
Trinity University
Digital Commons @ Trinity
,"#/01,"',% 5#0'%,-+.*#1#-**#!1'-, ,"#/01,"',% 5#0'%,
2++#/
Investigating Atoms and the Periodic Table
Yvee Muniz
53#8#+2,'6%+'*!-+
-**-41&'0,"""'1'-,*4-/)01 &8.0"'%'1*!-++-,01/','15#"2#"2!2,"#/01,"',%0
7'0,01/2!1'-,*1#/'*'0 /-2%&11-5-2$-/$/##,"-.#,!!#00 51&#,"#/01,"',% 5#0'%,1'%'1*-++-,0/','15-/+-/#
',$-/+1'-, -211&'02,'#.*#0#!-,1!11&-/0 53#8#+2,'6%+'*!-+-/',$-/+1'-, -211�#/'#0',!*2"',%.#/+'00'-,0.*#0#
!-,1!11&#"+','01/1-/ (!-01,61/','15#"2
#.-0'1-/5'11'-,
2,'63#8#,3#01'%1',%1-+0,"1&##/'-"'! *# Understanding by Design: Complete Collection
&8.0"'%'1*!-++-,01/','15#"2#"2!2,"#/01,"',%0

Investigating Atoms and the Periodic Table
Stage 1 – Desired Results
Established Goals
8.5 (A) describe the structure of
atoms, including the masses,
electrical charges, and locations,
of protons and neutrons in the
nucleus and electrons in the
electron cloud;
8.5 (B) identify that protons
determine an element's identity
and valence electrons determine
its chemical properties, including
reactivity;
8.5 C interpret the arrangement
of the Periodic Table, including
groups and periods, to explain
how properties are used to
classify elements
6.6(A) compare metals,
nonmetals, and metalloids using
physical properties such as luster
conductivity, or malleability
Transfer
Students will independently use their learning to…
• Analyze patterns in order to infer and make predictions
• Recognize how to use resources and tools to solve a problem or locate an
answer
• Construct a concise and intriguing explanation for a phenomena
Meaning
Understandings
• The atomic structure of an
element determines the
properties of the element and
determines how the element
interacts with other elements.
• The periodic table has
recurring patterns that are
seen in the properties of
elements
• Scientists identify patterns in
order to make sense of
otherwise chaotic information
Essential Questions
• How can we use patterns and
properties to make sense of what we
cannot see?
• Why do we seek structure out of
chaos?
• How small can we go? What is the
value in deconstructing the makeup
of our world?
Acquisition
Knowledge
Students will know…
• All matter is made up of atoms
• There are 3 subatomic particles
that make up an atom: proton,
neutron, and electron
• Subatomic particles can be
distinguished based on mass,
charge, and location within the
atom
• Protons and electrons make up
the charge of an atom
• Protons and neutrons make up
the mass of an atom
• Protons determine an
element’s identity
• Valence electrons determine
how reactive an element will
be
• Valence electrons are located
on the outermost energy level
• Elements in the same group on
the Periodic Table have the
same number of valence
Skills
Students will be able to…
• Identify and calculate the number of
protons, neutrons, and electrons
given an element square
• Draw a Bohr model of the atom
• Identify an element as metal,
nonmetal, or metalloid based on its
location on the periodic table
• Predict physical and chemical
properties of an element based on its
location on the periodic table

electrons and will therefore
react the same
• Elements in the same period
on the Periodic Table have the
same number of electron shells
• Metals, nonmetals, and
metalloids have distinguishing
physical properties such as
luster, malleability, etc…
• The periodic table is organized
by increasing atomic number
Stage 2 – Evidence
CODE
(M or T)
Evaluative
Criteria
(for rubric)
T , M
A
Rubric
Menu
Performance Task(s)
Students will demonstrate meaning-making and transfer by…
Textbook Page
Students will be creating an engaging “textbook” page that both illustrates
and explains atoms, the periodic table, and why people should care about
the periodic table. Students will work in groups of no more than 3 to create
an engaging “textbook” page on poster paper. Students will begin the
performance assessment by engaging in a constructive analysis of how a
textbook describes elements and the periodic table versus how author
Theodore Gray describes the different elements found on the periodic table
in his book The Elements: A Visual Exploration of Every Known Atom in the
Universe. This will inform the student’s decision on a template for their page
and also encourage them to consider how to portray information to others.
Students will then present their product through a gallery walk.
Atoms Color Board Assignment
Students will have the opportunity to select from a menu the assignments
they wish to complete in order to demonstrate their understanding of the
content. The menu is a suite of performance tasks based on the 8 multiple
intelligences. Students will select one performance task from the reading
section, one from the Thinking section, and another from the vocabulary
section.
--------------------------------------------------------------------------------------------------
Other Evidence (e.g., formative)
• DUGI’s (Did U Get It?) Exit Tickets
• Quick Write Prompts
Stage 3 – Learning Plan
CODE
(A, M, T)
Pre-Assessment
Students will complete the What Do I Know About Atoms and Periodic Table mini-quiz. This consists of a list of
statements that the students have to decide individually if they agree or disagree with on their own. Then they will
discuss with their group. I will call on students to share any thoughts on the statements and upcoming unit.
Students will return to these statements at the end of the unit.
• WDIKA Atoms and Periodic Table

A
Learning Activities
Lesson 1: New American Lecture on Parts of an Atom
• Students will watch/listen to the video “Powers
of 10, then they will write down and discuss
observations about 3 different atom models.
Students will then listen to an interactive lecture
on the atom and its subatomic particles
Progress Monitoring
Student’s responses to questions
asked during the lecture.
T, A
Lesson 2: Positive ID Mystery!
• Students will be tasked to solve a mystery: Some
poor people died from an accidental ingestion of
elements! The students will be tasked with
figuring out which element each person ingested.
They will be presented with the “bodies” of the
people that have a major clue on them (plus signs
which represent protons). This lesson will
reiterate that protons identify elements and that
atomic number on the periodic table is the
number of protons.
• Positive ID
• Positive ID men
Quick write prompt: Why is the
proton the most important
subatomic particle in the atom
T, A
Lesson 3: Petri Dish Atoms (2 days)
• Students will observe models of atoms (different
types of beans in a petri dish) and identify their
numbers of protons, neutrons, and electrons.
Students will use data collected from previous
lesson and identify the atomic number, mass
number, and element name. They will use their
data to begin drawing Bohr models of atoms
themselves
• Petri Dish Atoms Data Collection
• Petri Dish Atoms Practice
Petri Dish conclusion questions
and Bohr model drawings will be
checked for accuracy
T, A
Lesson 4: Counting and Atom Building
• Students will learn to use the periodic table to
identify numbers of protons, neutrons, electrons.
Now they can build atoms and identify valence
electrons.
• Counting Notes and Practice
DUGI/Exit ticket (3-5 STAAR like
questions)
A
Lesson 5: Magnetic Atoms
• Students will use magnetic atom boards to create
atom models and compare/contrast them
• Worksheet
Quick write prompt: Explain how
you find the numbers of protons,
electrons and neutrons using a
periodic table.
A
Lesson 6: Atoms Model Day Scavenger Hunt
• There will be various atom models scattered
throughout the room, students are given clues
and must identify the atom model correctly
• Scavenger Hunt
• DUGI
DUGI/Exit ticket (3-5 STAAR like
questions)
A
Lesson 7: Color Board Performance Task (2-3 days)
• Assignment will be explained and students will
choose which 3 assignments from each category
(Reading, Thinking, Vocabulary) they wish to
complete. They will gather the materials they will
Check in with students on their
progress and understanding.
Students will submit their 3
assignments which will be

need and begin working. They will have 2-3 whole
class period work days.
• Atoms Color Board Materials
checked for accuracy and
completion.
T, A
Lesson 8: Concept Attainment (The Strategic Teacher)
• Students will be provided with yes and no
examples of atoms that belong to the same group
and then atoms that belong to the same period.
They will identify what all the yes examples have
in common and the critical attributes they have.
Exit Ticket: Students will be
presented with various atoms
and determine which atoms go
in the same group and which go
in the same period
T, M, A
Lesson 9: Periods and Groups on the Periodic Table Notes
(2 days)
• Students will draw in electron configurations for
the first 20 elements on the periodic table. This
periodic table will serve as notes in their
interactive notebook. This will reiterate what we
learned previously about groups and periods but
now they will see the pattern on a full size
periodic table. Students will identify patterns of
VE from their drawings. Students will take notes
on group names, group number, number of
valence electrons per group, and level of
reactivity. They will color code their notes.
Quick write prompt:
Identify a group on the PT and
explain how each element fits in
to the group
T, M, A
Lesson 10: Reactivity Lab
• Students will complete a reactivity lab in which
they test 6 elements in water, hydrochloric acid
and copper chloride. They are looking for
examples of reactivity
• Reactivity Lab
Lab conclusion questions
T, A
Lesson 11: Metaphorical Expression (The Strategic
Teacher)
• Students will learn the difference between what
makes a reactive atom and a nonreactive atom. I
will present them with a metaphor I came up with
to remember the difference. (Reactive atoms are
“hangry” and nonreactive atoms are billionaires)
Students will then be tasked to make up a
metaphor that will help them remember the
difference. They may work with a partner or
alone.
• Graphic Organizer
• Powerpoint
Students will create a metaphor
for reactive and nonreactive
atoms. This will be checked for
accuracy and creativity.
A
Lesson 12: Metal, Nonmetal, Metalloids Review
• Students will create a mini periodic table in their
interactive notebook where they shade in where
you find metals, nonmetals, and metalloids on the
periodic table. They will then construct a foldable
in their notebook going over the 5 main physical
properties.
• Mini Periodic Table
• Foldable Outside
• Foldable Inside
DUGI/Exit ticket (3-5 STAAR like
questions)

A
Lesson 13:Practice with Metals, Nonmetals, and
Metalloids Sort
• Students will be tasked with sorting physical
properties as either that of metals, nonmetals,
and metalloids
• Metals, Nonmetals, Metalloid Chart
Students charts will be checked
for accuracy
T, M, A
Lesson 14: Practice Using the Periodic Table
• Complete independent practice utilizing the
periodic table to identify location on the periodic
table, relationships with other elements, and
properties of elements. I will do a think-aloud,
then we will do a few as a class, and then
students will complete the rest independently
• Independent Practice
Students will turn in their
worksheet which will be checked
for accuracy.
T
Lesson 15: Pattern Maker Lesson (The Strategic Teacher)
• Students will identify the key parts of what makes
a good and interesting explanation by comparing
their textbook’s writing style to the writing style
of Theodore Gray’s book The Elements: A Visual
Exploration of Every Known Atom in the Universe
• Pattern Maker Graphic Organizer
Think-Pair-Share discussion on
what makes for a good
explanation
T, M, A
Lesson 16: Performance Assessment - Workday I
• Students will make a rough draft of what they
want their final product to look like
• Textbook Page Instructions
• Rubric
Rough draft of textbook page
T, M, A
Lesson 17: Performance Assessment - Workday II
• Students will create their textbook page on poster
paper
Final textbook page
T, M, A
Lesson 18: Performance Assessment - Gallery Walk
• At the start of class students will hang their poster
up and the students will walk around, observe,
and provide feedback on each other’s work.
Students will reflect on what they have learned in
this unit and return to the What Do I Know About
Atoms and Periodic Table statements they were
presented with at the start of this unit.
• Feedback Forms
Students will return to the What
Do I Know About Atoms and
Periodic Table Statements and
reflect on their new level of
understanding the content.
Resources and Materials
*Lesson materials in this unit made in collaboration with Brandy Bagnall and Jennifer Alford at Corbett Jr High

What do I know about
NOW
LATER
Agree Disagree
1. Different elements are composed of the same kind of atoms
Agree Disagree
A D
2. It is possible to determine the EXACT location of electrons at any given
time
A D
A D
3. Almost all of the mass of an atom is found in the center
A D
A D
4. An atom’s volume is mostly the empty space of where the electrons fly
around.
A D
A D
5. All atoms are composed of only protons, neutrons, and electrons
A D
A D
6. Neutral atoms have the same number of protons and electrons
A D
A D
7. The protons determine the identity of the element
A D
What do I know about my
NOW
LATER
Agree Disagree
1. Most elements are considered nonmetals
Agree Disagree
A D
2. Elements in the same period (going across) have similar properties
A D
A D
3. Elements in the same group (going down) have the same number of
valence electrons
A D
A D
4. Valence electrons are located on the outermost energy level
A D
A D
5. Reactivity of an element depends on its number of valence electrons
A D
A D
6. You can predict properties of an element just by knowing where an
element is on the periodic table
A D
A D
7. The periodic table is organized by increasing atomic number
A D
A D
8. The element above or below oxygen is more similar than the element to
the right or left of oxygen
A D
A D
9. Conductivity is a chemical property
A D
A D
10. Reactivity is a chemical property
A D

Name: ________________________________ Period: ________
Part 1: Textbook
Read and analyze the page from your textbook. Then consider the following questions.
1. Was it easy to read? Was it interesting?
2. Where is there room for improvement?
Part 2: The Elements: A Visual Exploration of Every Known Atom in the Universe
Question
DESCRIBE what the author does
PROVIDE specific examples
How does the author grab the
reader’s attention?
How does the author use
language to make their points
quickly?
How does the author use
pictures to make their message
more appealing?
Name: ________________________________ Period: ________
Part 1: Textbook
Read and analyze the page from your textbook. Then consider the following questions.
3. Was it easy to read? Was it interesting?
4. Where is there room for improvement?
Part 2: The Elements: A Visual Exploration of Every Known Atom in the Universe
Question
DESCRIBE what the author does
PROVIDE specific examples
How does the author grab the
reader’s attention?
How does the author use
language to make their points
quickly?
How does the author use
pictures to make their message
more appealing?
Name: ________________________________
Textbook Page Instructions
Background: Yesterday we looked at how a textbook describes atoms and the periodic table versus how the book
“The Elements: A Visual Exploration of Every Known Atom in the Universe” describes atoms found on the periodic table.
As a class we decided on the most important information to be included on our textbook page. Now it is your turn
to teach others in the class what you know about atoms and the periodic table!
The Project: You will be creating an engaging “textbook” page that both illustrates and explains atoms, the periodic
table, and why people should care about the periodic table. You will work with your group to create an engaging
“textbook” page on poster paper. You will have 2 days to complete your textbook page. Before the end of Day 1 you
must show me a rough draft of your textbook page. Day 2 will be the day you put it on the big poster paper. It must
contain the list of things below. You may also refer to the rubric on the back of this paper for more specifics.
Checklist for a Complete Project:
Explanation of how the periodic table is organized (Minimum 1 paragraph)
Relevant and engaging hook to your explanation
Concluding statement on why the periodic table matters in the real world
Sketch of the periodic table with groups and periods labelled
Draw Bohr models of at least 2 elements in the same group
Draw Bohr models of at least 2 elements in the same period
I Worked Today! – I will check your work before you leave class.
Day 1
Day 2
Due at the end of class
Teacher’s Approval: _______
Rough Draft of textbook page that includes
everything in the checklist
Something I’m proud of doing today:
__________________________________________
Something I need to improve on:
__________________________________________
Teacher’s Approval: ______
Final product was turned in and includes everything
listed on the checklist
Something I’m proud of doing today:
__________________________________________
Something I need to improve on:
__________________________________________
Presentation: You are going to hang your poster up and the class will engage in a gallery walk around the room to
look at everyone’s poster and provide some feedback on each other’s work.
TURN OVER FOR RUBRIC!

I can...
Novice
Proficient
Artisan
Start my
explanation with a
hook.
_____ / 5 points
My hook is too long, not relevant,
or not engaging.
I start with a hook that is short,
relevant, and engaging.
My hook is very well done and
relevant to our lives as middle
school students.
Illustrate the
periodic table.
_____ / 5 points
My illustration is small or messy.
My illustration is not accurate.
My illustration is not labeled.
My illustration is large, neat, and
legible.
My illustration is accurate to the
number of rows and columns.
My illustration labels periods and
groups.
My illustration is professional-
level.
My illustration is exceptionally
accurate.
My illustration is easy to read with
a quick glance.
Illustrate atoms in
the same period.
_____ / 10 points
My reduced Bohr models are
messy, not labeled, or hard to
read.
My reduced Bohr models are
clean, labeled, and easy to read.
My reduced Bohr models have
perfect circles and are very easy to
compare.
Illustrate atoms in
the same group.
_____ / 10 points
My reduced Bohr models are
messy, not labeled, or hard to
read.
My reduced Bohr models are
clean, labeled, and easy to read.
My reduced Bohr models have
perfect circles and are very easy to
compare.
Explain how the
periodic table is
organized.
_____ / 60 points
My explanation is missing
vocabulary or does not use the
vocabulary to explain how the
periodic table is organized.
My explanation correctly uses
atomic number, period, group,
energy level, and valence
electron to explain how the
periodic table is organized.
My explanation shows I know all
the vocabulary well, gets to the
point quickly, and is easy for even
younger students to understand.
Explain why people
should care about
the periodic table.
_____ / 10 points
My explanation is shallow, not
convincing, or missing this part.
I end my explanation by showing
the reader why the periodic table
matters in the real world.
My real-world connection would
make Theodore Gray proud to
have me as a co-author for his
next book.
Team Member
What did I do?
Be detailed!

Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …
Creator of the Poster: _____________________________
I loved that …
Next time you might consider …

Atoms Color Board
You must have at least two different colors!
See individual paper for more specific instructions
This is a test grade
Reading worth 25 points, Thinking worth 50 points, Vocabulary
worth 25 points
Reading
GREEN-R: Read the article “shiny
diamonds may make screens
stronger on smartphones” and
answer the attached questions
BLUE-R: Read the article “If
diamonds are forever, maybe they
can help protect smartphones” and
answer the attached questions
PINK-R: Analyze the given data
and do a smidge of research
Thinking
GREEN-T: Draw a labeled model of
the atom Beryllium, compare that
to an atom with 9 protons, 8
electrons, and 10 neutrons
BLUE-T: Form a performance
group. Design models or a
production about atoms. Make a
video or perform in front of the
teacher or class.
PINK-T (GT): How does the small
word of atoms and atomic
theory interact with CERN’s
Large Hadron Collider in
Switzerland?
Vocabulary
GREEN-V: Complete 3 acrostic
poems: one for proton, one for
neutron, and one for electron
BLUE-V: Complete two papers “I
Am” and a “Thinking Triangle”
PINK-V : Create a poem, rap,
song, or story about atoms. You
must include every atoms
vocabulary word listed.

Name: _______________________________________ Period: _____ DUE: ____________________
GREEN-Vocabulary: Acrostic Poems
Using the words “PROTON” “NEUTRON” and “ELECTRON,” create 3 acrostic poems. For an acrostic poem, you write a
statement relating to the entire word that stars with each letter. Statements must be written with your best handwriting
using correct grammar (capitalization and punctuation) and spelling. You also need to draw a related picture under each
word. Your science teachers did an example for you ☺


Name: ______________________________________________ Period: _____ Due______________
BLUE-Vocabulary: I Am and Thinking Triangle
Part I: Complete the I Am using any atoms vocabulary word you choose. You are trying to get the reader to guess your
vocabulary word without telling them what it is.
My Word: ________________________________________________

Part II: Complete the Thinking Triangle.
Each line of the triangle is also how many words should be in each line; for example, on line 5 you should use 5 words.
Line 1 is the subject: atoms Lines 2, 3, and 4 may be phrases Lines 5, 6, 7, and 8 must be complete sentences
Regardless if you are using phrases or complete sentences, each line must make sense as a whole. This means you cannot
just list words!

Name: ______________________________________________ Period: _____ Due______________
PINK-Vocabulary: Poet, Author, Rapper
Think about all we have learned about atoms. Create a poem, story, rap or song filled with facts or memories about
atoms. Regardless of your chosen piece, you must include the following: (proton, neutron, electron, nucleus, electron
cloud, identity, atomic number, atomic mass, atom). All works must be original (no cutting/pasting, copying, or
plagiarizing). Works may be handwritten (best handwriting!) or typed.
Important note: make sure as you are letting your words flow you also slide in some characteristics/definitions of each of
the above words. You may use more paper than this, below is just starter paper.
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
___________________________________________________________________________________________________
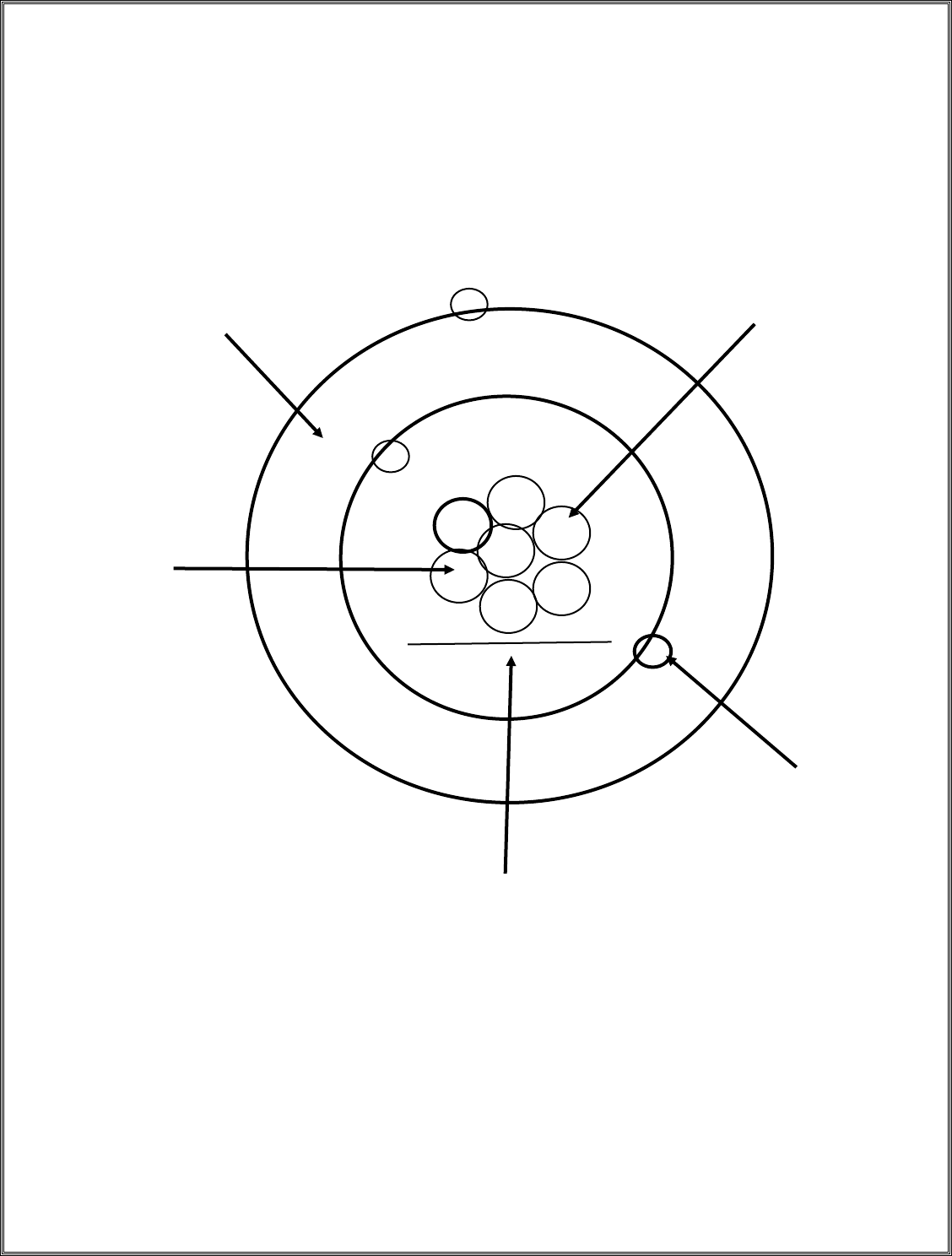
Name: _______________________________________ Period: _____ DUE: ____________________
GREEN-Thinking: Models – Teacher Approval Required
Part I: Interpreting a Model
Label the parts of the model below then answer the questions
1) What is the atomic number? ________________
2) What is the mass of the atom? ______________
3) What is the charge of the atom? _____________
4) What is the identity (name) of the atom? _________________________
5) What part of the atom contains ALL the mass? _______________________________
(more on back)
-
+
+
+
-
-

Part 2: Creating a Model
Your turn! Draw a model of the element Beryllium (Be); you will need a periodic table. Make sure it is neat (round circles,
straight lines, etc…). Make sure the correct subatomic particles are in the correct places. Extra credit if you design and
build your own model!
Pre-drawing questions
1) How many protons does Beryllium have? __________________
2) How many electrons does Beryllium have? _________________
3) How many neutrons does Beryllium have? _________________
Explain how you figured that out _________________________________________________________________
4) What subatomic particle tells us this is Beryllium? ___________________________
Name: __________________________________________________ Period: ___________ Due________
GREEN-Thinking: Comparing and Contrasting Atom Models
Compare and contrast two different atom models.
When drawing make sure make sure it is neat (round circles, straight lines, etc…) with 3 different colors and a key. Make
sure the correct subatomic particles are in the correct places with the correct numbers and charges. Extra credit if you
design and build a 3
rd
model of your own choice (no Hydrogen or Helium)
Model A: Draw a model of the atom Beryllium (Be)
Model B: Draw a model of an atom with 9 protons, 8 electrons and 10 neutrons

Compare and contrast Model A and Model B from the front. This means there are things that the models have that are
different and some things that are the same. Fill out the Venn Diagram; remember similar items go in the common space
in the middle. Make sure to use your best handwriting AND your vocabulary words (mass number, atomic number, etc).
Helpful hint: be specific (see the example below), also don’t forget about identity and charge! There is 1 thing these
atoms have in common (hint: look on the outside)
Finally, what would happen if subatomic particles were gained or lost? You can discuss in general terms or you may use
Model A or Model B to help explain your answer.
Remember: Use your best handwriting and write in complete sentences with correct spelling and proper grammar
(capitalization and punctuation).
__________________________________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
Model A
Model B
Mass number = ___
Mass number = ___

Name: _________________________________________________ Period: ___________ Due______________
BLUE-Thinking: Performance Group
Part I: What Am I Doing?
• Form a performance group to complete an interactive atom teaching
Part II: What Do We Have to Have?
• Explain locations and properties of subatomic particles
• What happens when particles are gained or lost
• Build or act out two different atom models (not Hydrogen and/or Helium)
• Compare and contrast atom models (similarities and differences)
Part III: Product Options (choose 1)
• Build models (human or 3D) and explain your components to the class
• Write a play and perform for the class or teacher
• Make a video and show it to the class or teacher
Group Maintenance
• Create a group name and list all members and their roles.
• Teacher approval is required for groups over 4 members, no group may exceed 6 members
• Teacher approval is required to work with students in other class periods.
Group Name: ___________________________________
Members Role
_______________________________________________ ________________________________________
_______________________________________________ ________________________________________
_______________________________________________ ________________________________________
_______________________________________________ ________________________________________
_______________________________________________ ________________________________________
_______________________________________________ ________________________________________
We will be developing…
_____ Class Instruction
_____ Play
_____ Video

Name: _______________________________________ Period: _____ DUE: ____________________
PINK- Thinking: CERN’s Hadron Collider and the Atom
Question: How does the small world of atoms and atomic theory interact with CERN’s Large Hadron Collider?
Answer: You find it!
Part 1: Background
1) Research CERN: where are they based, what is their mission, what are their areas of study?
2) CERN Experiments: what are some past and current experiments they are working on, what was learned, where do they
conduct their experiments
3) Where do you want to focus your study? You have 2 options, choose 1
Option A: What is 1 experiment you are interested in: why is it important, how is the experiment run, what have been the
results, what are the missing pieces, if you were one of these scientists what other experiments would you want to
completed based on the results
Option B: What are some ethical concerns of particle research, atomic theory, nuclear power/energy? Select 1 or 2 and
lay it out: what are the pros, what are the cons, what do you personally think????
Part 2: Product
Now you must share this information with the world (or at least your teacher)! Design a product to showcase/highlight
your findings. You can write a research paper, design a PowerPoint, make a poster, make a collage, make a model, make
a video, make a diorama, use Adobe Spark, use Canva, design an infographic; it is completely up to you!

Name: _______________________________________ Period: _____ DUE: ____________________
GREEN- Reading: Shiny Diamonds May Make Screens Stronger on Smartphones
Before Reading: What do you think when you hear the word "diamond"? Write down all of the words that come to
mind. Draw a picture of what you see when you hear this word.
During Reading: Highlight facts you already know about diamonds in blue. Highlight new or unknown information about
diamonds in yellow.
After Reading: Take the quiz below
1A. Read the paragraph from the section "Some People Wonder If It Will Work."
Diamonds are not unbreakable, says Jim Butler. He worked in a lab for the U.S. Navy. Diamonds can crack under
enough force.
What does the author mean by saying diamonds are "not unbreakable"?
(A) Diamonds never break. (B) Diamonds can break.
(C) Diamonds are perfect. (D) Diamonds get scratched.
2A. Read the paragraph from the section "Screens Must Be Made Carefully."
As diamonds are made, lots of energy is created. Energy makes heat. A diamond is very good at deflecting heat.
It can handle up to 2,200 degrees Fahrenheit of heat.
Which word could replace "deflecting" in the paragraph above WITHOUT changing its meaning?
(A) blocking (B) moving (C) changing (D) losing
3A. Read the paragraph from the section "Some People Wonder If It Will Work."
A study came out recently. It said that 3 out of every 10 U.S. smartphone users have cracked screens.
Many people keep using those screens anyway.
What information do you get from this paragraph?
(A) how many people crack the screens on their smartphones (B) what causes smartphone screens to crack
(C) how smartphones are still able to work with cracked screens (D) what date the study was published on
4A. Which section, in the introduction, gives information about what diamonds are commonly used for? __________
5A. What is the main idea of the article?
(A) A great number of people crack their cell phone screens
(B) Diamonds may be able to help solve the problem of cracked screens
(C) Diamonds are very strong, but they still can crack
(D) It requires a tremendous amount of heat to make a diamond

Name: _______________________________________ Period: _____ DUE: ____________________
BLUE – Reading: Diamonds are Forever, Maybe They Can Help Protect Smartphones
Before Reading: What do you think when you hear the word "diamond"? Write down all of the words that come to
mind. Draw a picture of what you see when you hear this word.
During Reading: Highlight facts you already know about diamonds in blue. Highlight new or unknown information about
diamonds in yellow.
After Reading: Take the quiz below
1B. Read the selection from the section "Tricky Problem Must Be Solved."
The glass and its diamond coating will have different reactions, even at those temperatures. Temperatures always
change, he says. As temperatures change, this causes pressure between the glass and the diamond film above it.
Which of the following sentences uses "film" in the SAME way as the selection above?
(A) The college professor showed a historical film about World War II.
(B) The photographer could not wait to develop the roll of film she had shot.
(C) The student had to film a short video for his final project.
(D) After a very cold night, there was a thin film of frost on the grass.
2B. Read the sentence from the section "Gems Can Be Made In A Lab."
The company says it will be less likely to scratch or shatter.
Which phrase from the article helps you understand what happens when smartphones "shatter"?
(A) difficult to scratch (B) spider web-like cracks (C) change in temperature (D) doesn’t do well in heat
3B. Overall, the article is organized around:
(A) an idea and a process (B) a company and a problem
(C) a person and a product (D) a gemstone and a location
4B. Which number in the section "Gems Can Be Made In A Lab" uses problem and solution in its structure? _______
5B. What is the main idea of the article?
(A) A great number of people crack their cell phone screens
(B) Diamonds may be able to help solve the problem of cracked screens
(C) Diamonds are very strong, but they still can crack
(D) It requires a tremendous amount of heat to make a diam

Name: _____________________________________________________________ Period: ___________
PINK-Reading – Data Analysis
Look at the graph below. Make sure you read the title, x-axis label, and y-axis label. Interpret the graph and answer the
questions below. You may have to look up the following words to analyze correctly: atomic radius, atomic number,
metals, metalloids and you probably want to have a periodic table handy to find out where the elements are…
Atomic Radius versus Atomic Number for Metal and Nonmetal Elements
Questions (when necessary, use your best handwriting and write in complete sentences with correct spelling and proper
grammar (capitalization and punctuation).
1) Describe the relationship between atomic radius and atomic number.
__________________________________________________________________________________________________
__________________________________________________________________________________________________
__________________________________________________________________________________________________
2) Generally, what happens as you move down (not across) the periodic table for the elements shown?
A) Atomic radius increases B) Atomic radius decreases C) There is no relationship
3) Generally, what happens as you move across the periodic table for the elements shown?
A) Atomic radius increases B) Atomic radius decreases C) There is no relationship
4) Find the element Cesium (Cs) on the graph. What is its approximate atomic number and atomic radius?
A) 55 and 275 B) 55 and 225 C) 52 and 145 D) There is not enough information to answer
5) On the back of this paper, draw a representation of a periodic table. Draw in arrows to show the pattern of atomic
radii. Explain why this pattern exists.
