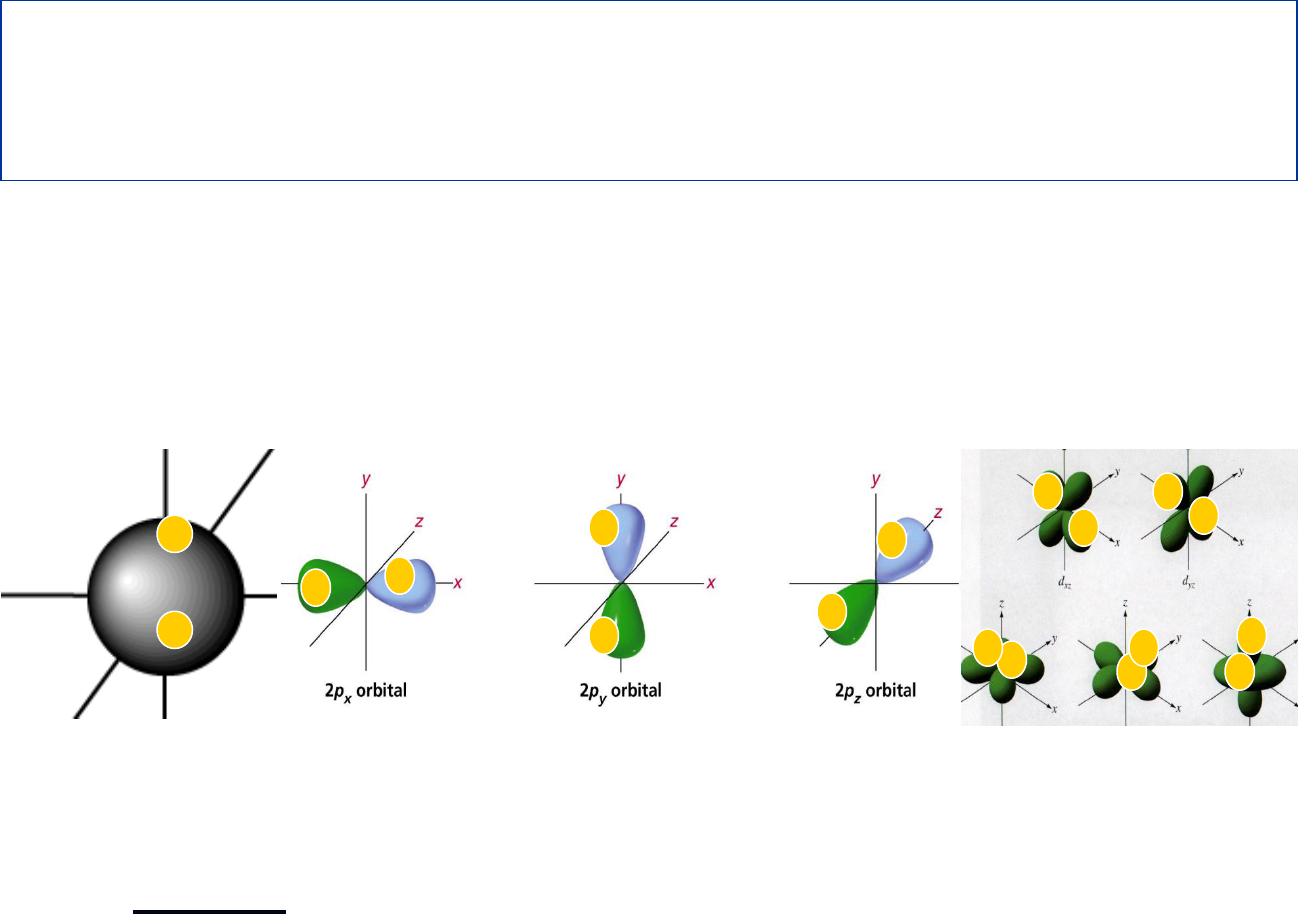
Each energy level has specific orbitals (3- D
pathways) where electrons can be found
Each energy level can hold a specific # of electrons
- Puts them in “S” shaped orbital (always
only hold 2 electrons).

2
nd
Energy Level
Can hold up to 8 electrons
Has an s-shaped orbital & a p-shaped
orbital
The s-orbital always fills up 1
st
!
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
2s
2
2p
6
3
rd
Energy Level
Can hold up to 18 Electrons
Has s, p and d orbitals
NOTE: The 4s orbital is actually at a lower energy,
so electrons will fill it before the 3d orbital!
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
3s
2
3p
6
3d
10

4th Energy Level-
Has 4 different types of orbitals (s,p,d & f)
f- orbitals are
the most
complicated
7 possible “f”
orientations
14 electrons can fit in f orbitals (2
electrons x 7 orientations = 14)

One little trick…
Here’s the order that
an atom will fill it’s
electrons.
Starts with the easiest,
lowest energy level to
put electrons and
moves up.
Diagram on pg 150 of your text book
Whoa… skips
from 3p to 4s
to 3d?
Based on energy, it’s
easier to fill the “s”
orbital on 4
th
energy
level then the
complicated “d” on
the 3
rd
.
Look further up the
fill chart and you’ll see
more of this.

n=7
n=6
n=5
n=4
n=3
n=2
n=1
Order that Orbitals Fill Up…
don’t memorize!!!
7p
6
6d
10
5f
14
7s
2
6p
6
5d
10
4f
14
6s
2
5p
6
4d
10
5s
2
4p
6
3d
10
4s
2
3p
6
3s
2
2p
6
2s
2
1s
2
•You will
learn to use
your periodic
table to
figure this
out!

Writing Electron
Configurations

Orbital Notation & Electron
Configuration Notation
Ex: E. Config. for Fluorine (9 electrons)
1s
2
2s
2
2p
6
Write the order that they fill the electrons

More Practice (a harder one)
Ex: Titanium (22 electrons)
E. Configuration Notation
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
2
Remember the fill order,
4s before 3d!

Using the Periodic Table for E. Config.
S Block
d Block (n-1)
(Energy level -1)
p Block
f block (n-2)
Noble
Gases
(last
column)

E. Configuration with Periodic Table

Practice Writing E. Configs.
Carbon
Magnesium
Iron
Iodine
Challenging:
Gold
Even more
challenging:
Plutonium
1s
2
2s
2
2p
2
1s
2
2s
2
2p
6
3s
2
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
6
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
6
5s
2
4d
10
5p
5
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
6
5s
2
4d
10
5p
6
6s
2
4f
14
5d
9
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3d
10
4p
6
5s
2
4d
10
5p
6
6s
2
4f
14
5d
10
6p
6
7s
2
6d
1
5f
5

Noble Gas Notation- the “short cut”
For Na
E. config: 1s
2
2s
2
2p
6
3s
1
Noble Gas Not.: [Ne] 3s
1
For Cl
E. config: 1s
2
2s
2
2p
6
3s
1
Noble Gas Not.: [Ne] 3s
2
3p
5
*Start at the noble gas ABOVE the element and
do the configuration from there.

Noble Gas Notations through the D
and F Blocks
Noble Gas Notation for Br
[Ar] 4s
2
3d
10
4p
5
Remember d block is n-1
(row 4 -1 =3)
Noble Gas Notation for Pb
[Xe] 6s
2
4f
14
5d
10
6p
2
Remember f block is n-2 (row
6 -2 =4)

Using Noble Gas Notation
The noble gas notation can tell you the identity
of an element
[He]2s
2
2p
2
Period #
block
Element identity = Carbon
Which element in
the block

Decoding Noble Gas Notation
Period Block Group Identity
[Ne]3s
2
[Ar]4s
2
3d
8
[Xe]6s
2
4f
14
5d
10
6p
2
3
4
6
S
d
p
2
10
14
Mg
Ni
Pb

Electrons

History Behind Electron
Configuration
Certain elements emit distinct, visible light
when heated in a flame. But why?
Copper
Strontium

What is light?
It’s a form of energy
The Electromagnetic Spectrum (see image)
shows other types of energy in our environment.
Violet Light =
highest frequency
Red Light =
lowest frequency
Visible light makes up only a small portion of the spectrum

Spectroscopic Analysis
When white light is scattered through a
prism (or spectroscope), all of the colors of
the visual spectrum can be seen.
This is seen as a “continuous spectrum” (without
breaks)

Electron Transitions
Electrons can “jump” to
higher energy levels when
atoms are exposed to an
energy source
This is known as the
“excited state”
When the electrons fall
back down, they release
that energy in the form of
light
This is known as the
“ground state”
Energy
Light

Energy Transitions for Hydrogen
Shows how the 1 electron in
hydrogen can go up and
down in different energy
levels.

Emission Spectroscopy
Because atoms have different numbers of
electrons, different types of atoms emit
specific wavelengths and have a different
pattern of spectral lines
This is the “line-emission spectrum”

Spectroscopy
Argon Hydrogen
Elements have a
unique set of spectral
lines that allows us to
identify them
This is how we
know the sun
contains H and He,
even though we’ve
never been there.

Valence Electrons, Octet
Rule, and Ions

These 3 atoms have similar reactivity and chemical
behavior.
A) where are they located on the periodic table?
B. What do you think might be responsible for their
similar properties?

Valence Electrons
Valence Electrons -
electrons in the outer-
most energy level
These are the electrons
that interact with other
atoms
They determine an atom’s
chemical reactivity

Valence Electrons & E. Congfig.
In the electron configuration,
the valence electrons are
found in the s & p orbitals of
the highest energy level.
Examples:
Cl: [Ne]3s
2
3p
5
Has 7 valence electrons
Fe: [Ar]4s
2
3d
6
Has 2 valence electons
Sn: [Kr]5s
2
4d
10
5p
2
Has 4 valence electrons
1s
2
2s
2
2p
6
3s
2
3p
7
Chlorine

Valence Electrons and the
Periodic Table

Figuring out # of Valence Electrons
Using the Periodic Table (Short Cut)
The column that they are in is the number of valence
electrons an atom has.
(EXCEPTION: This does not work for the D Block)
2 Valence Electrons

The Octet Rule
Atoms tend to gain, lose,
or share electrons to
“fill” their valence shell.
Exceptions: H & He
abide by the “duet” rule.
They only need 2
electrons in their valence
shell because the 1
st
energy level only holds 2
electrons

Ions
Ions are charged particles or
atoms that have gained or lost
electrons to “fill” their octet.
Anions have a negative charge.
They have gained electrons &
electrons are negative.
They have more electrons than
protons.
Cations have a positive charge.
They have lost electrons.
They have more protons than
electrons

Ion Examples
Potassium has 1 valence
electron, what ion will it
form?
It will lose 1 electron and
form the ion K
+
Sulfur has 6 valence
electrons, what ion will it
form?
It will gain 2 electrons
and form S
2-

Joke

joke
Two atoms walk into a bar.
One atom stops and says to the other,
"I think I just lost an electron."
The second atom asks "Are you sure?"
The first atom replies, "I'm positive!"

Organization of the Periodic
Table

Characteristics of the Periodic
Table
Elements are arranged in order of
increasing atomic number
Elements with similar properties appear
at regular intervals (“periods” or rows)
Elements with similar properties fall in
the same column (“group” or “family”)

Periods (rows)
Families/ groups

Organization of the Periodic
Table
Metals – excellent conductors of heat & electricity
Alkali metals – Group 1
Alkaline-earth metals – Group 2
Transition metals – Groups 3-12
Metalloids – properties of metals & non-metals
(along zigzag)
Non-Metals- poor conductors of heat &
electricity. Usually brittle solids or gases.
Halogens – Group 17
Noble gases – Group 18
Other solid non-metals – above metalloids

Alkali Metals
Group 1 of the Periodic Table
All have 1 valence electron
Highly reactive (with water)
Silvery in appearance
Soft enough to be cut with a knife
e-

Group 2 of the Periodic Table
All have 2 valence electrons
Harder, denser, stronger the alkali
metals
Also reactive, but not as much as
alkali metals
e-
e-
e-
e-
Alkaline-Earth Metals

Groups 3-12 of the periodic table
All have 2 valence electrons
Transition Metals
e-
e-
e-
e-

Group 17 of the Periodic Table
All have 7 valence electrons
Despite chemical similarities, some
are solids, liquids, and gases
Most reactive non-metals
React with metals to make salts
Halogens
e-
e-
e-
e-

Group 18 of the Periodic Table
All have 8 valence electrons, a
complete octet
Total lack of reactivity, inert
“too
noble
to react with anyone
else”
Noble Gases
e-
e-
e-
e-

PERIODIC TRENDS

Periodic Trends
Characteristics of elements are predictable based
on their location on the Periodic Table.
These characteristics are dependent on the
structure of the atom and the location of its
electrons.
Periodic Trends include:
Reactivity
Atomic radius (size)
Ionization energy
Electronegativity

Atomic Radii
Size of the atom –
measured using half the
distance between the
nuclei of two identical
atoms bonded together
Trend – decreases across
a period, increases down
a group
•Largest atom = Francium
•Smallest atoms = Helium

Atomic Radii
As you move across a period,
you are adding electrons to the
same energy level, but also
adding more protons
These protons attract the
electron cloud closer,
decreasing the atomic
radius.
As you move down a
group, you add energy
levels.
Each energy level is farther
away from the nucleus,
increasing the atomic radius.

Ionization Energy (IE)
The energy required to remove one
electron from a neutral atom
Trend – increases across a period,
decreases down a group
Ex: Noble Gases require a ton of energy to lose an
electron because they are “happy” with their full
shells
Ex: Alkali Metals have low ionization energies
because they want to lose their outer electron.

Ionization Energy
Increase
Decrease

Electronegativity
The ability of an atom to attract
electrons (how “greedy” it is)
Trend – increase across a period,
decrease down a group
Ex: Flourine really wants another electron
to get to the octet rule so it has a very high
electronegativity. Anything close to
Fluorine will have a high electronegativity

Electronegativity
Increase
Decrease

Heavy metal (joke)
thinkgeek.com

Mental_Floss magazine
