
ERNST R. BERNDT
Massachusetts Institute
of Technology and
National Bureau of
Economic Research
IAIN M. COCKBURN
University of British
Columbia
and
National Bureau of
Economic Research
ZVI GRILICHES
Harvard University
and
National Bureau of
Economic Research
Pharmaceutical
Innovations
and
Market
Dynamics:
Tracking
EJfects
on
Price
Indexes
for
Antidepressant
Drugs
THE CONSTRUCTION
AND PUBLICATION of measures
of
price
inflation are
important tasks carried
out
by governmental
statistical
agencies.
In
the
United
States
the
Department
of
Labor's
Bureau of
Labor Statistics
(BLS) publishes price
indexes
measured at
the
point of final
consumer
demand (the
consumer
price index, CPI)
and
at the initial transaction
We
gratefully
acknowledge
the
cooperation
of
officials
from the
U.S. Bureau
of
Labor
Statistics,
in
particular
Commissioner
Katharine
Abraham,
as
well
as Assistant
Commissioner John
M.
Galvin,
Irwin
Gerduk,
and
Douglas
Kanoza in the
Office
of
Industrial Prices
and
Dennis
Fixler,
Division of Price
and Index
Number Research.
We
have also benefited
from
the
timely
and able research assistance of Gillian Currie and
Mark Moore.
We
thank
Martin
Baily,
Stan
Finkelstein,
Richard
Frank,
Theodore Keeler,
Peter
Reiss,
Jack
Triplett,
and Cliff
Winston for
comments.
Research
support
from
the
National Science Foundation,
the
U.S.
Bureau
of
Economic
Analysis,
the
Alfred
P.
Sloan
Foundation,
and
Eli
Lilly
Inc.
is
gratefully
acknowledged,
as
is
the considerable
data
support
from
Stephen Chappell
and Robert Plefka at IMS
International
and from
Rhea
Mihalison, Phyllis
Rausch,
Ditas
Riad,
and Paul
Snyderman
at
Merck & Co.
The
opinions
and
conclusions
expressed
in
this
paper
are those of the authors and do
not
necessarily
reflect views
or
positions
of
any
of the
organizations
with
which the authors
are affiliated or
those of
any
of
the
research
sponsors.
133

134
Brookings Papers: Microeconomics 1996
point,
that
is, prices
received
by producers
from
whomever makes the
first purchase (the producer price index,
PPI). These price measurement
tasks are difficult ones, particularly because
new goods embody scien-
tific discoveries and technological
progress; inherent difficulties exist
in measuring the output
of
services that themselves
combine goods and
time, and dynamic structural
and
compositional changes occur in the
underlying markets
for
production,
distribution, and sale.
The
marketplace
for health
care contains all these features
and
pre-
sents
particularly
difficult
challenges
for
price
measurement.
Health
care expenditures represent
a
significant
portion
of
gross
domestic
prod-
uct
(GDP) and are likely
to become
increasingly important
as
the U.S.
population ages.
The
conceptual
foundations for
a health care-related
CPI are
clouded,
not
only
because
physicians typically
act as
agents
for
consumers, but
also because insurance
plans pay
for
many,
but not
all, health care products
and
services.
Thus,
for
example,
the
CPI
for
prescription pharmaceutical products
currently weights only cash pay-
ment transactions from drugstores and mail-order
outlets;
it
excludes
prescription drugs purchased by managed
care
plans, Medicaid,
or
other
third
parties on behalf
of an
individual.'
Here
we focus
attention
on the
measurement
of
a
health-care-related
PPI, which,
while
arguably simpler
than
a
CPI, nonetheless presents
enormous measurement
difficulties
and obstacles.2 A PPI
measures
changes
in
selling prices
that domestic
producers
receive
for
their out-
put.
It
is
frequently
used
in
deflating
current
dollar
expenditures
to
obtain a
measure
of real
output growth
by industry.
The
reliability
and
accuracy
of
PPIs
are therefore critical
to
understanding
the
substantial
growth
in
health
care
expenditures
during
the last
ten
years.
Growth
rates
in
PPIs
by industry
are also
used
to assess
inflationary pressures
and
pricing
behavior
in
the health care sectors or to
make international
comparisons.
While
the PPI is
an
output price
index for a
specific
industry, say, pharmaceuticals,
it
is also
an
input price
index for whole-
salers who in turn sell
to
retail
drugstore
chains, hospitals,
mail-order
1. For further discussion, see
Cleeton, Goepfrich,
and
Weisbrod
(1992), and
U. S.
General Accounting Office
(1996).
2. For a recent discussion
on
problems
involved in
interpreting
various
measures
of
wholesale prices such as the
average
wholesale
price (AWP, also
known
as "Ain't
What's Paid"), see Bill
Alpert, "Hooked on Drugs: Why Do Insurers Pay such Outra-
geous Prices for Pharmaceuticals?"
Barron's,
June
10, 1996, pp.
15-19.

Ernst
R.
Berndt,
Iain M.
Cockburn, and
Zvi
Griliches
135
firms, and
managed care
organizations.3
Because issues of
pharmaceu-
tical pricing and health care cost containment
are
currently
of
great
importance to
public policy
analysts, government
statisticians, con-
sumers'
groups, and
industry
officials,
it
is
particularly timely
to
audit
closely
the
accuracy
and
reliability
of
one of the BLS health
care-
related PPIs.
That is
our
purpose
in this
paper.
Although
we
focus on
the PPI, many of
the
issues we
address
are also
germane
to
concerns
cited
by
the
Advisory
Commission To
Study
the Consumer
Price
Index
in its
final
report, released
in
December 1996.
The market on which we focus
our
audit is
that for
antidepressant
prescription
pharmaceuticals
sold between
January 1980
and
February
1996. We have
chosen
this market
segment
and
time
period
for
several
reasons,
all
relating
to the
high likelihood
of
there
being substantial
challenges
here
in
tracking price
changes.'
First,
several
very
successful new
products
have been
introduced
in
the
antidepressant drug class, with well-known
brand names such
as
Prozac, Zoloft,
and
Paxil
having
combined annual sales of
more than
$3
billion
in
the mid-
I
990s
.5
Eight
of the
twenty-one
currently marketed
chemical
entities
(molecules)
are new branded
products
launched
since
1988. Thus,
issues concerning
the
incorporation
of
new
goods
into
price
measurement,
as well as
adjustments
for
quality change,
could be
very
important
in
this
market
class.
Second,
not
only
has new
product
entry
been
substantial,
but
within
the
last
ten
years, seven branded
antidepressants
lost
patent
protection,
and
each
has
subsequently
faced
competition
from
lower-priced
generic
entrants.
Those
buyers
who
regard
the branded
and
generic
versions of
a chemical
entity
as more
or less
perfect
substitutes
realize a
substantial
price decline after
generic
entry. Although
the
BLS
has
been
making
changes
in its
CPI
procedures
for several
years,
until
mid-1996
its PPI
methods did not
adequately
link
generic products
to their
patented
an-
tecedents and
instead
generally
treated
generics
as
entirely
new
goods;
3. In
the United
States, the vast
majority of
pharmaceutical
manufacturer
sales are
to
wholesalers, not to
hospitals,
drugstore chains,
or
managed
care
organizations.
4. For
related
studies on issues in
the economics
of
mental
health,
see
Frank
and
Manning
(1992), and
Jonsson
and
Rosenbaum
(1993).
Keith
and Berndt
(1994)
provide
an overview of
price
measurement
issues in the
pharmaceutical
industry.
5.
Ellen Joan
Pollock, "Side
Effects: Managed
Care's Focus on
Psychiatric
Drugs
Alarms
Many Doctors,"
Wall Street
Journal, December
1, 1995,
p.
Al.

136 Brookings Papers. Microeconomics 1996
thus these older PPI methods failed to record price declines realized by
some purchasers of generic drugs.
Recently the BLS
announced that the
May 1996 pharmaceutical PPIs
would incorporate linking procedures for generic drugs that treat ge-
nerics and their branded antecedents as perfect
substitutes.
The overall
implications of this significant change are not yet clear. Our analysis
of
1980-96 data
in
the antidepressant prescription drug marketplace
provides important information
on
what BLS-measured price growth
for
antidepressants
would have been
had
these
changes
been
introduced
earlier. We also assess the
sensitivity
of
measured
aggregate price
growth to alternative linking and weighting assumptions
that the
BLS
could
have employed.
Because
we
report findings
for an entire
thera-
peutic class, namely, antidepressants,
this
research extends that of Gril-
iches and Cockburn, who provided illustrative empirical evidence con-
cerning
two
systemic
anti-infective
drugs.6
A third
reason for focusing
on
antidepressant drugs
is that
they are
but one
component
in the treatment of
depression, along
with
psycho-
therapy and medical management.
To some
extent, psychotherapy
and
antidepressant drugs
are substitutes for each
other; indeed, controversy
surrounds the extent
to which
managed
care
organizations
are substi-
tuting prescription drugs
for talk
therapy.7
The research
findings
re-
ported here compose
one element of a
larger
research effort
in
which
we
are
creating
a
price
index for the
treatment of
depression
that
incor-
porates
both
drug
and talk
therapy components.
In
this
paper
we
begin
with a
background
discussion on the
nature
of the
medical
condition called
depression
and
provide
a historical
overview on the
evolving
medical
understanding
of
psychotherapeutic
drugs
used for the treatment
of
depression.
We
then outline data sources
and describe the
changing marketplace
for
antidepressant drugs
from
1980 to 1996, particularly
new
product
introductions and
postpatent
expiration entry by generic
firms. We review BLS
procedures
for track-
ing producer prices
in
general
and
antidepressant drugs
in
particular.
We
next
consider issues
from
economic
theory
and then
present
results
6. Griliches and Cockburn (1994).
7. See, for example, Carol Hymowitz
and Ellen Joan
Pollock, "Cost-Cutting
Firms
Monitor Couch Time as Therapists
Fret," Wall Street Journal, July 13, 1995, p.
Al;
and Pollock, "Managed Care's
Focus
on
Psychiatric Drugs
Alarms
Many Doctors,"
p.
Al. For empirical evidence, see Berndt,
Frank, and McGuire (forthcoming).

Ernst R. Berndt,
Iain
M.
Cockburn,
and
Zvi Griliches
137
on alternative
procedures
for measuring
price inflation,
including those
involving hedonic
price adjustment.
Finally
we discuss
implications
of
our results
and offer suggestions
for
further
research.
Depression:
Diagnosis
and Prevalence
Whether
depressive disorders
are discrete
and distinguishable
from
'subclinical" depressive
symptoms
is
a question
clinicians
and
re-
searchers
have long debated;
it still has no definitive answer.
Almost
everyone
at some
time or another
has
experienced
melancholy
or
been
depressed
as
a mood,
affect,
or emotion.
To be human is to know about
a
variety
of emotions, including
sadness,
disappointment,
and
despond-
ency.
Many such
affective
occurrences are
within the normal
range
of
human experience.
It is only
with
greater
degrees
of
severity
or
longer
durations
that
such affective
states come
to be viewed
clinically
as
symptomatic
of depression.
The American
Psychiatric
Association has
issued
and updated
clin-
ical guidelines
for diagnosing
depression.8
The current
guidelines,
known as
DSM-IV,
list nine symptoms
of
depression:
(1) a depressed
mood; (2)
diminished
interest
or
pleasure
in most
activities; (3) signif-
icant unintentional
weight
loss or weight
gain,
or a decrease
or
increase
in
appetite;
(4) insomnia
or hypersomnia
nearly every
day; (5) psycho-
motor agitation
or
retardation
nearly
every day; (6)
fatigue
or loss of
energy
nearly every day;
(7) feelings
of worthlessness
or
excessive
or
inappropriate
guilt; (8)
diminished
ability
to think or
concentrate,
or
indecisiveness;
and (9)
recurrent thoughts
of death or suicide.
To
be
diagnosed
as having
a
major depressive
episode,
a
person
must show
at
least five of
these
symptoms
(including
either
a
depressed
mood or
diminished interest
in
most
activities)
for
two or more
weeks.9 These
symptoms
must
also
represent
a
change
from the individual's
previous
functioning.
A
chronic
but milder form
of
depression
is known
as
dysthymia
and
is diagnosed
when the patient
has a
depressed
mood that
persists
for
at
8.
See
American
Psychiatric
Association
(1968, 1980,
1987, 1993).
9.
It must also
be the
case
that
an organic
factor cannot
be established
as
initiating
and maintaining
the
disturbance
or
that the disturbance
is not a normal reaction
to
the
death of
a loved one.

138
Brookings
Papers:
Microeconomnics 1996
least two years
and has at least
two other
symptoms.
'"
Both
forms of
depression are
serious. Even
moderate
levels of
depression
significantly
impair
functioning
in work
and
school settings and in
social
situations.
"
Survey
evidence suggests that
in a
given
year, 9 percent
of
the
employed labor force
experiences
a
depressive
episode
and
that 80
percent
of
these workers are below the
age
of
45.1
Depression
is
widely
believed to be
an
underdiagnosed
condition;
patients
suffering
from
depression
often
present
themselves to clinicians
as
having
other
med-
ical symptoms
such as lower
back pain,
gastrointestinal
disorders, and
headaches.
1'
Depression is a
treatable condition;
modern
treatment suc-
cess rates
approach
80 to
90
percent.
'
Episodes
of illness
come and
go,
last from
several weeks to several
months,
and are
followed
by
periods
of
relatively
normal mood and behavior.
Untreated,
the
average
depressive episode lasts about four to
six
months.
Between 50 and 85
percent
of
patients
who seek
treatment
for
depression
will have
at
least
one
subsequent
episode
of
depression
in
their
lifetimes, usually
within
two or
three
years.'5
The
lifetime
average
for
depressive episodes is
five
to
seven,
but
as many as
forty episodes
have been
reported."6
Although
the
reasons are
still
not
fully
understood,
women
are about
twice as
likely
to
suffer
from
depression
as
are men.
17
Alternative
Drug
Treatments for
Depression
Before
discussing
alternative
drug
treatments for
depression,
we
briefly
review
several
medical terms.
A
synapse
is
the
point
of
contact
between
adjacent
neurons,
where
nerve
impulses
are transmitted from
one
to the
other. Neurotransmitters are
the
chemical
"messengers"
in
10.
A
tenth
symptom
associated
with dysthymia
is
feelings of hopelessness.
11.
See, for example,
the
studies and clinical
trial
findings referenced by Nolen-
Hoeksema (1990, p. 5).
12. For further
discussion
and
references, see
Greenberg, Stiglin, and others (1993)
and
Greenberg, Kessler,
and others
(1996, p. 328).
13. See
Eisenberg (1992)
for
discussion and references
documenting
the
somatiza-
tion
phenomenon, and Katon
and
others (1992)
for a
discussion of the underdiagnosis
of
depression.
14.
Regier and others (1988).
15.
American Psychiatric
Association
(1993, p. 11).
16.
Papolos
and
Papolos (1992, p. 7).
17. For an
extended discussion,
see
Nolen-Hoeksema
(1990).

Ernst R.
Berndt, Iain M.
Cockburn, and
Zvi
Griliches
139
the brain
that transmit signals
across synapses,
setting
in
motion com-
plex
neural interactions that
shape behaviors,
feelings, and thoughts.
Although
there are many different
neurotransmitters,
the
vast majority
of them
monoamines, three
of
particular importance
are
norepineph-
rine,
serotonin, and dopamine.
Today
it
is
known
that low levels of
these
monoamines are associated
with
depression.
Moreover, after per-
forming
their messenger
activities,
these
monoamines are
eventually
destroyed by monoamine oxidase
(MAO),
a
liver
and brain
enzyme,
through
a bodily absorption
process
called
reuptake.
In
this reuptake
phase,
however, MAO also
destroys
another amine
called
tyramine, a
molecule that affects blood
pressure.
Modern
biological
theories
of
depression
apparently emerged
from
several
chance discoveries.
Clinicians testing
the
antituberculosis
drug
iproniazid in
the early 1950s
observed
that subjects
experienced
relief
from
any depression, and some
even experienced
euphoria. Several
years
later,
this
drug
was shown to
inhibit the
MAO
enzyme."
About
the same
time, clinicians
prescribing reserpine,
a
drug commonly
used
to treat
hypertension,
noted that about
15
percent
of
patients taking
this
medication
became seriously
depressed. Subsequent research demon-
strated
that
reserpine
led
to
the
depletion
of all three of
the
important
monoamine neurotransmitters.
In
1957 isoniazid was
introduced;
it
was a
more
effective
antituber-
culosis
drug
than
iproniazid
and did
not inhibit MAO.
Although
the
manufacturer had
planned
to
cease
production
of
the less
effective
iproniazid,
the
coincident publication
of
psychiatric
research
linking
MAO inhibitors to the treatment of
depression
resulted
in
an
unexpected
surge
in
demand for
it;
in
1957
alone,
unmet
needs were
so
large
that
physicians
prescribed iproniazid
for more than
400,000 depressed pa-
tients.19
Because the MAO
enzyme
also inhibited
tyramine, however,
it was
soon discovered that
iproniazid, by
inhibiting MAO,
could
in-
directly
increase the amount
of
tyramine present
in
the
body,
sometimes
with lethal
consequences.
Excess
tyramine
can cause a sudden
increase
in
blood
pressure
so severe
it
on
occasion
hemorrhages
blood
vessels
in
the
brain and causes death.
The
potential
frequency
with which
this
fatal
response
could
occur
for
patients taking
MAO inhibitors was
quite
18. Baldessarini
(1990, pp.
414-18); Hyman,
Arana,
and
Rosenbaum
(1995, p. 82).
19.
Turkington-Kaplan (1994, p.
49).

140
Brookings Papers: Microeconomics
1996
large, for tyramine is present
in
common foods such as chicken
liver,
aged cheese, broad-bean pods,
soy sauce, and pickled
herring. For this
reason, MAO inhibitors (MAGIs)
were taken
off the
U.S.
market for a
time. Eventually modified MAOIs
were reintroduced,
in large part be-
cause some depressed patients
did not
respond
to
any
other medication.
Today the
MAOIs
are used
most often when other antidepressant
drugs
yield unsatisfactory results
and when electroconvulsive
treatment is
inappropriate
or
refused.20
Because of these complexities,
psychiatric
specialists currently
write about 90 percent
of
MAOI prescriptions;
general practitioners
or
internist physicians
write
only
a small
portion.2'
During the 1950s much
pharmaceutical research began
to
focus on
various mental illnesses.
Although initially analyzed by Swiss
research-
ers for
use as
an
antihistamine,
a tricyclic drug called
imipramine was
tentatively hypothesized to
be
successful
in
treating
schizophrenia. Re-
searchers soon found
that
although imipramine
was
relatively
ineffec-
tive
in
quieting agitated patients,
it
apparently
bestowed
remarkable
benefits upon
certain
depressed
individuals.22
Instead of
stimulating
the
central
nervous system (which
amphetamines do)
or
inhibiting
mono-
amine
oxidase reuptake (a
property
of the
MAGIs),
imipramine
in-
creased the brain's supply
of
norepinephrine
and
serotonin; remarkably,
about
70 percent
of
depressed
patients responded
to this
drug.
The
introduction of imipramine
(brand name Tofranil)
in
1958 was
soon
followed
by
market introductions
of numerous
related
tricyclic
com-
pounds.
These
compounds
include
amitriptyline (Elavil,
1961),
nor-
triptyline (Aventyl, 1963),
protriptyline (Vivactil, 1967),
trimipramine
(Surmontil, 1969),
and
doxepin (Sinequan, 1969).
The
tricyclic antidepressant
class of
drugs
has been
enormously
suc-
cessful
in
treating depression,
and
experience
with
these
drugs
has
been
extensive.
Today
it is known
that the various members
of
this
class
of
drugs
differ
in the
extent to
which
they
affect
the
three
monoamines.
Although
on
average
there
is
no
statistically significant
difference
in
efficacy
rates
among
the
various tricyclics,
often
patients
who
do
not
respond
to
one tricyclic
do respond
to another. About two-thirds of
people
find relief
with the first
tricylic they
are
prescribed.23
20. Baldessarini (1990,
p. 414);
American.
Psychiatric
Association (1993, p.
2).
21. [MS America (1993).
22. Baldessarini (1990,
p. 405).
23. Turkington and Kaplan
(1994, p. 91).

Ernst R. Berndt, Iain M. Cockburn, and Zvi Griliches
141
Not all patients can tolerate these drugs, however. Because they
affect several neurotransmitters other than serotonin, dopamine, and
norepinephrine, as well as receptors, the tricylic drugs are often asso-
ciated with
side effects. Although
the
side-effect
profiles
of the indi-
vidual tricyclic drugs differ slightly, common side effects include an-
ticholinergic effects (dry mouth, constipation, urinary hesitance,
blurred vision), weight gain,
increased heart
rate,
drowsiness
(which
may be a beneficial side effect initially for those depressed patients
experiencing insomnia),
increased heart
rate,
decreased blood
pressure,
dizziness
when
standing up,
and sexual
dysfunction;
side-effect
profiles
are
given
in
table 1.
The
tricyclics
also differ
in their
half-lives and
in
daily dosing frequency.
Patient
compliance
in
taking
medications
is of
course
negatively affected by
adverse side
effects
and
more
frequent
required daily dosing. A significant unattractive characteristic of the
tricyclic drugs is that overdoses are potentially lethal,
a factor
quite
important for depressed patients with suicidal tendencies.24
The
most
recent major therapeutic development
is
the 1988
launch
of
fluoxetine (brand
name
Prozac),
the
first of the
selective
serotonin
reuptake inhibitors (SSRIs); subsequent
SSRI introductions include ser-
traline (Zoloft, 1992), paroxetine (Paxil, 1993),
and
fluvoxamine
(Lu-
vox, 1994). In contrast to
the
MAOIs
and
tricyclics
that affect several
neurotransmitters, the SSRIs are selective
and
specific
in
that
they
inhibit the reuptake only
of
serotonin. Thus,
side effects associated
with the
reuptake
of
norepinephrine
or
dopamine
are reduced with the
SSRIs, and serotonin
levels are increased.
The
70
percent efficacy
rates
of the
SSRIs are
not
statistically significantly
different
from
the
MAOIs
and
tricyclics,
but adverse
interactions
with
other
drugs
occur less
frequently,
and
the
consequences
of overdoses
are
much less severe.25
With the
SSRIs, anticholinergic effects, drowsiness,
dizziness
when
standing up,
interaction
with the
cardiovascular
system,
and
weight
gain
side effects are
very
rare. Nausea is still a common side effect of
the
SSRIs, as are headaches, nervousness, anxiety,
and
various forms
24. American Psychiatric Association (1993, p. 9);
as the same
article notes,
how-
ever, "the vast majority
of
studies suggest
that all available
antidepressants decrease,
rather than increase, suicidal thoughts and indicate
no
predilection on the part of a
particular agent to
either ameliorate or
aggravate
suicidal tendencies." Also see
Potter,
Rudorfer, and Manji (1991, p. 636).
25. American Psychiatric Association (1993, pp.
7-10).
Also see
Potter, Rudorfer,
and Manji (1991).

Table
1.
Characteristics
of
Drugs
Prescribed
for
the
Treatment
of
Depression
Typical
Ha
lf-
Index
of
side
effects
Chemical
daily
dose
life
Daily
FDA
(0
=
rare,
4
=
common)
entity
(milligrams)
(hours)
frequencv
OCD
AC
DR
IA
OH
CA
GI
WTG
MAOIs
isocarboxazid
20
2
1
0
1
1
2
2
0
1
2
phenelzine
45
2
1
0
1
1
2
2
0
1
2
tranylcypromine
50
2
3
0
1
1
2
2
0
1
2
TCAs
amitriptyline
75
24
1
0
4
4
0
4
3
0
4
amoxapine
200
10
1
0
2
2
2
2
3
0
1
clomipramine
100
24
1
1
3
4
1
2
2
0
3
desipramine
150
18
1
0
1
1
1
2
2
0
1
doxepin
100
17
1
0
3
4
0
2
2
0
3
imipramine
100
22
1
0
3
3
1
4
3
1
3
maprotiline
100
43
1
0
2
4
0
0
1
0
2
nortriptyline
100
26
1
0
1
1
0
2
2
0
1
protriptyline
30
76
3.5
0
2
1
1
2
2
0
0
trimipramine
100
12
1
0
1
4
0
2
2
0
3

SSRIs,
related
drugs
fluoxetine
20
168
1
1
0
0
2
0
0
3
0
paroxetine
30
24
1
0
0
0
2
0
0
3
0
sertraline
50
24
1
0
0
0
2
0
0
3
0
fluvoxamine
100
15
1
1
1
3
3
1
0
3
0
nefazodone
300
18
2
0
3
4
1
1
0
3
0
Others
bupropion
225
14
3
0
0
0
2
0
1
1
0
trazodone
300
8
3
0
0
4
0
1
1
1
1
venlafaxine
112.5
5
3
0
2
3
2
1
1
3
0
Sources:
Depression
Guideline
Panel
(1993.
tables
7,
8,
pp.
56.
59);
for
clomipramine,
fluvoxamine,
nefazodone,
and
venlafaxine,
Physicians'
Desk
Reference
Generics
(1996.
pp.
735-39.
1383-1683,
2246-50,
3071-76).
Notes:
See
text
for
discussion
of
typical
daily
dosages
in
milligrams.
Half-life
is
average
of
elimination
half-lives
in
hours.
Daily
frequency
is
that
recommended
for
maintenance
therapy
alter
titration
has
determined
daily
dosages.
FDA
OCD
=
I
if
FDA
has
approved
obsessive-compulsive
disorder
indication.
For
side
effects,
AC
=
anticholinergic
(dry
mouth.
blurred
vision.
urinary
hesitancy,
constipation);
DR
=
drowsiness,
IA
=
insomnia-agitation;
OH
=
orthostatic
hypotension
(abnormally
low
blood
pressure):
CA
=
cardiac
arrhythmia:
GI
=
gastrointestinal
disease:
and
WTG
=
weight
gain
(more
than
6
kg).

144
Brookings
Papers:
Microeconomics
1996
of sexual dysfunction;
some
patients
encounter insomnia,
while a small
portion experience drowsiness.
In addition to their use
as
antidepressants,
two of the SSRIs-Prozac
and
Luvox, along
with a
tricyclic,
Anafranil-have received Food and
Drug Administration (FDA)
approval
for use in
treating
obsessive-
compulsive disorders (OCD).
Within
the class of
SSRIs,
Prozac has
the
longest
half-life
(see
table 1);
this has
disadvantages
for
those who
experience negative side effects
but
can be beneficial
for
those who
occasionally might forget
to
take
their medication.
Three related
drugs
have
recently
been introduced
into
the antide-
pressant
market: nefazodone
(brand
name
Serzone),
a
serotonin-related
compound
that
may
cause less sexual dysfunction;
venlafaxine
(Ef-
fexor),
a
compound
that inhibits
reuptake
of
norepinephrine
and
sero-
tonin, but not dopamine,
and thus exhibits some of the features
of both
the tricyclics and SSRIs;
and
bupropion (Wellbutrin),
a
compound
whose mechanisms
of action
are
still
not
well understood. More
gen-
erally,
researchers of the central nervous system
still do not
understand
precisely
how
the SSRIs
affect
depressive
moods and
the role of
sero-
tonin
in
this
process. Although
serotonin
levels increase within
several
days
of
taking
SSRI
(and
other
antidepressant) medications,
typically
a
change
in
depressive
moods manifests itself much
later,
after
two,
four,
or
perhaps
even
six
weeks. It
is
possible
that serotonin
causes
slight effects
in
other
neurotransmitter
systems,
which
in
turn
relieve
depression. Apparently
the serotonin neurotransmitter
system
is
very
complex.
Although
much
progress
has been
made in
developing psychothera-
peutic drugs
for
treating depression,
the
causes
and
optimal
treatments
of
depression
remain unresolved.
This has lead the American
Psy-
chiatric Association
to
issue the
following
current medical
practice
guidelines:
No one
medication
can be recommended
as
optimal
for all
patients
be-
cause
of
the substantial
heterogeneity among patients
in their
likelihood
of beneficial
response
to these medications
and
the
nature,
likelihood,
and
severity
of side
effects.
Furthermore, patients vary
in the
degree
to
which
particular side
effects and other
inconveniences of
taking
medi-
cations
(e.g.,
cost and
dietary
restrictions)
affect
their
preferences.26
26. American Psychiatric
Association
(1993, p. 7).

Ernst R. Berndt, Iain M.
Cockburn, and
Zvi
Griliches
145
Finally,
it
is
widely believed that
psychotherapy, drug
therapy, or
their
combination is
an effective
treatment
for
cases of mild to
moderate
depression. Although this consensus is based on extensive
clinical ex-
perience,
and on clinical trial data
for
drugs,
evidence
concerning
the
efficacy
of
psychotherapy based
on
controlled
experiments
is not as
extensive,
in
part
because controlled
experiments
involving
uniform
and consistent
forms
of
psychotherapy
have
proved
difficult to
design
and conduct.27 For
the more
severe forms of
depression, both
drug
treatment
and electroconvulsive treatments
appear
to
be more effica-
cious
than
psychotherapy
alone.28
The Changing
Marketplace
for
Antidepressant Drugs
Our
description
of the
changing
marketplace
for
antidepressant
drugs
is
based on the
following data sources.
Monthly price and
quantity data
for
drugstore purchases
of
antidepressant
drugs
are from
IMS
America,
a
Pennsylvania
firm that
collects
and
sells
data on the
sales and mar-
keting
of
pharmaceutical
products.
The transactions monitored
by
this
data are from wholesalers and manufacturers to
drugstores
(or
their
purchasing agents)
and are based on actual
invoices;
IMS tracks
more
than
99 percent
of
manufacturer
and wholesaler
transactions and thus
provides a near-census universe
of
drugstore
purchases.29
These in-
voices
reflect slightly
imperfectly
the
prices
manufacturers receive.
The
invoice data provide a
dollar
sales amount and
quantity
number for each
type
of
transaction;
they
include
chargebacks
(credits
to
wholesalers for
any
special price agreements
negotiated
among drug stores,
manufac-
turers,
and
wholesalers),
but rebates
(direct
payments
from
manufac-
turers to health
care providers
and
others,
such as health
maintenance
organizations
and
pharmaceutical
benefit
management
firms)
are not
always included,
nor do
the dollar
purchase
amounts on the
invoices
reflect
prompt payment
cash discounts
(usually
2
percent off).30
Further
27. See, however, the seminal
study by Elkin, Parloff, and others (1985) and
Elkin,
Shea, and others (1989).
28. See Depression Guideline Panel
(1993).
29. lMS America (1996b, p.
39-6).
30. Rebates occur in part because
health maintenance organizations and
pharmaceu-
tical benefit management companies
can affect market shares, but often these organi-
zations do not
actually take possession
of
drug products.

146
Brookings Papers. Microeconomics
1996
discussion
of the IMS
price
data is
given
in
Berndt,
Griliches,
and
Rosett,
who report that from
1986 through
1991,
the
period covered in
their
study,
the IMS data and
price
data
provided
them
by
four manu-
facturers had
very
similar
growth
rates.3'
In the
paragraphs that
follow,
we
report
sales
data,
measured
in
both
dollars and
daily dosage
units.32
Frequently
a
drug
is available in
var-
ious
strengths; considerable differences
also
occur
in
the total
daily
dosage
taken
by
individuals. To
develop
a
quantity
measure
providing
some
comparability
across diverse chemical entities and
dosage
strengths, we first take
the
midpoint
of
the
normal recommended
daily
milligram
dosage range
during
the
maintenance
phase,
as
specified
for
each chemical
entity
in
the
1996
Physicians'
Desk
Reference
and then
assess
what
integer
number
of
equal-strength
tablets at
recommended
daily
frequencies
could
feasibly
make
up
the
total
daily dosage
closest
to
this
midpoint.33
In cases of
ambiguity,
we consulted
IMS data on
volume
sales by tablet
strength. The resulting
"typical daily
dosages"
are listed
in
table
1
for each
chemical
entity.
To
express quantities
in
total number of
daily
dosages,
we divide the total
number
of
milligrams
of
active
ingredient
sold over the various
presentations
of
the
drug by
this
typical daily dosage.
The
typical daily
dosage price
is
then
com-
puted
as
sales
in
dollars divided
by
total
typical
daily dosages.
The
Overall Market
for
Antidepressant
Drugs
Growth
in
the overall market for
antidepressant
drugs
since
1980 has
been sustained and substantial.
In
1980
about 452 million
daily
dosage
units
of
antidepressant
drugs
were
sold; by
1995 this number
had
in-
creased to
about
2.44
billion,
a
factor
of more
than
five;
the
implied
average
annual
growth
rate
(AAGR)
is
11.9
percent.
Growth of
dollar
revenues
has been even
stronger,
from
a
$128
million market
in
1980
to
$3
billion in
1995,
for
an AAGR
of
23.5
percent; using
the
GDP
deflator to
convert into constant
1980
dollars,
the
1995
sales are
$1.65
billion, implying
an
AAGR of
18.6
percent.
Growth has
accelerated
31. Berndt,
Griliches, and
Rosett (1993, p. 255).
32. Because
their uses are
often for very
different purposes and
because their
vol-
umes
are
relatively small,
all
liquid
forms,
such as oral
solutions and
injections,
are
excluded.
33. The
midpoint dosage was often an infeasible
number, unless
patients broke up
tablets into
smaller units. Thus we
sought
an
integer
value.

Ernst R. Berndt,
Iain
M. Cockburn, and Zvi Griliches 147
dramatically since 1988, the year
in
which
the first SSRI
was intro-
duced.
From
1980 to
1987,
for
example,
the
AAGR in
daily dosage
quantities
was about 5.3
percent,
but
from
1987
to
1995
this
AAGR
more than tripled to 18.3 percent;
in
real dollars, these AAGRs are 9.5
percent
and
26.9 percent.
Entry and Exit
There has been much
entry
and some
exit
in
the market for
antide-
pressant drugs. Two types of entry occurred,
one
involving introduc-
tions of
patented products
and
products newly approved by
the
FDA,
and the other
involving generic
introductions after
patent protection
expired.
In
some cases branded
products
left the
market,
while both
entry
and exit
occurred
for
generic products.
This
entry
and exit
behav-
ior is
summarized
in
table 2.
Of
the twenty-one antidepressant chemical
entities on the market
in
February 1996,
fifteen were either new branded
products
or
generic
versions introduced
within the
past
ten
years.
All
three
MAOI products
were introduced
in
the
1959-61 time
pe-
riod,
and
although patent protection
has
expired,
the
market for these
products
is
apparently so
small and
unattractive that generic entry has
not been
induced.
Among
the ten
tricyclics
and
related
tetracyclic (hereafter, TCA)
chemical
entities,
the two oldest
are
imipramine
and
amitriptyline.
The
branded
pioneers,
Elavil and
Tofranil,
not
only
faced
competition
from
generic entry beginning
in the
1970s,
but from 1975 on
they
also ex-
perienced
branded
competition
from
other
major pharmaceutical
man-
ufacturers
(Endep
for
Elavil,
and
Janimine for
Tofranil).34
The
com-
petition
these
secondary
brands
encountered from the
primary
branded
products and the generics must
have been
considerable,
for
Janimine
exited
in
1985,
and
Endep
in
1988.35
The
TCA
class of
drugs
attracted considerable branded
entry, espe-
cially
in the
1960s,
but in
the
1980s
generic entry
was
predominant,
reflecting
in
part
the
reduced
costs of
generic entry made possible by
34.
Although
the distinction
is not
completely clear,
we
distinguish
branded
products
from those
generics sold primarily by
their chemical
entity name,
often under a
private
label; thus Endep is distinguished from, say, Walgreen imipramine.
35.
It
is
possible
that
these brands exited
only
from the
IMS data
base,
not from
the
market,
in
that their sales
may
have fallen below
a
minimum
reporting
threshold
imposed
by
IMS.

Table
2.
Entry
and
Exit
in
the
Antidepressant
Drug
Market
Orginator
brand
Seconidary
brand
Genieric
distributors
Generic
name
Name
Year
Name
Entry
Exit
Entry
1988
1996
MAOIs
isocarboxazid
Marplan
1959
None
0
0
phenelzine
Nardil
1959
None
0
0
tranylcypromine
Parnate
1961
None
0
0
TCAs
amitriptyline
Elavil
1961
Endep
1975
1988
1977
13
24
amoxapine
Asendin
1980
1989
0
14
clomipramine
Anafranil
1990
None
0
0
desipramine
Pertofrane
1971
Norpramin
1975
1989
1987
9
20
doxepin
Sinequan
1969
Adapin
1973
1991
1986
12
22
imipramine
Tofranil
1958
Janimine
1975
1985
1975
12
16
maprotiline
Ludiomil
1981
1988
7
11
nortriptyline
Aventyl
1963
Pamelor
1977
1992
0
17
proptriptyline
Vivactil
1967
None
0
0
trimipramine
Surmontil
1969
1988
5
0
SSRIs,
related
drugs
fluoxetine
Prozac
1988
None
0
0
fluvoxamine
Luvox
1994
None
0
0
nefazodone
Serzone
1995
None
0
0
paroxetine
Paxil
1993
None
0
0
sertraline
Zoloft
1992
None
0
0
Others
bupropion
Wellbutrin
1989
None
0
0
trazodone
Desyrel
1981
1986
22
22
venlafaxine
Effexor
1994
None
0
0
Sources:
IMS
America,
Inc.,
and
Food
and
Drua
Administration
(annual).

Ernst R. Berndt,
Iain M. Cockburn, and
Zvi Griliches
149
passage
of
the
1984
Waxman-Hatch Act.36
By
1996
eighteen
or so
distributors were
offering generic products
for
each of the
TCA drugs
facing generic
competition, up
sharply
from about ten in
1988. Not
all
generic entry has been
sustained;
although
Surmontil
faced
generic
entry in 1988, in
1992
the
generic
competition
exited,
and
none has
emerged since then.
The introduction
of Prozac
in
1988
marked
the
entry of an
entire new
class of
antidepressants,
the
highly
successful SSRIs. Other
SSRI
branded drugs were
Zoloft,
introduced
in
1992, Paxil
in
1993,
Luvox
in
1994, and
Serzone
in
1995;
Effexor,
a
related
product, was also
introduced
in
1994.
Prices and
Market Shares
Next we
look at
market share
and
price movements,
first
among the
four classes of
antidepressant drugs
listed
in
table 2.
During 1980-88
the
MAOIs
had
only
a
very
minor unit and
revenue
market
share,
between
1.4
percent
and
2.4
percent,
and after 1988 this share
dropped
even
further;
the
1996
share was but
0.3
percent.
In
1980
the
TCAs
accounted for about
98
percent
of
both the
daily
dosage quantities
sold and
total
antidepressant
revenues.
By
1987 the
TCA
unit share
fell
slightly,
to
90
percent,
as trazodone
(from
a differ-
ent class of
drugs)
increased
its unit market share to
about
8
percent;
the
corresponding
TCA
revenue
shares
were 77
percent
and 21
percent.
Among
the
TCAs,
three dominated
in
1980:
amitriptyline
had a 50
percent
unit
share,
doxepin
22
percent,
and
imipramine
18
percent,
for
a combined share of
90
percent. By
1987
this
combined share
fell to
80
percent,
as sales of
products
such as
desipramine,
amoxapine,
and
nortriptyline
(having fewer
and less
severe
side effects-see
table
1)
increased to a combined
14
percent
unit share. The three
largest
TCAs
accounted
for
about
82
percent
of total
TCA
dollar
sales
in
1980,
but
only 49 percent
in
1987,
in
large part
because
all three
products
faced
increased
generic
competition
in
the
1980s.
The launching of Prozac was a
huge
success. Not
only
did
this first
SSRI
take market share
away
from the
TCAs,
but
it
also
expanded
enormously
the size of the overall
antidepressant
drug marketplace.
36. For
discussion of
this legislation and its
consequences, see Grabowski
and Ver-
non
(1992).

150 Brookings Papers: Microeconomics 1996
General practitioners and internists, not just psychiatrists, were now
able to prescribe antidepressants comfortably,
for
concerns about side
effects and adverse interactions
with
Prozac were much less intense
than with the TCAs. Moreover, because the daily dosage for Prozac
was
the same
for
almost everyone, specialist knowledge
and
experience
concerning optimal patient-specific dosages, typically required for
many of the TCA drugs, were
no
longer necessary.
At
the end of
its
first year on the market (1988), the Prozac daily dosage share among
all
antidepressants
was
11
percent,
and
given
its
higher price,
its dollar
market share
was 21
percent; by
1991 these shares
had
increased to
29
percent and 51 percent, respectively.
The SSRI market continued
to
grow rapidly following entry by ad-
ditional
SSRIs,
and
by 1996
the SSRI market share
among
all antide-
pressants was 63 percent
in
daily dosage
units and a
remarkable
84
percent
in
dollars;
unit market shares
for
the
TCAs
fell
from 90 percent
in 1987 to 27 percent in 1996, while revenue shares dropped even more
dramatically, from 77 percent
to 7
percent. Clearly, for many physi-
cians and patients dealing with
the
treatment
of
depression, the SSRIs
were enormously successful
in
fulfilling unmet
needs.
Within the SSRI subclass of drugs, unit
sales of
Prozac continued to
grow, from 340 million daily units
in
1991 to 645 million in 1995. But
the
great success
of
Zoloft
and Paxil in
expanding the overall SSRI
market has implied
a
loss
in Prozac's
market share;
in
1996 SSRI daily
dosage market shares for Prozac, Zoloft,
and Paxil were
41.6 percent,
41.5 percent, and 12.6 percent, respectively,
while
corresponding
dol-
lar market shares
were
48.0
percent,
29.8
percent,
and 17.8
percent.
Moreover,
the unit shares
of
Prozac, Zoloft,
and Paxil
prescriptions
written
by nonpsychiatrists
were
39
percent,
51
percent,
and
49
percent,
respectively, indicating proportionally
more
nonspecialist prescriptions
written
for Zoloft and Paxil than
for
Prozac.37
Prozac and other SSRI
entrants have been
tremendously
successful
despite
their
higher daily dosage prices.
When Prozac was launched
in
1988,
for
example,
its
daily price
was about
$1.18,
almost double the
$0.60 daily price
of
the
branded version
of the
leading selling tricyclic,
amitriptyline,
and more
than
twenty
times
the
$0.05 daily price
for
generic
versions
of
that chemical
entity; doxepin,
the second
best-
37. IMS (1996a).

Ernst R. Berndt,
lain M. Cockburn,
and Zvi
Griliches
151
selling tricyclic,
was also
much cheaper
than Prozac-$0.70
a day in
its branded
version and $0.21
in generic form.
When Zoloft,
the second
SSRI entrant,
was launched
in 1992, its
daily price was set at about
25
percent
lower
than that of
Prozac-$1.26
compared
with
$1.69.
Ser-
zone, the most
recent
SSRI, is priced
in between Prozac and Zoloft.
In
constructing
a price
index,
what happens
following entry
of
ge-
neric competition
is very
important.38 In
table
3
we summarize
price
and market
share developments
at twelve,
twenty-four,
and thirty-six
months following
initial
generic entry
for the seven
chemical entities
experiencing
initial
generic
competition
since 1980. The
top panel
shows
that
although
considerable variability
is
present,
unweighted
average generic
prices
are
about
57
percent,
43
percent,
and
35
percent
of
brand prices
after
one,
two,
and three
years.3
Substantial differences
in
market share
penetration
are
also
present.
Measured in
daily units,
generic
market
shares
vary
from 5
percent
to
68
percent
of brand shares
after
one year
and
average
about
27
percent,
while
they
average
about
44
percent
and
54
percent
after
two
and
three
years,
respectively.
There does
not
appear
to
be
any
dominant
time
trend
to
generic
penetration
rates, although
the market share
of
the most recent
generic
entrant, nortriptyline,
is the largest
after
one, two,
and
three years.
Because generic
prices
are
lower than brand
prices,
dollar shares are
smaller
than
unit
shares;
even
so,
after
just
one
year
the
nortriptyline
dollar share
is
56 percent.
The generic
price
can
fall relative to
the
brand
price
if
the
generic
price decreases,
the
brand
price
increases,
or both.
As
the second
panel
of table 3
shows,
manufacturers
have tended
to
increase
the
price
of
branded
products
following generic
entry, apparently
focusing
on the
price inelastic
market
segment
and
letting generics gain
market share
from the elastic
segment;
after
one,
two,
and
three
years,
the
average
38. For
discussion
of generic
pricing and
responses by
incumbents,
see Caves,
Whinston,
and Hurwitz
(1991); Frank
and Salkever
(1992);
Grabowski
and Vernon
(1992);
Griliches and
Cockburn (1994);
Hurwitz
and Caves
(1988); and
Masson and
Steiner (1985).
39. These trends
in
prices
of generic drugs
for treatment
of a
relatively
chronic
condition such as depression
differ
considerably
from
those
reported by
Griliches and
Cockburn
(1994)
for systemic infectives,
which tend
to be used in the treatment of
more
acute conditions.
For
generic antidepressants
(except nortriptyline),
the initial price
discount
is larger, but
after that the
relative
price
is
flatter
than that
of
generic
systemic
anti-infectives.

Table
3.
Relative
Prices
and
Market
Share
Penetration
of
Generic
Antidepressant
Drugs
Introduced
since
1986
IWelve,
TWenty-four,
and
Thirty-six
Months
after
Introduction
Percentage
Relative
price
generic
Generic
market
share
Generic
market
share
Chemical
Entry
to
brand
in
units
in
dollars
entity
year
12
24
36
12
24
36
12
24
36
doxepin
1986
38
30
25
39
54
67
20
26
34
trazodone
1986
62
42
18
23
44
70
16
25
29
desipramine
1987
61
37
31
29
61
58
20
37
30
maprotiline
1988
61
54
42
10
22
33
6
13
17
trimipramine
1988
60
53
58
5
11
11
3
6
7
amoxapine
1989
58
51
50
14
37
51
9
23
35
nortriptyline
1992
61
36
22
68
80
85
56
58
56
Pioneer
brand
price
after
generic
entry
(Generic
entry
date
=
1.00)
Nominal
price
per
day
Real
GDP-deflated
price
12
24
36
12
24
36
doxepin
1986
1.11
1.35
1.50
1.08
1.27
1.35
trazodone
1986
1.01
1.22
1.58
0.98
1.14
1.42
desipramine
1987
1.13
1.35
1.60
1.09
1.25
1.42
maprotiline
1988
1.14
1.21
1.28
1.08
1.11
1.12
trimipramine
1988
1.14
1.23
1.43
1.09
1.12
1.25
amoxapine
1989
0.97
1.39
1.45
0.94
1.33
1.29
nortriptyline
1992
1.04
1.06
1.11
1.01
1.01
1.05
Source:
IMS
America,
Inc.

Ernst R.
Berndt, lain M.
Cockburn, and
Zvi
Griliches
153
nominal
price increases
for
the
branded
products
are
about
1 1
percent,
26 percent, and
42
percent
(average
real
price
increases are
about 7
percent,
18
percent,
and
27
percent,
respectively).
With
this data as
background, we now
summarize
procedures
the
BLS has
used to
track and
measure
price indexes in
this
rapidly
chang-
ing
antidepressant
drug
marketplace.
BLS
Procedures
and
Samples for
Tracking
the
Antidepressant
Drug
Market
Currently
the PPI
program at the BLS
encompasses
the
construction
of
monthly
aggregate
price
indexes
for almost five
hundred
mining
and
manufacturing
industries,
including
approximately ten
thousand
in-
dexes for
specific product
categories,
based on
reports
from
approxi-
mately
twenty-five thousand
companies that
respond
voluntarily.
For
the
specific
product
category
called
prescription
pharmaceutical
prep-
arations,
the BLS
has
been
publishing
a PPI
since
January 1961.
In
June
1981
the BLS
began
publishing
a
price
index for
a
category
of
drugs
called
psychotherapeutics.
The
specific products
the BLS sam-
pled
for
this price
index were
drawn
in
1980
and
are known
as
"Cycle
A"
items.
Although
the
psychotherapeutic
category
consisted
of
sub-
categories
for
tranquilizers
and
antidepressants,
separate
price
indexes
for
these
distinct
and
more
disaggregated
subcategories were not
offi-
cially published.
Unfortunately,
the
BLS
has not
kept
files
on
which
particular
psychotherapeutic
drugs
and
presentations
made
up
the
Cycle
A
sample
and what
their
index
weights
were.
About six
years
later,
in
December
1987, the
BLS
drew
up
a
new
sample,
implementing
where
possible
a
sampling procedure
in
which
items
were
chosen
in such
a
way that
the
probability
of
selection was
proportional
to
a
product's value
of
shipments.40
A
separate
antide-
pressant
drug
subcategory
was
created,
and
specific
items
were
chosen
for
that
subcategory
in
what the
BLS
calls
its
"Cycle
B"
sample.
For
six
years
beginning
in
December
1987,
the BLS
computed
and
pub-
lished a
PPI for
antidepressant
drugs
based on
this
Cycle
B
sample.
In
0
.-
C#7
0
0
~0
~0
ID
0
0
.-
X _E;
Cd
0
It
0_
C0
;o
'C7
'I
_E _7E

154
Brookings Papers: Microeconomics
1996
December 1993 the BLS
again updated its sample; the items
making up
this new
sample of
antidepressant drugs
are
called "Cycle C"
products.
Under
strict
confidentiality agreements,
BLS officials
have made
available to
us
information
concerning
the set
of
antidepressant
drugs,
and their
item weights, that
make
up
the
Cycle
B
and
Cycle
C
samples.
As best we can
determine, six items were originally
in
Cycle B, and
one
additional item
was linked
in
around
May 1990.
Two of
the seven
items may be
misclassified, because the FDA has not
approved them
for treatment of
depression,
nor does
the American Medical Association
list them as as
antidepressant treatments.4'
All seven
Cycle
B
items
apparently
were branded
products;
when the
Cycle
B item
sample
was
implemented, three of the six brands faced
generic competition.
Ge-
nerics as a group accounted
for
11
percent
of total
antidepressant
rev-
enues and 44
percent
of
total
daily dosage
units sold.
Prozac,
the
pi-
oneer
SSRI,
did
not
enter
the market
until
January 1988,
and thus none
of
the
new
generation
of
SSRIs
was
included
in
the
Cycle
B
sample.
During the six-year Cycle
B
period (1987-93),
an
additional two of
the
seven
branded
drugs
in
the
sample
lost
patent protection
and faced
competition
from
generic
entrants.
Thus
at the
end of the
Cycle
B
era
(December 1993),
while five
of
the
Cycle
B
items faced
generic
com-
petition,
all seven
sample
items
were branded
products.
Details
concerning procedures
used
to construct
the
Cycle
B
sample
are no
longer
available.
BLS officials
have, however,
informally
de-
scribed how the
Cycle
C
sample
was
drawn
and how its
item
weights
were
determined.
In
early
1993
the
BLS
contacted
a
private data source
to
provide
1991
and
1992
annual sales
data
by drug,
separately
for
several market
segments
such
as
drugstores
and
hospitals.
Based on
this and related
FDA
data,
the BLS
chose a
preliminary
set of thera-
peutic
classifications
and,
using
a
sampling procedure designed
to en-
sure that a
manufacturer's
probability
of
being
selected was
propor-
tional
to its
sales,
selected about
120 manufacturers for
sampling,
of
which
approximately
75
percent cooperated voluntarily.
Item
weights
were then
constructed based
on information
these manufacturers
pro-
vided to
the
BLS. The
resulting Cycle
C
sample
of
products
used to
41. American Medical Association
(1991).
Both of these
products are known to be
prescribed "off-label" infrequently by
some physicians
for
treatments occasionally
associated with depression. BLS officials
have
suggested
that these
products may have
been selected
as antidepressants by
the
responding firms,
rather
than
by
the BLS.

Ernst R. Berndt,
lain M.
Cockburn,
and Zvi Griliclies
155
construct
PPIs
for
prescription
pharmaceuticals
numbered
between
500
and 520.42
Within
this Cycle C
sample,
first
used
in December
1993,
the
BLS
retained five
of the seven
Cycle B chemical
entitities (each
with
differ-
ent
weights,
and one switched
from brand to
generic,
with
a
changed
milligram
strength),
including one
drug
not normally considered
an
antidepressant.
Two
Cycle
B
items were
dropped,
and five new items
were added.
Of the ten
Cycle
C items, three
are
generic
and
seven
are
branded. Among the
latter,
three faced competition
from
generic
entry
at the time the Cycle
C item
sample
was drawn.43
Because
Prozac
is
manufactured
in
Puerto
Rico
(along
with
many
other drugs,
in
part because
of
provisions
in
the federal
tax code)
and
because Puerto
Rico is
not
considered part
of
the United States
for
purposes
of PPI calculations,
Prozac could not
be part of the
Cycle C
sample even
though it
is the largest-selling
SSRI.
More generally,
unlike the
CPI, which
includes drugs
manufactured
in
Puerto
Rico for
use in
the
fifty states and
the District
of
Columbia,
the PPI
excludes all
Puerto Rican
production.
Government
statistical
agencies do
not all
deal with Puerto Rican economic
accounts
in
the same
way.
For ex-
ample, the
national income
and
product
accounts
from
the
Bureau of
Economic
Analysis
exclude
Puerto Rican
production
and
that
of
other
dependencies,
but
in the balance
of
payments
accounts,
Puerto
Rico is
treated as domestic.44 The Census
Bureau defines
the
United
States as
the U.S.
customs
territory,
which consists
of the
fifty states,
the
District
of
Columbia,
and Puerto
Rico, plus
U.S.
foreign
trade
zones
and the
U.S.
Virgin
Islands.45 There
appears
to be
some
ambiguity,
however,
in
determining
what constitutes
Puerto
Rican
production
from
the
view-
point
of
the
BLS. One
of the
products
in
the
current
Cycle
C
sample,
for
example,
is
produced
both
on the mainland
(45
percent
of
domestic
consumption)
and in
Puerto
Rico
(55
percent
of
domestic
consumption).
42. In
December 1995 the
BLS supplemented
the original
Cycle C sample
by intro-
ducing fifty-one
additional products,
based
in
part on
data from new drug products
introduced after
1992, as published
in the
FDA's "Orange
Book." None
of these
products is in
the antidepressant
drug class (but
see footnote 47).
Kanoza ( 1996) provides
further details.
43. In December
1993 generics
accounted
for about
8
percent
of
total
antidepressant
market revenues,
and
37 percent
of daily dosage
units.
44. Bureau
of
Economic
Analysis (1985a,
p. 2; 1985b, p.
10).
45. Bureau
of
Economic
Analysis (1987).

156
Brookings
Papers:
Microeconomics
1996
The BLS
includes this
product in its sample,
even though
most of its
domestic
consumption
emanates
from
Puerto
Rico.46 This issue
of how
one
treats
Puerto Rican production is
important,
for
Puerto Rican
pharmaceutical
production is
about 20 to 25
percent of
mainland U.S.
production .
The current
Cycle C
sample incorporates items
from
several of
the
subclasses of
antidepressant
drugs displayed
in
tables
1
and
2, but the
weight given the
SSRI subclass
item(s)
is
(are) considerably
less
than
IMS data
would indicate
appropriate
(ignoring Puerto Rico
production
complications).
Moreover,
the older
antidepressants
appear
to be over-
weighted.
Specifically,
when
one assigns
each
antidepressant
chemical
entity
in
the
IMS data base the date of its
initial market
introduction,
calculates its
age
as of
1993:12
(the
beginning
of
the
Cycle
C
sample),
and
then
sales-weights each
entity's age using
IMS
sales of
daily
units
as
weights,
one obtains a
sales-weighted
average age
for each
entity.
In
1993:12 the
sales-weighted average age
of the IMS
universe of
antidepressant
drugs was 15.18
years,
while
that of the new
BLS
Cycle
C
sample
was an older 18.50
years;
if
one excludes
Prozac from the
IMS universe,
however, the
sales-weighted
average age jumps
to 18.53
years, virtually
identical
to
that
of the BLS
Cycle
C
sample.
In
February
1996, the last month in our
data
series, the
sales-weighted
average ages
for
the IMS
universe,
BLS
Cycle
C
sample,
and
IMS
universe
excluding
Prozac were
12.97, 16.58,
and
14.78 years,
respectively.48
Based
on
the
information it
collects,
the BLS
calculates
the
PPI
according
to a modified
Laspeyres formula,
in
which the value
of base-
46. This information was
provided
to
us
by
the manufacturer of the
product.
Note
that
the
weight employed by the BLS for this
product
could reflect
only
the
mainland
production.
47. When
the BLS supplemented
its Cycle C sample in December
1995 (see footnote
42
above), it chose four additional
antidepressant drugs.
All
four of these were found
to
be
manufactured
in
Puerto Rico, and thus
they
were
not included in the
supplemental
sample.
Regarding relative
importance,
it is not clear how best to
measure the Puerto
Rican
production proportion of U.
S. pharmaceutical
consumption.
If
one simply em-
ploys
value of shipments (VOS) data
from
the
1994 Economic
Census of Outlying Areas
(table 4,
p. 32) and from the 1992 Census
of
Manufacturers, Industry Series Drugs
(table 5b,
p. 28C-14),
both
published
by
the Bureau of the
Census,
one finds that
Puerto
Rican
VOS
is 22
percent
of
"domestic"
VOS-$1
1.1
billion, compared with $50.4
billion.
48. The
IMS
universe and BLS
Cycle
B
sales-weighted
average ages
at
the
beginning
of
Cycle
B
were 21.82 and
20.42
years, respectively;
six
years
later,
at the
end of
Cycle
B, the
respective average ages were
15.18
and 28.17.

Ernst
R.
Berndt,
lain M.
Cockburn, and
Zvi
Griliches
157
Figure 1.
BLS
Pharmaceutical Price
Indexes, 1980-96
Index
(1987:12
=
100)
210
All
prescriptions
............Antidepressants
165
-
Psychotherapeutics
120-
75-
30
I
1980:1
1987:12
1996:2
Source:
BLS
producer
price
indexes.
period
quantities at
current-period
prices
is
divided
by
the value of
base-period
quantities
at
(perhaps
temporally
different)
base-period
prices, that
is,
(1)
1t,=
[
Y
Q",P,
/
Y
Qb,PO]
100=
[={I
I
Q
(P/IP,)}/
E
QaPo,]
100,
where
Qb,
represents the
quantity
shipped
during
the
weight-base
period,
P,
is
the
current
price
of the
commodity,
and
Po
is the
price
of the
commodity
in
the
comparison
period;
the summation is over
i
goods,
but
i
subscripts
are omitted. Note that this index can be
written
as
a
weighted
average
of
price
relatives
P,IP,,
where the
weights
are
fixed
within
each
cycle.
The
monthly
time series
for the BLS PPI for all
prescription
phar-
maceutical
products,
for the
aggregate
class
of
psychotherapeutics,
and
for
the
antidepressant
subcategory
of
drugs
are
displayed
in
figure
1;
selected AAGRs are
given
in table
4.
For the
period
covered
by Cycles
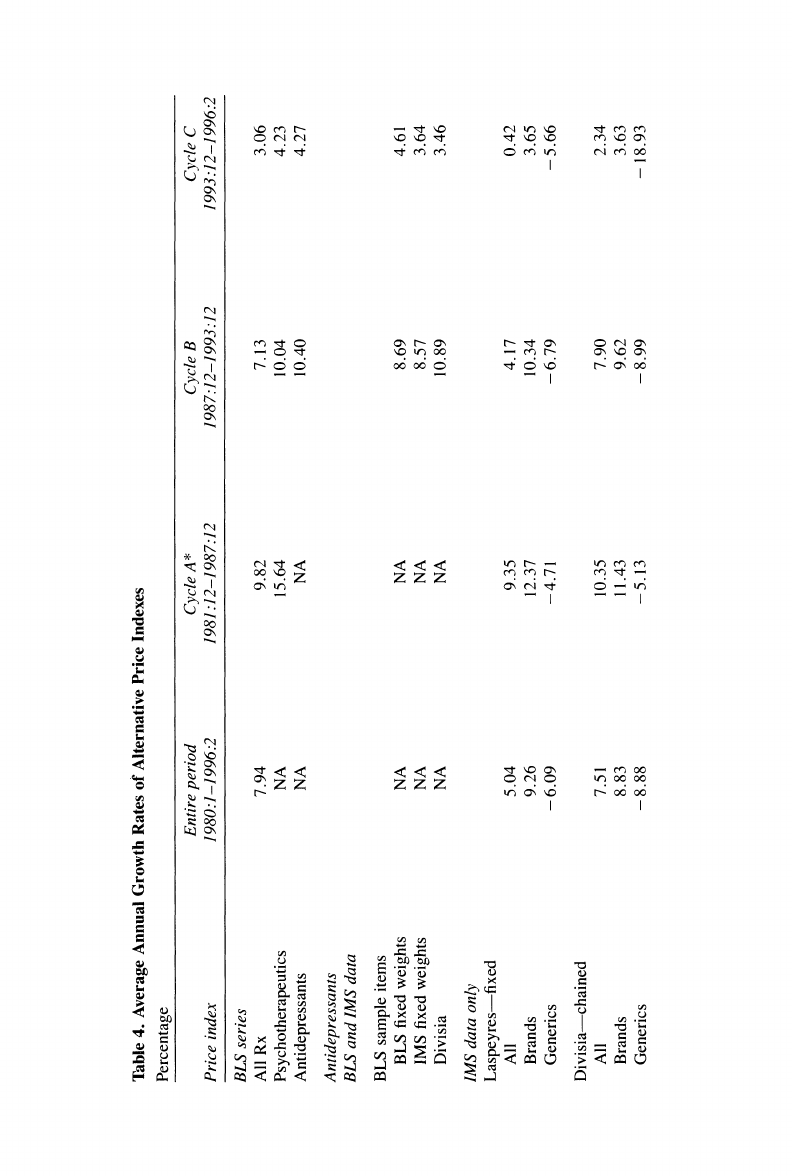
Table
4.
Average
Annual
Growth
Rates
of
Alternative
Price
Indexes
Percentage
Entire
period
Cycle
A*
Cycle
B
Cycle
C
Price
index
1980:1-1996:2
1981:12-1987:12
1987:12-1993:12
1993:12-1996:2
BLS
series
All
Rx
7.94
9.82
7.13
3.06
Psychotherapeutics
NA
15.64
10.04
4.23
Antidepressants
NA
NA
10.40
4.27
Antidepressants
BLS
and
IMS
data
BLS
sample
items
BLS
fixed
weights
NA
NA
8.69
4.61
IMS
fixed
weights
NA
NA
8.57
3.64
Divisia
NA
NA
10.89
3.46
IMS
data
only
Laspeyres-fixed
All
5.04
9.35
4.17
0.42
Brands
9.26
12.37
10.34
3.65
Generics
-6.09
-4.71
-6.79
-5.66
Divisia-chained
All
7.51
10.35
7.90
2.34
Brands
8.83
11.43
9.62
3.63
Generics
-8.88
-5.13
-8.99
-18.93

Paasche-all
7.11
9.87
7.45
2.29
Divisia
brands
SSRIs
NA
NA
NA
3.94
TCAs
9.22
11.02
10.82
4.31
MAOIs
9.63
10.96
11.72
5.63
Others
10.89
12.77
15.29
4.11
Laspeyres
brands
SSRIs
NA
NA
NA
3.62
TCAs
9.02
12.41
9.40
4.28
MAOIs
9.07
10.69
12.53
1.07
Others
NA
NA
16.11
3.13
Divisia
generics
TCAs
-9.96
-4.88
-10.72
-22.30
Others
NA
NA
-6.40
-6.88
Laspeyres
generics
TCAs
-6.08
-4.71
-6.81
-5.57
Others
NA
NA
-6.40
-6.88
Source:
Authors'
calculations;
see
text
for
explanation.
NA:
Not
available.
*Cycle
A
is
defined
here
as
1981:12-1987:12.

160
Brookings Papers:
Microeconomics 1996
B
and C (1987:12
through
1996:2), the
PPI
for
all prescriptions
in-
creased by about
63 percent (an
AAGR
of
6.08
percent), much
less
than the
PPIs
for
psychotherapeutics (96 percent,
AAGR of 8.53
per-
cent) and for
antidepressants (101
percent,
AAGR
of 8.80
percent).
This faster growth
of
psychotherapeutics compared with
all
prescription
drug prices
continues
a trend
going
back at least to the
beginning
of
Cycle
A;
from
1981:12
through
1987:12,
the
price
index of
psycho-
therapeutics increased 139
percent
(AAGR
of 15.64
percent), compared
with a PPI for all
prescription
products
of
75 percent
(AAGR
of
9.82
percent).49
Finally, annual average
growth rates
for
all three
price
in-
dexes
(all prescription
drugs,
psychotherapeutics,
and
antidepressants)
are
greater during
the
Cycle
B
era
(7.13 percent,
10.04
percent,
and
10.40
percent,
respectively) than
during
the
Cycle
C
time
span
to
date
(3.06
percent,
4.23
percent,
and
4.27
percent).
Given its fixed
weights
in
the context
of
a
rapidly
changing market,
the
reliability
with
which the BLS
PPI
for
antidepressants
could be
expected
to track
actual
marketplace
developments
is
ambiguous
at
best,
but
whether the BLS
sampling procedures
impart
a
systematic
bias
to
the
index
is
unclear.
The
undersampling
of
generics
would likely
impart an
upward
bias, given
the
substantial
price
reductions
they
have
experienced,
but the revenue shares of
generics
in
total are small and
falling (11 percent
in
December 1987,
8 percent
in
December
1993,
and 3
percent
in
February
1996).50 The BLS
lags marketplace
developments
in
the choice of its
sample,
and the net effect of
this
lag
on
an
aggregate price
index is
therefore
an empirical issue. But
a
different
consideration unrelated to
sampling
issues-namely,
the
absence,
until
recently,
of a
link
between
generics
and their
patented
antecedents-can more
clearly
be
expected
to
result
in
an upward bias
to
the
BLS
index.
Alternative
Price
Indexes:
Theory
and
Evidence
The
price
of a
good
before its market
introduction cannot be
ob-
served.
After
a new
good
enters
the
market,
it
may
take
quite
some
49. Of the
twenty-five
seven-digit products
in
SIC
2834-1, only
two
have a
greater
rate
of
price increase than
psychotherapeutics-central nervous system
stimulants and
antiobesity preparations,
and sedatives. See
Bureau
of Labor
Statistics
(1996, p. 61).
50. The
corresponding
daily
unit
dosage
shares are 44
percent,
38
percent,
and
27
percent, while daily dosage
levels of
generics are
32
million, 56 million, and
58
million.

Ernst R. Berndt, lain M. Cockburn, and
Zvi
Griliches
161
time for statistical agencies
to track
its
price.
As
Early
and Sinclair
have discussed, the BLS periodically
revises the sample items and
"links in" new commodities.5' For example,
in December 1995 the
BLS supplemented its Cycle C prescription
pharmaceutical sample with
fifty-one items, thereby incorporating
selected
market
developments
since the original sample (based
on 1991-92
data)
was drawn for
im-
plementation
in
December
1993. Once
items are selected for an
updated
sample, the
BLS
includes
their
price
changes
in its
price
index com-
putations.
Because this
procedure
makes no
comparisons
between new
and incumbent goods, however, changes
in
the aggregate price index
reflect only changes in the prices
of
the
products and ignore any absolute
price differentials between the
new and
comparable
incumbent
prod-
ucts.
Although
such
a
procedure may
perhaps
be
appropriate
for
truly
new goods, it surely is not appropriate
for many goods such as phar-
maceuticals for which some forms of
substitute goods or services are
available.
Considerations
from
Economic
Theory
The
theoretical solution
to this "new
goods problem"
has
long
been
known: for the time period just before
the introduction
of
the
new
good,
find that price at
which
quantity
demanded
is
just equal
to
zero and
put
this "reservation price" into the price
index calculation for the time
period just
before the
new
product
is
launched.52
This theoretical
insight
is
informative, but
it is also
challenging
to implement empirically,
for
it
requires
estimation
of demand models that
may
have
burdensome
data
requirements,
it
may
entail
making strong assumptions,
the esti-
mated reservation prices might
be sensitive to
the
choice of functional
form,
and the issue
of
proper
item
weights
is left
open.
The
special
characteristics of
generic
drugs provide
an
opportunity
to
modify price
index
computations
in a
relatively simple way, thereby
taking
into
account
the
implicit price
declines
experienced by
those
consumers
who switch from
brand
to
generic
versions of a
chemical
entity. Specifically, generic drugs
can be
envisaged
as a
particularly
simple
case of the
new
goods problem,
because
a
generic
is
a
variety
of an
existing product
identical
in
almost
all
respects
to the "old"
51. Early and Sinclair (1983).
52. Hicks (1940), Rothbarth (1940-41), and
Fisher and Shell
(1971, 1972).

162
Brookings Paipers:
Microeconomics 1996
version. In the United States,
the FDA publishes
an
"Orange
Book,"
Approved Drug Products with
Therapeutic Equivalence
Evaluations,
that certifies therapeutic equivalence.
Although the generic
versions
differ from the branded product
in
packaging (including
the inert matter
enclosing the active ingredients),
labeling,
and
provenance,
the FDA
certifies that the generics are
equivalent to
the
branded product
in
two
senses: pharmaceutical equivalence,
that
is,
the active
ingredient
is
chemically identical,
has the
same
strength, dosage form,
and route of
administration, and is manufactured
in
compliance
with
Current
Good
Manufacturing Practice regulations;
and the
generic
version
is
"bio-
equivalent"
in
that it is statistically indistinguishable
from
the branded
product in key pharmacological
aspects of therapeutic use,
such as
blood concentration profiles.
The
extent to which
generics
and branded
products
are
in
fact "al-
most perfectly substitutable"
is a hotly debated topic. Therapeutically
equivalent products may
still
vary
in characteristics such
as
inert ma-
terial, shape, color, flavor, scoring,
packaging, labeling, shelf
life, and
stability under adverse storage
conditions.
Insofar
as
any
of these char-
acteristics affects patients'
ability
to
distinguish
between
different tab-
lets and dosages, their readiness
to
take
the medicine at
the
time and
in
the amounts prescribed,
or their
possible
reactions to coloring
or
pre-
servative ingredients,
these
apparently
trivial factors
may
in
fact influ-
ence
the
realized
effectiveness
of the
generic drug
relative
to the
branded
product. Moreover,
variations
in the inert
matter
encasing
the
active
ingredient
can
affect the
speed
of
absorption
of a medication.
If one takes the
FDA at its word-"a
pill
is
a
pill
is a
pill"-the
reservation
price
is the branded price just
before the
generic
enters the
market, and in this case the
appropriate price
index for
a
particular
chemical
entity
is
straightforward,
being
the
weighted average
price
of
a
tablet across all
generic
and branded manufacturers.
If,
however,
one
takes
the
opposite
extreme position-that
taken until
recently by
the
BLS
for its
PPI-then one implicitly
treats
generic
versions of a
drug
as
entirely distinct,
nonsubstitutable
commodities.
In that
case,
the
generic
launch
price
is
also
its reservation
price.
As we noted
earlier,
the BLS has
recently changed
its
policy
and is now
introducing
a
linking
procedure
consistent with
perfect
substitutability:
"...the
predecessor
brand-name
drug price
and
successor
generic drug price
will
always
be
directly compared
without
quality
adjustment.
The direct
comparison

Ernst R.
Berndt, lain M.
Cockburn, and Zvi
Griliches
163
is predicated
on the
assumption that the
two products
are of equal
quality,
because the FDA has determined
them to be
therapeutically
equivalent.
"53
Generic
prices tend to be
considerably
lower than that of
the branded
version, and
the spread
between them tends
to increase
over time. Given
the magnitude of the
price
differential,
it is
striking
that not all
con-
sumers
switch
to the cheaper
variety.
Apparently
consumers,
or rather
physicians
who prescribe for
them, differ
in
their
perceptions concern-
ing the
efficacy and quality
of generics,
despite
FDA
certification, and
some
consumers,
prescribers,
and
insurers
prefer
the
much
higher-
priced branded
versions.
Some
consumers, prescribers,
and
insurers,
however,
do switch
to the
cheaper generic
version,
either
perceiving
no difference
between
brand and generic varieties or
taking the price
differential as more than
sufficient
compensation
for
any
difference in
quality.
Clearly,
there are considerable differences
in
information
and
knowledge
and in tastes and
preferences
among
consumers, prescribers,
and
insurers
concerning
brand-generic
differences.
Alternative
Procedures for
Incorporating
Generics
We now
consider alternatives to these two
extreme
positions,
var-
iants
we believe more
accurately reflect
the
price
declines
realized by
intermediate
purchasers of
prescription
drugs such as
pharmacies.
These
alternatives
vary
in
how diverse ultimate
consumer, physician,
and
insurer choices are taken into
account when reservation
prices
are
being computed.
Fisher and
Griliches
have
shown that even
when con-
sumers are
heterogeneous,
aggregate
Paasche and
Laspeyres
price
index
computations
provide
bounds
for
a
hypothetical
social
planner's
ideal
index, giving the minimum amount needed to
keep
all
individuals on
their
base
utility
level when
prices change.54
Griliches and
Cockburn
present
formulas for such
aggregate
indexes
in
a
world
in
which
either
the
branded or the
generic
version
of a
particular
chemical
entity
is
purchased.55
Let
Pb
be the
unit
price
of a branded
drug,
and let
pg
be
the
generic
price.
In
a
simple
linear random
utility
framework, purchaser
h
chooses
53. Kanoza
(1996, p. 9). The italics
are
in
the original.
54.
Fisher and
Griliches (1995).
55.
Griliches
and
Cockburn
(1994).

164
Brookings Papers: Microeconomics 1996
the
generic
version
if
pb
>
pg
+
b,,
where
b,
is
the
subjective premium
required by purchaser h when
buying the generic
to
compensate for the
putative
loss in
security
or
quality
associated
with
the switch.
If
one
knows the reservation price p,;
for
each
purchaser,
then Griliches and
Cockburn show
that the
aggregate
Paasche
price
index between
periods
0 and
1
is
appropriately
calculated
as
H
(2)
Pl = Qipo +
Q,j, wherep- P
"=
_
where the 0 and
1
superscripts
refer to
time
periods, Q
denotes
aggre-
gate quantities
over
all
H
consumers,
and
qg,,
is the number
of
units
of
the
generic
version
bought
on behalf of consumer h.
Although elegant, this theoretical framework
requires estimation of
reservation prices, a nontrivial
task. One feasible approach involves
making
an
assumption
concerning the distribution of preferences for
brandedness. In
the linear random
utility framework,
the
probability of
any purchaser switching
from
brand
to
generic depends on
Pb
-
Pg
>
b,,
and thus the share
of
generic
users
in
the total is
s,
=
F(b,,),
where
F(b,,)
is the cumulative distribution of
reservation prices, given a fixed
Pb*
If
no
buyer
is
willing
to
pay
more for a
generic
version than
for a
branded
one
when the branded one
is
available,
then
b,,
?
0, and in this
case
the
average
reservation
price
for
switchers
must
be
bounded be-
tween
pb
and
pg,
with the
precise
location
depending
on
the shape of
F(b,). Following Griliches and
Cockburn, one can assume that unob-
served
tastes
for
brandedness
among purchasers
are
uniformly distrib-
uted,
in which case the
average
reservation
price
is
half
way between
Pb
and
p?,
thus
"splitting
the
difference" between the two extremes
of
the
old BLS
approach,
which
assigns
all of the
brand-generic price
differential to
quality
differences
(pg being
the
reservation
price), and
the FDA
approach,
which
assigns
none of it
(Pb
being
the
reservation
price).
One notable feature
of these markets
is that a new
generic product
typically
takes several months
to achieve
significant
sales. The
product
may
take time
to move
through
distribution
channels,
and it
may
take
time
for
physicians
and
purchasers
to become
aware of its
availability
or
to
acquire
other
information
germane
to
prescribing
and
buying
decisions.
Regardless
of its
causes,
the
lagged response
of demand to

Ernst
R. Berndt,
lain M. Cockburn, and Zvi Griliches
165
price changes has
important implications for price
index computations,
particularly
at
monthly frequency,
since
weights of
new generic prod-
ucts are
typically
initially low.
This "diffusion
problem" can be approached in
several ways. One
way
is
to link
in
the new generic good after
sufficient time has passed
(say,
six
months to
a year), thereby allowing much of the
early diffusion
of
generics
to
be
completed
before
evaluating
their direct
contribution.
We discuss the BLS variant
on
this
approach
below.
Alternatively, as
Griliches
and
Cockburn have proposed
and
implemented,
the
Paasche
index formula can
be adjusted
to
reflect
the
assumption that those shift-
ing later on to
generics do so from the branded
good, with an average
reservation price
that
is
half way between the prices
of the branded and
generic good.
In
such a
case,
the Paasche
equation
2
becomes
I
V~Q)p),
+
Q""p"
(3) ,
Q),P57 + Q+Q Q ,'=
(p-
+
p')/2.
Thus shifters from
the branded to the generic
version are assumed to
have experienced
a price decline equal to half of
the branded-generic
price differential
also
in
periods subsequent to the initial
appearance of
56
generics.
The new BLS
approach
to this diffusion
issue, given
its
fixed-weight
Laspeyres index,
is
considerably
more
parsimonious
in its
data
require-
ments than are
the
above alternatives
and
addresses the choice of res-
ervation
price
and item
weights simultaneously.
Based
on
a review
of
published
research
materials,
data from the
FDA
"Orange Book,"
and
consultations with various
industry experts,
the
BLS has
determined
that
in
the month when initial
generic entry
occurs for a
chemical
entity
in
its
sample,
the
previous
fixed branded
quantity
weight, say, x,,
will
be
split
into
two
components,
with a 0.642
x,,
quantity weight assigned
to the
generic,
and
a 0.358
xo
weight given
the
branded
version. These
weights
are
then
fixed
until a new or
supplemental
sample
is drawn
and
are
the
same
for
all
generic
entities. The 64.2
percent
generic weight
turns out to
be
quite
close to
the
generic
quantity
share of the
seven
antidepressants
experiencing
initial
generic entry
after 1980
(see
table
3); specifically, the 64.2
percent
share falls
in
between
the
daily dosage-
56. Ibid. The procedure used here is referred
to in
Griliches and Cockburn (1994)
as "Paasche-UD."

166 Brookings Papers:
Microeconomics 1996
weighted average generic quantity share of 57.0 percent
after twenty-
four months and 68.9 percent after thirty-six months.
Finally, yet another alternative approach
to
deriving
reservation
prices, one that we plan to pursue
in
subsequent research, is to
estimate
demand curves from data on
prices
and
quantities
and then
to
project
the
estimated demand function
to
find
the
brand-generic
price
differ-
ential
that
would choke
off demand
for
the
generics
to zero. A related
research project involves using
similar data to estimate the
shape
of
F(b,,)
consistent with observed relationships between
prices and market
shares.
New Products and Hedonic
Regressions
As
noted earlier, generics
are a
special
case
of
the
new
goods prob-
lem, for with generics the FDA has certified
equivalence. In general,
however, new goods differ in significant ways
from
older products,
reservation prices are more difficult to quantify, and thus
incorporating
new goods into price indexes
is
more complex.
One
way
in
which
the
effects of new goods
could
be incorporated
into a
price
index is
simply
first
to
regress for each branded product-month, say,
the
logarithm
of
daily dosage price
on time
dummies,
and a
dummy
variable for each
distinct
brand.
One could then use the
predicted price
for
the month
prior
to
a good's introduction
as an
approximation
of
the
reservation
price, thereby linking
in
the price of the
new
good.
The
hedonic price approach employs
instead
quality
attributes as
regressors,
in
effect making parameters
on
the
brand
dummy
variables
functions
of
quality
attributes.57
The hedonic
approach
is
particularly
relevant for branded
products,
because
their
prices
are set
by
firms
having some
market
power.
For
generic products,
the
hedonic
approach
might
be
less useful, particularly
if
competition
drives
prices
down to
marginal production-distribution costs,
and when
such
marginal
costs
are
not
dependent
on
quality
attributes.
It is
important
to note that
price
indexes
linking
in
new
products
using predicted prices
from hedonic
price equations
can
grow
at
rates
less
than, the
same
as,
or
greater
than
those
that
entirely
ignore
the link
and instead
incorporate only
the
price changes
after new
product
launch,
that
is,
those indexes
that treat the reservation
price
as
equal
to the new
57. See Griliches (1971,
1990) for a discussion of this
methodology.

Ernst R. Berndt,
lain M. Cockburn,
and Zvi Griliches
167
product's launch
price. The relationship
between
the two growth rates
depends critically
on whether the
actual price of the
new product at
the
time of its launch
is above or below
that predicted
by the hedonic price
equation, given
the new product's
quality characteristics.
Any
differ-
ence between
the linked and
nonlinked series
will
emerge only
if
there
is
positive or
negative nonpriced
quality
at launch.58
If
the new product
has a launch price
that just compensates
for
its hedonic-estimated qual-
ity, then the
launch date hedonic
residual
would
be
zero, and
AAGRs
of
price indexes
based on the
nonlinked and
the
hedonic quality-
adjusted linked
procedures would
coincide.
If the
launch
price
of the
new product
were set above (below)
its estimated
hedonic quality,
however, then
hedonic residuals
would be positive
(negative), and
the
hedonic quality-adjusted
linked
price index would
grow at a greater
(lesser) rate than
the nonlinked index.
The magnitude
of any difference
would also depend
of course on
how
quickly
the quantity weights
be-
come substantial,
that
is, the speed
of diffusion.
Launch date
residuals
from
an estimated
hedonic
price equation
are
therefore quite
important, for they
purport
to measure deviations
from
reservation prices.
Whether
these residuals
in
fact
reflect price
dis-
counts or premiums
relative
to "true" quality
is
unknown,
however,
for
hedonic residuals
could
instead
be manifestations
of
important qual-
ity aspects that
have been omitted
or other specification
or measurement
errors. Evidence
on
unmeasured
quality
as a
possible
specification
error
could be obtained
by examining
relationships
between hedonic
equation
residuals and
realized market quantity
shares;
a
negative relationship
between
them
would be consistent
with the
notion
that residuals instead
reflect unobserved
quality differentials.
Later
we
present
findings
based on hedonic
price equations
for
branded
products.59
First, however,
we
focus on alternative
price
in-
dexes where issues
of
sample
selection and
item
weighting
are central.
Empirical
Evidence:
Sampling
and
Weighting
Issues
In
table 4
we
report AAGRs
for
several
alternative
price
index
cal-
culations. In
the
top panel
we
list official
BLS
growth
rates,
which for
58. For examples and further discussion,
see Berndt and
Griliches
(1993) and
Berndt, Griliches, and Rappaport (1995).
59. For earlier attempts to estimate
hedonic
price equations
for branded
prescription
pharmaceuticals, see Berndt and Finkelstein (1992) and
Suslow
(1996).

168
Brookings
Papers: Microeconomics
1996
the
antidepressant class of drugs is 10.40
percent (Cycle B) and
4.27
percent (Cycle
C). Based on
the
BLS
sample information provided
us,
we
employ
the
IMS price data but the BLS item
weights
to
construct
an
aggregate
index, mimicking BLS
fixed-weight Laspeyres proce-
dures, with
weights updated
at
1987:12 and
1993:12.
It is
not
possible
to obtain an exact
correspondence, because two
of
the seven items in
the BLS
Cycle
B
are not
generally
considered
antidepressants,
nor is
one
of
the ten
in
Cycle C, whereas the
IMS data
are confined to anti-
depressants.
If
these items
are
removed, we
obtain
AAGRs for the
partial BLS
item
sample
that
are
lower
than those
reported by
the BLS
during Cycle
B
(8.69 percent, compared
with 10.40
percent),
and close
but slightly
larger during Cycle
C
(4.61
percent, compared
with 4.27
percent).
The
relatively
close
correspondence
during Cycle
C
is
reas-
suring,
particularly because
the one omitted item has
a
relatively low
item weight,
whereas the two omitted
items during Cycle B have
a
larger combined
relative weight.
If
one retains
the
BLS
sample
items
but
uses
instead
of
the BLS
weights those based
on
IMS
data,
the
resulting
difference
in
AAGRs is
small
during
Cycle
B
(8.57 percent, compared
with
8.69
percent),
but
larger during
Cycle
C
(3.64 percent
using
the
December 1993 IMS
weights, compared
with 4.61
percent
using
the
necessarily
older
1992
or 1993 BLS
weights). Finally,
if
one uses the
partial
BLS item
sample
and
allows
weights
to
change monthly
with the Divisia
index,
one
obtains
rather different
results, suggesting
that
weights
do
matter.
Spe-
cifically,
as seen
in the
bottom row
of the second
panel
of table
4,
during Cycle
B
the
AAGR of the Divisia index is
10.89
percent.
It is
useful to
distinguish
between
the
choice and
accuracy
of
fixed
weights
and
the
effects
of
changing weights.
We
begin by
comparing
AAGRs
of the BLS official PPI
for
antidepressants
with those
based on
the IMS
universe, using comparable
fixed-weight Laspeyres
index
pro-
cedures. As the row marked
Laspeyres-All
shows, during Cycle
B
the
Laspeyres index based on the universe
of
antidepressants grows much
less
rapidly
than
does
the
BLS
index,
4.17
percent
a
year (compared
with
10.40
percent).
In
Cycle
C the IMS universe
data have an AAGR
of
only
0.42
percent,
while
the BLS
grew
at
ten
times this
rate,
4.27
percent.
This
large disparity
is
surprising,
given
that the
only
underly-
ing difference
is one of
weights
drawn from
a
sample
rather
than the
universe,
and not the
Laspeyres
index.

Ernst R.
Berndt, lain M.
Cockburn, and
Zvi
Griliches
169
Figure
2.
Daily Doses of Antidepressants
Percentage
Millions of dollars
100
25
90
80
i
Brand shares
20
70-
60
-15
50-
40
1
I0
30-
20 -
.0Total
daily
doses
-
5
10
1980:1
1982:1 1984:1 1986:1 1988:1 1990:1 1992:1 1994:1
1996:1
Source: IMS
America,
Inc.
Recall
that the BLS
Laspeyres fixed-weight
procedure entails a
change
of
weights only
at
six-year
intervals.
If
the sizes of the
weights
selected
at
1981:12, 1987:12,
and 1993:12
do
not
accurately portray
actual
data
trends
during
the
subsequent
six
years,
the
resulting
indexes
can
yield
very misleading
AAGRs,
with
the
sign
of
the
bias
being
generally
indeterminate.
Apparently,
that is what
has
happened.
To
see this, notice
in
figure
2
that the time
trend
of
the brand share
of
daily
dosages
over
the
sixteen-year period
is
approximately U-
shaped,
starting
at 77
percent
in
1980:
1, falling
to 47
percent
in
1988:8,
and then
increasing
to
73
percent
by
1996:2. When the
BLS drew its
Cycle
B
sample
in
1987,
the
generic
share was
near its
peak
at
47
percent,
but
by
the end of
Cycle
B in
late
1993,
it had
fallen to
about
37
percent,
and
by 1996,
to
27
percent;
the
corresponding
brand
shares
increased with
time,
53
percent
in
1987,
63
percent
in
1993,
and
73
percent
in
1996.
Thus,
if the
BLS
fixed-weight procedure
had
been
applied
to the
universe
of
antidepressant drugs,
over the
entire
Cycle
B
and
in
Cycle
C to
date, generic products
would have been
overweighted

170 Brookings Papers: Microeconomics
1996
(their unit share fell as brand dosages grew more rapidly, led
by new
SSRIs such as Prozac),
and brands would have been
underweighted.
Recall from our earlier discussion that prices
of
(overweighted)
generics
have been falling, while (underweighted) brand prices have
generally
been increasing. Together
these trends
imply
that had the
six-year
fixed-
weight procedure been applied to the universe
of
drugs,
the
resulting
AAGRs
would
have
severely
understated
price growth
in
both
Cycles
B
and C. The much higher AAGR
for the BLS
published
PPI
based on
its sample than for the Laspeyres
based on the universe reflects the fact
that the BLS
sample
was in
fact
nonrepresentative,
fortuitously weight-
ing generics less and brands more than
the
then-current
market condi-
tions warranted. (Recall
that none of
the
seven items
in
the
Cycle
B
sample
was a
generic.) Clearly, using six-year
fixed
weights
in
a rapidly
changing
environment
can
lead to
highly
unreliable results.
One
way
to
assess
the role of
changing weights
is
to
compute
a
Divisia
index, which weights percentage price changes by
the
average
share
in the
current and previous month,
in
contrast to the fixed-weight
BLS procedure in which the weights are changed only every
six years.
Alternatively,
one
can employ
a Paasche
index that
sequentially
updates
the
weights monthly.
As
seen
in
the
row marked
Divisia-All,
based on
the
IMS
universe, the
Divisia
grew
more
rapidly
than
the
Laspeyres
during
all
three cycles-10.35 percent compared
with 9.35
percent
in
Cycle A,
7.90
percent compared
with
4.17
percent
in
Cycle
B,
and
2.34 percent compared with 0.42 percent
in
Cycle C;
for the
entire
sixteen-year period,
the Divisia has an AAGR
of
7.51
percent,
com-
pared
with
5.04
percent
for the
Laspeyres
index.
Finally,
as the row
marked Paasche-All
shows,
the AAGR
of
the chained Paasche index
over the sixteen
years is,
at
7.
11
percent, slightly
smaller than
the
Divisia
index,
but two
percentage points larger
than the
fixed-weight
Laspeyres.
Differences between
the
Laspeyres
and
Divisia also
persist
when
antidepressants
are
disaggregated
into brands and
generics.
Although
Laspeyres-Divisia
differences are modest
for brands
(9.26 percent
com-
pared
with 8.83
percent
over the
sixteen-year
time
frame),
for
generics
the
disparity
is
larger
in
Cycle
B
(
-
6.79
percent compared
with
-
8.99
percent),
and
in
Cycle
C
it is
dramatic
(-5.66 percent
compared
with
-
18.93
percent).
Note that the
Divisia index
incorporates
new
gener-
ics,
unlike the
fixed-weight Laspeyres.

Ernst
R.
Berndt, lain M. Cockburn, and Zvi Griliches
171
If
one
disaggregates even further to
within brands and within
gener-
ics, for the
branded SSRIs, TCAs,
and others,
Laspeyres-Divisia dif-
ferences
are
modest, but for the generic
TCAs,
these
differences are
larger
in
Cycle
B
(-6.81 percent
compared
with
-
10.72
percent) and
enormous
in
Cycle
C
(-5.57 percent
compared
with
-22.30
percent).
It is also
interesting to note that
during Cycles
B
and C,
the oldest
branded
products, the MAOIs,
generally
have
larger Divisia
price
in-
creases than the
younger TCAs,
which
in
turn have
larger price
in-
creases than
the most recent SSRIs.
This
age-price pattern
is
consistent
with
the
product life cycle pricing
results reported by Berndt,
Griliches,
and
Rosett
for
all branded
prescription
products.60
Finally, no matter what index
procedure
is
employed, the
prices
of
generic products
clearly
have been
declining throughout
the
sixteen
years and,
as measured
by
Divisia
indexes,
the rate of
decline has
sharply
accelerated
over time.6'
Empirical
Evidence: Effect of
Linking Generics to Brands
We
now
consider effects
of
linking generic prices
to their
branded
versions, rather than
following the
old BLS
procedure
of
not
linking
them
at all.
There
are at
least
three
ways
to
introduce
a link
between
generics
and
their
patented
antecedents.
In
what we call
the "FDA
Average
Price
Procedure," generics
and branded versions
of the same
chemical
entity
are treated as
perfect
substitutes,
and the
average price
of the
entity
is
simply
a current-month
weighted average
of
generic
and
branded versions.
An
alternative
is
to assume that
preferences
for
brandedness are
uniformly
distributed and then to
adjust
for
diffusion
using equation
3. We call this the
Griliches-Cockburn
adjusted
"Paasche
Diffusion"
method
(GCPD). Finally,
we
mimic the new
procedure
recently adopted by
the
BLS,
in
which
the
Laspeyres fixed
branded
weight
is
split
into a 64.2
percent
generic component and a
35.8
percent
branded
component upon
initial
generic entry
and is
fixed
thereafter;
we call this the "New BLS Procedure with
Fixed
Split
60. Berndt,
Griliches,
and
Rosett (1993).
61. An
implication is that private sector price
indexes based only on brand
prices,
such
as the
PRIME index published by the National
Association of Chain Drug
Stores
(1995),
are
likely
to overstate
price
inflation
significantly.

172
Brookings
Papers:
Microeconomics
1996
Table 5. Average Annual
Growth Rates of Price
Indexes that Link
Generics to Their Patented
Antecedents
Percentage
Entire Period
Cycle
A
Cycle
B
Cycle C
1980:1- 1981:12-
1987:12-
1993:12-
Procedure
1996:2
1987:12
1993:12 1996:2
All
drugs, no link:
Paasche
7.11
9.87
7.45
2.29
Divisia
7.51 10.35 7.90
2.34
Laspeyres
5.04
9.35 4.17
0.42
FDA
Average Price
Procedure
All with
generics
-2.98
5.33
-6.49 -
17.22
All drugs linked
2.95
5.71
1.33
1.10
Griliches-Cockburn
Adjusted Paasche
Diffusion Procedure
All with
generics
0.96
6.97 0.42
-
12.68
All
drugs linked
4.73
7.08
4.44
1.69
New BLS
procedure
with fixed
split
generic/brand
weights
All with
generics
2.69 7.18
1.97
-4.15
All
drugs
linked
3.71
7.41
2.49 0.42
Source: Authors'
calculations,
see text for
explanation.
Generic-Brand
Weights.
"62 AAGRs
based
on
these three
linking pro-
cedures are
given
in
table
5. In each
panel
we
report
AAGRs of
price
indexes where generics have been
linked to
their
patented
antecedents,
first for the
subset of
drugs
that
experienced
generic entry
and then for
all
the
drugs
in
the IMS
antidepressant
universe with
generic
versions.
For
purposes
of
comparison,
at the
top
of
table
5,
we
give
AAGRs for
Paasche, Divisia, and
Laspeyres
indexes
with
generics
not
linked in at
all.
Of
particular
interest is
the
effect
that
linking
in
generics
has on
growth of the overall
antidepressant
drug price index,
and
how
this
effect varies
among
the three
alternative methods.
When
generics
are
linked
in
using
the FDA
Average
Price
Procedure,
AAGRs are affected
62. New
generics
are introduced within
cycles
with
split weights,
with the brand
portion
retaining
the base
price
of
the brand and with
the
generic portion
having
as its
base
price
the
price
of the brand in the time
period
prior
to
entry
date.

Ernst
R.
Berndt,
lain
M. Cockburn,
and
Zvi Griliches 173
dramatically, and the
overall price
index
grows
less than half as
fast as
it does when no
attempts are made to link in
generics.
In
particular,
during the entire
sixteen-year period, the generic-linked AAGR
grows
at
only 2.95
percent, compared
with
7.
11
percent
for
the
unlinked
Paasche index or
7.51 percent for the unlinked Divisia index.
This
difference is
particularly large during Cycle
B
when
considerable ge-
neric entry occurs,
with the linked AAGR being 1.33
percent, dramat-
ically lower than the
unlinked
Paasche
(7.45
percent)
or
unlinked
Div-
isia (7.90
percent); during Cycle
C
they
are somewhat
closer
at 1.
10
percent, compared
with 2.29
percent
and 2.34
percent.
Even under the
more conservative
GCPD
"split-the-difference"
as-
sumptions, the
impact
on
aggregate growth
rates of
linking
in
the
ge-
neric drugs is very
substantial.
As
seen
in
table 5,
AAGRs are about a
third lower than
they are
in the unlinked indexes.
Specifically, for the
sixteen years, the
generic
linked
price
index
grows
at
4.73
percent,
compared
with 7.11
percent
for
the unlinked
Paasche
index
and 7.51
percent
for the unlinked Divisia
index; again
the difference
is
largest
during Cycle B,
still
large
in
Cycle A,
and smallest
in
Cycle
C.
Finally, when
we mimic the new
BLS
procedure
of
splitting
the
Laspeyres
fixed
weight
into 64.2
percent generic
and
35.8
percent
brand
components
following generic entry,
we obtain
results
that
generally
yield
an
impact
in
between the
FDA and GCPD
procedures;
the
all-
drug
linked index
has an AAGR over the sixteen
years
of
3.71
percent.
Had the BLS
implemented
the
procedures
it will now
employ,
the fixed-
weight Laspeyres
AAGR would have been
only
2.49
percent
for
the
IMS
universe
of
antidepressant drugs during Cycle
B, substantially
lower than
the published
10.40
percent
based
on the
BLS
sample (which
entirely neglected
generics).
These results
dramatically
illustrate that the old
BLS
policy
of not
linking prices of
newly
introduced
generic goods
to
the
prices
of
their
branded
predecessors
exerted a
very
substantial
upward
bias
on
the
overall
price
index
for
antidepressant drugs.
The effects
of
the new
BLS
procedure,
beginning
in
January 1996, might
well be
expected
to
result
in
lower measured rates
of
price inflation,
all
else
being equal, although
the
magnitude
of
this effect
will
depend
on
which
products
will
lose
their
patent protection
and how
important they
are.
It
is worth
noting
that
in
the
antidepressant
market,
no
drugs
are
currently
scheduled to
lose U.S.
patent protection
before
the
year
2000.

174
Brookings
Papers: Microeconomics 1996
Empirial Evidence:
Hedonic
Regressions
As we
noted
earlier,
differences
in
the
efficacy rates
among the
various
antidepressant
drugs are
statistically
insignificant, but the
side-
effect and
adverse interaction
profiles
vary
considerably,
with
the
newer
generations
of
drugs
generally
having superior
characteristics;
these
side-effect
profiles
were summarized
in
table
1.
To
capture and
quantify
these
quality
improvements over
time, we have
undertaken
a hedonic
price
analysis. The
results we report here
represent
ongoing
research.
As the
dependent variable
in
the hedonic
regression,
we
compute
for
each
of
the branded
drugs
in
our
sample
the
monthly price
per daily
dose
equivalent for the
period 1980:1
to
1996:2;
this
price
is not
the
same as a
Laspeyres,
Paasche,
or
Divisia
index,
for
those indexes do
not
provide absolute
conmparisons
across
drugs.
Because
their charac-
teristics
are
so
different
from
other
antidepressant
drugs
and their
mar-
ket shares
are so small
(less
than 1.5
percent), the MAOIs
are excluded
from the
sample
of
branded
drugs.
That
leaves
up
to
nineteen branded
drugs
in
any
one month and
a
total of
2,478
observations.
The
specification
of
attributes or characteristics in
hedonic
price
equations
is
always
somewhat
problematic,
and that
is the
case here as
well.
Considerable
collinearity
frequently occurs
among
possible attri-
butes,
which is to
be
expected
in
this
case because of the
biological
and
chemical
relationships.
Another
specification
issue is that
knowledge
about
the attributes of
drugs
diffuses at
different rates even
though
these
attributes
are
relatively
constant
(indeed,
here we
have
them
fixed
over
time).
Further,
how one scales attributes
such
as
drug
side-effect
pro-
files
is
not
without
ambiguity,
because
frequency
and
severity
are not
necessarily
related.63
Given
these
difficulties,
we have chosen to
pursue
a
relatively simple
and
parsimonious
specification,
in
which
we
regress
the
logarithm
of
the
price
of the i"
antidepressant
drug
in
month
t
on a
constant,
on
193
monthly
time
dummies
(that
for
1980:1
is
omitted),
and on
several
attribute measures that
proxy
for
quality.
We
consider
six
quality
attri-
butes,
whose
values
are
given
in
table 1: HALF
(mean
half-life of
elimination,
measured in
hours),
DR
(a
drowsiness side
effect,
scaled
zero
for
rare
and
four
if
frequent),
AC
(anticholinergic
side
effects,
63. For an extended discussion
of
related
issues in the
specification of hedonic
equations
for
antihypertensive drugs,
see Berndt
and
Finkelstein
(1992).

Ernst R.
Berndt, lain M.
Cockburn, and Zvi
Griliches
175
zero to
four), GI
(gastrointestinal side effects,
zero
to
four), and
WTG
(weight
gain greater than
six kilograms, zero
to four). When
greater
frequency of occurrence of an
attribute such
as anticholinergic
side
effects is
usually considered
as
being
undesirable,
we
expect the
cor-
responding hedonic price
coefficient to be
negative. Our
specification
also
includes dummy variables
GEN
for
whether
the
brand
faced com-
petition
from
generics,
and OCD
if
the FDA had also
approved
the
antidepressant
drug
for treatment
of
obsessive-compulsive
disorders.
Estimation by ordinary
least squares
(OLS) yielded the
following
equation, with
heteroskedasticity-robust
standard errors
in parentheses:
ln
Pi,
-
0.217 + time
dummies
+
0.245
GEN -0.005 * HALF
(0.089)
(0.014)
(0.0002)
+
0.577 OCD
+
0.056
*
DR
-
0.056 AC
-
0.069 * GI
(0.027)
(0.006) (0.006)
(0.007)
-
0.277
WTG,
R2
= 0.8489.
(0.008)
Estimation with a random effects variance
components specification
yielded
same-signed coefficient
estimates, but
generally larger
standard
errors:
64
ln
Pi,
-
0.391
+
time dummies
+
0.125 GEN
-0.002 * HALF
(0.155)
(0.010)
(0.002)
+
0.312 OCD
+
0.064
*
DR
-
0.012 AC- 0.130
*
GI
(0.169)
(0.050) (0.081)
(0.053)
-
0.191 WTG.
(0.060)
Several results are worth
noting.
Consistent
with
previous
findings,
all
else
being equal, branded
products
have
higher prices after
facing
generic
competition;
here this effect
is
estimated at
between
12
percent
and
24
percent.
The
a
priori
expectation
on the
sign
of
HALF is am-
biguous,
for short half-life
is
beneficial
to
those
experiencing
serious
side effects
or
adverse
interactions,
but
longer
half-life
may
be
prefer-
able for those who
might
forget
to take
medication,
such as
the
elderly.
64. Note that
fixed-effect
estimation is not
feasible,
because
the
quality
attributes
are fixed over time for
each
drug.

176 Brookings Papers:
Microeconomics 1996
In
this market, the estimated impact is negative,
but it has
statistical
significance only
with
the OLS
estimates. The estimated
parameter
on
OCD is
positive
and
substantial
but of
marginal
statistical
significance
in
the random effects estimation. The a priori
expectation on the sign
of
drowsiness
is
ambiguous,
for a
considerable
number of
depressed
patients
with an
acute episode initially experience
insomnia,
and
thus
for
them the
DR
side effect
is
beneficial;
for
others, however,
it
may
be unwanted. The 0.06 positive estimates here
suggest
that DR
is,
on
balance, valued as beneficial
in
the marketplace.
The a priori sign expectation on the remaining
three attributes is
clearer, and
in
each case both OLS and random effects
parameter
esti-
mates are negative as expected. Specifically, more
frequent
anticholi-
nergic side effects (such as dry mouth, constipation,
urinary hesitance,
and
blurred
vision), gastrointestinal impacts,
and
substantial
weight
gain are each perceived as negative attributes
in
the
antidepressant
marketplace. The estimated impact
of
WTG
is
particularly large and
significant.
With
these estimated hedonic price equations
relating prices
of anti-
depressant drugs
to
their
quality
attributes and
time,
we
have the build-
ing
blocks
necessary
to
construct
a
price
index that links in
quality
change.
Changing Weights,
Hedonic
Adjustments for
Nonpriced Quality
Change,
and
Linking
Generic
Products:
Results
from
a
Merged
Analysis
As noted
earlier,
one
way
to link
nonpriced
quality improvements
into a
price
index is to use as an
approximation
to
the reservation
price
the
predicted price
of a
product just
before
its
market
introduction,
based
on
the estimated hedonic
price equation.
This
predicted price
is
then linked to the actual launch
price
of
the new
product.
When the new product
is
priced
above
(below)
its estimated
hedonic
quality
at
launch,
then the hedonic
residual
in
that month will
be
posi-
tive
(negative),
and the hedonic
quality-adjusted
linked
price
index
will
grow
at a
greater (lesser)
rate than the unlinked index. This
implies
that
residuals
from
estimated hedonic price equations
have an
important
impact,
for
they
are a measure
of
unpriced quality
change.
In
our
sample
of
ten new
products,
the launch
period
OLS hedonic residuals of six

Ernst R.
Berndt, lain
M.
Cockburn, and
Zvi
Griliches 177
Table 6. Effects of Simultaneously Linking Generics and New Products-
Average
Annual
Growth
Rates of Price Indexes
Percentage
Index number Entire period Cycle
A
Cycle
B
Cycle C
procedure 1980:1-1996:2
1981.12-1987:12
1987:12-1993:12
1993:12-1996:2
Paasche-all 7.11 9.87 7.45 2.29
(no link)
Paasche-all
4.73 7.08 4.44
1.69
(GCPD link,
no
hedonics)
Paasche-all 4.33
7.08 3.99
0.52
(GCPD
link
and
hedonics)
Source: Authors'
calculations;
see text for
explanation.
antidepressants
turned out
to
be
negative:
-0.22 for
Asendin, -0.63
for
Luvox, -0.62
for
Wellbutrin,
-0.31 for
Effexor, -0.45 for Zo-
loft,
and -0.07 for Serzone.
With
four new
products, however, the
launch
period
residuals were
positive:
0.03 for
Ludiomil,
0.05 for
Pro-
zac,
0.21 for
Paxil,
and 0.08 for Anafranil.65
Interestingly, while signs
of
residuals were
equally
mixed for
products
introduced
during Cycle
B,
in
Cycle
C all three
of
the new
products
had
negative
residuals
(that
is, positive nonpriced quality improvements).
Using
these
predicted prices
and
residuals,
we have
computed
a
Paasche
aggregate price
index over all
drugs, simultaneously linking
in
the
generics using
the GCPD method
and
accounting
for
nonpriced
quality
differentials
at launch month
for
the new
products;
as
quantity
weights
for
new goods
in this
merged index,
for
the
first
three months
in
the
market,
we
employ
the
average
over those
three
months.
Results
are summarized
in table 6.
The
net effect of our estimated
nonzero
residuals
on
the
growth of
the
aggregate
Paasche
price
index
depends,
of
course,
on
the
quantity
and
price growth paths
of all ten new
products;
note that six
new
products
had
negative
residuals
while four had
positive
ones at
launch
date,
and that all three
of
the new
products
introduced
during Cycle
C
had
negative
residuals.
As
seen
in table
6,
for
the
entire
1980:1-1996:2
time
period,
the
hedonic-adjusted
AAGR is
four-tenths
of
a
percentage
65. These
residuals
are based on the random
effects
model. Similar
findings
resulted
from OLS
estimates, as
well as from other
specifications involving
alternative
quality
attribute measures.

178 Brookings Papers: Microeconomics 1996
point
less
than one not incorporating
hedonic
quality adjustments (4.33
percent, compared
with 4.73
percent).
Because no
new
products were
introduced during Cycle A,
the
hedonic-adjusted
and
unadjusted
AAGRs
are identical
at 7.08
percent. Although the
AAGR of
the he-
donic-adjusted price
index is
about
half a
percentage point smaller
during Cycle
B
(3.99 percent, compared
with
4.44
percent), during
Cycle
C
the AAGR of
the
hedonic-adjusted price index lags more than
a
percentage point
behind the index
not
adjusted
for
quality (0.52 per-
cent, compared
with 1.69
percent). Thus,
somewhat
surprisingly,
link-
ing in generics has a much more substantial
effect on AAGRs of
price
indexes during Cycle B, when generic entry
was
substantial, than does
accounting
for
estimated nonpriced quality
differentials.
In
Cycle C,
however, the hedonic adjustment results
in
a
larger price decline than
does the
linking
in
of
generics.
Discussion
This detailed
audit
of
the IMS universe
of
antidepressant drug prices
reveals substantial differences between
the AAGRs
of the
published
BLS
price
indexes and
those
computed
in
a
variety
of
alternative
ways.
We
now
define our
"audited"
price
index with
generics
linked
in
using
the GCPD
procedure,
hedonic
nonpriced quality changes included,
chained
Paasche
weights,
and the IMS universe of
antidepressants
as
the
data
base. As the
bottom line of table 7
shows, during Cycle
B
this
audited
price
index
grows
at an
AAGR of
3.99
percent,
whereas
the
published
BLS
PPI has an
AAGR
of 10.40
percent.
For
Cycle C,
the
audited
price
index
has an AAGR of
0.52
percent,
much smaller than
the 4.27
percent
AAGR of
the
published
BLS
index.
In both
periods,
the
BLS
index overstates
price
inflation
by
a
very
substantial
amount.
It
is useful to summarize the sources
of
these differences
in
growth
rates,
which
we
do in tabular form
in
table
7.
The
first
source
of differences concerns
sample representativeness
of
the
fixed
weights.
If we
employ
the
BLS
Laspeyres fixed-weight pro-
cedures,
but instead
of
utilizing
the
BLS
item
sample,
we use
the IMS
universe
of
drugs,
we
obtain results
given
in
the second
row
of
table 7.
The
difference is
very large during
both
Cycles
B
and
C
and,
as noted
earlier,
reflects
the
fact that universe
item
weights
set
at
1987:12 and

Table
7.
Explaining
Differences
in
Average
Annual
Growth
Rates
of
Prices
for
Antidepressant
Drugs
Percentage
Entire
period
Cycle
A
Cycle
B
Cycle
C
Procedure
1980:1-1996:2
1981:12-1987:12
1987:12-1993:12
1993:12-1996:2
BLS
index
NA
NA
10.40
4.27
Sampling
procedures
Sample
vs.
universe
5.04
9.35
4.17
0.42
weights
Weights
Divisia-chained
7.51
10.35
7.90
2.34
Paasche-chained
7.11
9.87
7.45
2.29
Laspeyres-fixed
5.04
9.35
4.17
0.42
Laspeyres-chained
7.88
10.83
8.24
2.38
Linking
generics
FDA
average
price
2.95
5.71
1.33
1.10
GCPD
4.73
7.08
4.44
1.69
New
BLS
3.71
7.41
2.49
0.42
Linking
generics
and
hedonics
GCPD
4.33
7.08
3.99
0.52
Source:
Authors'
calculations:
see
text
for
explanation.

180
Brookings
Papers:
Microeconomics 1996
1993:12
would
subsequently
overweight
generics
whose prices
were
falling,
and
underweight
brands whose
prices
were
increasing. More
frequent
supplemental
sampling could
help
to reduce this
discrepancy,
although issues
concerning
Puerto Rican
production
would still
66
remain.
The
second
source of
differences
involves whether
fixed or
changing
weights
are used.
As
shown
in
the
third
panel
in
table
7, if
one
uses
the Divisia
or
Paasche index, based
on
monthly updates instead
of
the
relatively
fixed-weight Laspeyres
(changed
only
every
six
years),
over
the
IMS universe
of drugs, the AAGR rises from
3.3 to 3.7
percentage
points
during
Cycle B,
and about 1.9
percentage points
during
Cycle
C.
Weights
are
very important.
Obtaining
data
necessary
to
update
weights
more
frequently
is
of
course
possible,
but
not without costs. To obtain
some
evidence
on
the
possible
benefits
of
updating
weights
more
frequently,
we have
calcu-
lated AAGRs
of
Laspeyres price indexes over
the IMS
universe of
products
when,
like that for the chained Divisia
and
Paasche,
the
weights
are updated
monthly.
As
seen
in
the
bottom row
of
the
middle
panel
in
table
7,
when
Laspeyres weights
are chained on
a
monthly
basis,
AAGRs are
very
close but
slightly
larger
than those of the Div-
isia-7.88
percent, compared
with 7.51
percent over the
entire period.
Thus
what
is
empirically significant
is
failure
to
update
weights more
frequently, rather than choice
of
index number
formula.67
A
third source of differences concerns the
linking
of
generics
to
their
patented
antecedents.
As shown in the
fourth
panel
of
table
7,
the
effects of
linking
are substantial. Over the
last
sixteen
years,
the
AAGR
using
the FDA
procedure
is more than four
percentage
points
less than
that
of
a
comparable
Paasche index that
does
not link in
generics
(2.95
percent, compared
with
7.11
percent).
During
the same
period,
the
effect of
the
new BLS
procedure
is
only
slightly
smaller
(3.71
percent,
compared
with
7.11
percent).
During Cycle
B
the
new linked
procedure
would
have
generated
a
price
series
that
grew
at
2.49
percent
a
year,
almost
eight
percentage points
slower than its
published
fixed-weight
Laspeyres
(10.40
percent).
Because
patents
on
SSRI
drugs
in
the
United
66. See
footnote
47.
67. IMS America and the PRIME
Institute
construct
aggregate price
indexes
that
they
distribute
to
clients;
these indexes use
Laspeyres
index
number
procedures with
weights updated each
year, but generic products
are not linked to
predecessor brands.

Ernst R.
Berndt,
Iain
M.
Cockburn, and Zvi
Griliches
181
States all
expire after the year
2000, the
new
BLS policy that links in
generics
will not
have
any
measurable
impact
in this
therapeutic
class
for
some time,
but
in
other
therapeutic
classes
where patent
expiration
and
generic
entry
is more
extensive,
the effects could be
considerable.
The fourth
and final source
of differences
involves nonpriced
quality
differentials embodied
in
new
goods.
As shown in
the bottom
panel
of
table
7, during
Cycle
B
nonpriced
quality
results
in
a modest
difference
(less than half a
percentage
point for the
GCPD), but
during Cycle C
the effect of
accounting
for
nonpriced quality becomes
quite
substantial
(the
AAGR
is 1.2
percentage points
less than
the GCPD
index).
Al-
though
accounting
for
nonpriced
quality
change plays
a
significant mod-
erating role
in
the
growth
of
prices
in
the market
for
antidepressant
drugs,
the
impact
is
smaller
than that
in
other
high-tech markets.
For
example,
in
the
personal
computer market the
price
of
a
typical model
has been about
$2,500
for
more
than a
decade,
but that
$2,500
now
buys
much more
performance
in an
ever smaller
footprint.
As has
been
shown
elsewhere, quality-adjusted
personal
computer prices decline at
almost
30
percent
a
year,
more than double that for
price
indexes not
adjusted
for
quality change.68
Conclusions and Issues for Further Research
The market
for antidepressant
drugs
is
dynamic. Of the
twenty-one
entities
on
the market
in
1996, eight
are
entirely new,
having
been
introduced to the
market
within
the last
decade,
and an
additional
seven
brands
have
experienced
new
generic
competition
following patent
expiration.
Tracking prices and then
constructing
aggregate price
indexes
for
such
a
rapidly
changing
marketplace
are
challenging tasks,
particularly
for statistical
agencies
such as the
BLS
whose resources
are
tightening.
In
this
paper
we have
audited
the
reliability
of the BLS
producer
price
index in an
admittedly dynamic
market that
presents
enormous mea-
surement
challenges.
We
find
major
differences between the
published
BLS
numbers
and
the results
we obtain from
our
audit;
in
both
Cycles
68. For further discussion, see Berndt and Griliches (1993) and Berndt, Griliches,
and Rappaport (1995).

182
Brookings
Papers:
Microeconomics 1996
B
and C,
audited growth rates
are less
than
half those
published by the
BLS.
Of the
four
sources of difference that we have
examined
in
detail,
one-nonpriced
quality
changes
embodied
in
new
goods-plays
a mod-
est
to
significant
role
depending
on the time
period,
and
another-the
linking of new
generic products
to
their patented
antecedents-is
one
on
which the BLS has
just
recently announced
a
major
policy change.
The other two
sources
of
differences
(nonrepresentative
sampling, and
use
of
fixed-weight formulas)
could be
addressed
by
the BLS
obtaining
more frequent
(and more
costly)
information,
and
using
this data to
update
its
weights
more often.
Concerning
sample
representativeness,
one
issue that merits
greater
attention is
the treatment of
production
from
Puerto Rico. In
some of
the
national
accounts, national
production
includes Puerto
Rico;
in
others
such
production
is excluded. For
pharmaceuticals,
this
issue is
significant
because Puerto Rican
pharmaceutical
production
is
roughly
a fifth
to a
quarter
of
that
from
the U.S. mainland. If
Puerto Rico is to
be
excluded,
as is now the case for the
antidepressant PPI,
then to
the
extent
public
policy analysts
and others
seek
to
track the
price
growth
emanating
from U.S.
producers
(many
of whom
choose to
produce
significantly
in
Puerto Rico),
it will
be
necessary
to
collect
and
publish
"import" price series
from
Puerto
Rico,
and then to
combine those
data with
the more
narrowly
defined "domestic"
mainland
price
series.69
In
this
paper we have examined alternative measures of
price
growth,
but
we have not addressed the reasons
underlying
this
price
growth,
nor
have
we
attempted
to
model
quantities
sold
by
manufacturer and
the remarkable
growth
of the entire
therapeutic
class. It is
worth
em-
phasizing
that the measures of
price growth
presented
here
are
not
purported
to be
closely
related to measures
of
economic
welfare
and
consumers'
surplus.
There is reason to believe that
measures of
price
growth
employing predicted
prices
from
estimated hedonic
price equa-
tions could
differ
considerably
from
exact
price
indexes
that
employ
69. One incentive for Puerto
Rican
production
has
been
Section 936 of the
Internal
Revenue
Code,
which
has
provided
tax
benefits to firms
producing
in
Puerto
Rico. It is
worth
noting
that under the 1996 federal minimum
wage bill,
these
tax incentives will
be
phased
out over the next decade.

Ernst R. Berndt, Iain M. Cockburn, and Zvi Griliches 183
reservation prices based on estimated structural demand models.70
Pakes has argued that under a plausible set of conditions, the hedonic-
based prices provide an upper bound
to
growth
of
an exact price index .
It is worth noting that innovations providing new varieties of a product
such as antidepressant drugs have
an
unambiguous
beneficial
welfare
implication in that consumers are given the choice of another product,
one that
may
"work" for
them,
while
others
have not.
Although our analysis here has been confined
to
the market
for
an-
tidepressant drugs, our results
on
the importance
of
linking generics to
their patented antecedents may
have
implications
for
nonpharmaceuti-
cal markets.
For
example,
in the consumer electronics
industries,
new
products from branded manufacturers typically embody quality
im-
provements; these manufacturers subsequently often
sell a
virtually
identical product under a private label
or "knock-off" brand at a
much
lower price.72 To the extent that such lower-priced
versions are
not
linked to branded antecedents, the price indexes
will
fail to
incorporate
these implicit price
declines realized
by
some consumers.
Our
research
could be extended
in a number
of
ways. First,
our data
is from
the IMS, and
it
would
be useful
to
compare
the
IMS
price
data
with
actual transactions data
from the
pharmaceutical
manufacturers,
although
our
earlier
research
on this issue did not reveal
any systematic
differences.73
Second,
as
implied earlier,
it would be informative to
model much more completely
the diffusion
process
of
antidepressant
drugs, both
over time and
among
different market
segments,
such as
drugstores
and
managed
care
organizations.
It
would also be useful
to
assess how the various
price
indexes
change
when the
frequency
of
the
data
is
reduced
from
monthly
to
quarterly. Third,
we
have examined
here only
the
drug component
for the treatment
of
depression, entirely
ignoring
"substitutable"
inputs
such
as talk
therapy. Assessing
the
effects
of
the new
generation
of
SSRIs, along
with
increased
pressures
of
cost
containment,
on
the
changing
mix of
drug
and talk
therapy
in
the
overall treatment
of
depression
is
a most
interesting
avenue for
future research.
70. For
discussion,
see
Triplett (1983).
71. Pakes (1996).
72.
Several other examples of
this
brand-generic phenomenon
are
discussed
in
De-
neckere-McAfee (1996).
73. Berndt, Griliches, and Rosett
(1993), and Berndt and Greenberg (1996).

184
Brookings Papers: Microeconomics
1996
References
Advisory
Commission To Study the Consumer
Price Index. 1996. Toward a
More
Accurate
Measure of the Cost of Living.
Final
report
to
the Senate
Finance
Committee. Washington, D.C. December
4.
American Medical
Association. 1991. "Drugs
Used
in
Mood
Disorders."
Drug
Evaluations, 7th ed.
American
Psychiatric Association, Committee on Nomenclature and
Statistics.
1968.
Diagnostic and Statistical Manual of Mental
Disorders,
2d
ed. Wash-
ington, D.C.
American Psychiatric
Association,
Task
Force on
Nomenclature and Statistics.
1980. Diagnostic
and Statistical Manual of Mental
Disorders,
3d ed. Wash-
ington, D.C.
American Psychiatric Association. 1987.
Diagnostic
and Statistical Manual of
Mental Disorders:
DSM-III-R,
3d
ed.
rev.
Washington, D.C.
.
1993. "Practice
Guidelines
for
Major
Depressive Disorder
in
Adults."
American Journal
of Psychiatry
150
(4, April,
Supplement):
1-26.
Baldessarini, Ross
J. 1990. "Drugs
and the
Treatment
of
Psychiatric Disor-
ders."
In
The
Pharmacological
Basis
of
Therapeutics, edited by Alfred
Goodman Gilman and
others,
383-435.
8th
ed.
New
York:
Pergamon
Press.
Berndt, Ernst R., and Paul E.
Greenberg. 1996.
"An
Updated and Extended
Study
of
the Price Growth
of
Prescription
Pharmaceutical
Preparations."
In
Competitive
Strategies
in
the
Pharmaceutical
Industry,
edited
by
Robert
B.
Helms,
35-48.
Washington, D.C.:
American
Enterprise
Institute.
Berndt,
Ernst
R.,
Richard
G. Frank,
and Thomas G.
McGuire.
forthcoming.
"Alternative Insurance
Arrangements
and the
Treatment
of
Depression."
American Journal
of Managed Care.
Berndt,
Ernst
R.,
and Stan N.
Finkelstein. 1992.
"Price
Indexes
for Anti-
Hypertensive
Drugs
that
Incorporate Quality
Change:
A
Progress Report
on
a
Feasibility
Study." Unpublished working paper
6-92. MIT
Program
on the
Pharmaceutical
Industry, Cambridge,
Mass.
December.
Berndt,
Ernst
R.,
and Zvi
Griliches. 1993. "Price Indexes for
Microcomputers:
An
Exploratory
Study."
In Price Measurements and Their
Uses,
edited
by
Murray
F.
Foss,
Marilyn
E.
Manser,
and
Allan
H.
Young,
63-93.
University
of
Chicago
Press
for
the
National
Bureau
of Economic
Research.
Berndt,
Ernst
R.,
Zvi
Griliches,
and Joshua G.
Rosett. 1993.
"Auditing
the
Producer Price Index:
Micro
Evidence
from
Prescription
Pharmaceutical
Preparations.
"
Journal
of
Business and Economic Statistics
11
(3, July):
251-64.
Berndt,
Ernst
R.,
Zvi
Griliches,
and
Neal
J.
Rappaport.
1995.
"Econometric
Estimates
of
Price Indexes
for
Personal
Computers
in
the 1990's." Journal
of
Econometrics 68
(1, July):
243-68.

Ernst
R.
Berndt, lain
M.
Cockburn, and
Zvi
Griliches 185
Bureau
of
the Census,
U.
S. Department
of
Commerce.
1992.
1992
Census of
Manufactures, Industry Series Drugs. MC92-I-28C. Washington, D.C.
. 1994.
1992
Economic Census of Outlying Areas, Industry Statistics-
Puerto Rico. Washington, D.C.
Bureau of Economic Analysis, U.S. Department of Commerce. 1985a. "Busi-
ness Situation.
"
Survey of Current
Business 65
(10, October):
1-7.
. 1985b. "Revised
Estimates of the National Income
and
Product Ac-
counts of the
United States,
1929-85:
An Introduction."
Survey of
Current
Business 65 (12, December): 1-19.
. 1987. "Foreign Transactions."
In
Methodology Papers:
U.S.
Na-
tional Income and Product Accounts.
Washington,
D.C.
May.
Bureau
of
Labor Statistics, U.S. Department
of Labor.
1996.
Producer
Price
Indexes:
Data
for January
1996.
Washington,
D.C. June.
Caves,
Richard
E.,
Michael D.
Whinston,
and Mark A. Hurwitz.
1991. "Pat-
ent
Expiration, Entry,
and
Competition
in
the
U.S.
Pharmaceutical Indus-
try." Brookings Papers
on Economic
Activity,
Microeconomics:]. 1-48.
Cleeton,
David
L., Valy
T.
Goepfrich,
and Burton
A.
Weisbrod.
1992. "What
Does
the Consumer
Price Index
for
Prescription Drugs Really
Measure?"
Health
Care Financing
Review
13
(3, Spring):
45-51.
Deneckere, Raymond J., and
R.
Preston McAfee. 1996. "Damaged Goods."
Journal of Economics and Management Strategy
5
(2, Summer): 149-74.
Depression Guideline
Panel.
1993. Depression
in
Primary
Care.
Vol.
2:
Treat-
ment
of Major Depression.
Clinical Practice
Guideline,
No.
5, Rockville,
Md.:
U.
S. Department
of Health and Human
Services,
Public
Health Ser-
vice, Agency
for Health
Care Policy
and
Research,
AHCPR Publication
93-0551.
Early,
John
F.,
and James
H.
Sinclair. 1983.
"Quality Adjustment
in
the
Producer
Price Increases."
In The
U.S.
National
Income and Product Ac-
counts: Selected
Topics,
edited
by Murray
F.
Foss. Studies
in
Income
and
Wealth,
vol. 47.
University
of
Chicago
Press
for
the
National
Bureau of
Economic Research.
Eisenberg,
Leon.
1992. "Treating Depression
and
Anxiety
in
Primary
Care:
Closing
the
Gap
Between
Knowledge
and Practice." New
England
Journal
of
Medicine 326
(April 16):
1080-84.
Elkin, Irene,
Morris
B.
Parloff,
and others.
1985.
"NIMH
Treatment of
Depression
Collaborative Research
Program."
Archives
of
General
Psychia-
try
42
(March):
305-16.
Elkin, Irene,
Tracie
Shea,
and others. 1989.
"National
Institute of Mental
Health Treatment of
Depression
Collaborative
Research
Program:
General
Effectiveness
of
Treatments.
"
Archives
of
General
Psychiatry
46
(Novem-
ber):
971-83.
Fisher,
Franklin
M.,
and Karl Shell. 1971.
"Taste and
Quality Change
in
the

186
Brookings
Papers:
Microeconomics
1996
Pure Theory of
the
True-Cost-of-Living Index."
In
Price
Indexes and Qual-
ity Change:
Studies
in
New Methods
of
Measurement, edited
by
Zvi Gril-
iches, 16-54.
Harvard
University Press.
. 1972.
The Economic Theory
of
Price Indices: Two
Essays on the
Effects of Taste
Quality and
Technological
Change. New
York:
Academic
Press.
Fisher, Franklin
M., and
Zvi
Griliches. 1995.
"Aggregate Price
Indices, New
Goods,
and
Generics."
Quarterly
Journal
of Economics
110
(1, February):
229-44.
Food and Drug
Administration.
annual.
Approved
Drug Products
with Thera-
peutic
Equivalence
Evaluations ("The Orange
Book").
Washington, D.C.:
U.S.
Department
of Health and Human
Services,
Public Health
Service,
Center
for
Drug
Evaluation and
Research,
Office of
Management.
Frank, Richard
G., and David S. Salkever.
1992.
"Pricing, Patent
Loss, and
the
Market for
Pharmaceuticals."
Southern Economic
Journal
59 (2, Octo-
ber):
165-79.
Frank, Richard
G., and
Willard
G. Manning, Jr.
1992. Economics
and Mental
Health.
Johns
Hopkins
University
Press.
Grabowski,
Henry G.,
and John
M.
Vernon. 1992. "Brand
Loyalty, Entry,
and
Price
Competition
in
Pharmaceuticals
after the
1984
Drug
Act.
"
Journal
of
Law and
Economics
35
(2,
October):
331-50.
Greenberg,
Paul
E.,
Laura E.
Stiglin,
and
others.
1993.
"The
Economic Bur-
den of
Depression
in
1990.'"
Journal
of
Clinical
Psychiatry
54
(11,
Novem-
ber):
405-18.
Greenberg, Paul
E.,
Ronald
C.
Kessler,
and
others. 1996.
"Depression
in
the
Workplace:
An Economic
Perspective."
In
Selective
Seratonin
Reuptake
Inhibitors:
Advances in Basic Research and Clinical
Practice, edited
by
J.
P.
Feighner
and
W. F.
Boyers,
321-58. 2d ed.
Chichester,
U.K.:
John
Wiley
and
Sons.
Griliches, Zvi,
ed. 1971.
Price
Indexes and
Quality Change:
Studies
in
New
Methods
of
Measurement. Harvard
University
Press.
.
1990. "Hedonic Price Indexes and the
Measurement
of
Capital
and
Productivity: Some
Historical
Reflections.
"
In
Fifty
Years
of
Economic
Mea-
surement: The Jubilee
of
the
Conference
on Research in
Income and
Wealth,
edited
by
Ernst R.
Berndt
and
Jack
E.
Triplett.
NBER
Studies
in
Income
and
Wealth,
vol. 54.
University
of
Chicago
Press
for
the
National
Bureau
of
Economic Research.
Griliches, Zvi,
and lain
Cockburn. 1994.
"Generics and
New Goods
in
Phar-
maceutical Price Indexes." American Economic Review 84
(5,
December):
1213-32.
Hicks,
John R. 1940.
"The Valuation
of Social
Income." Economica 7
(26,
May):
105-24.

Ernst R. Berndt,
Iain
M.
Cockburn, and
Zvi
Griliches 187
Hurwitz, Mark A., and Richard E. Caves. 1988. "Persuasion
or
Information:
Promotion and the Shares of Brand Name and Generic
Pharmaceuticals."
Journal of Law and Economics
31
(October): 299-320.
Hyman, Steven E., George W. Arana, and Jerrold
F.
Rosenbaum.
1995. Hand-
book of Psychiatric Drug
Therapy,
3d ed.
Little,
Brown and
Company.
IMS America. 1993. National Disease and Therapeutic Index-Drugs,
July
1993-June 1994. Plymouth Meeting,
Pa.
.
1996a.
National
Disease
and
Therapeutic
Index-Disease, July 1995-
June 1996. Plymouth Meeting,
Pa.
.
1996b. Information
Services Manual.
Plymouth
Meeting,
Pa.
Jonsson, B., and
J.
Rosenbaum. 1993.
Health
Economics
of
Depression. Vol.
4:
Perspectives
in
Psychiatry.
Chichester,
U.K.:
John
Wiley & Sons.
Kanoza, Douglas. 1996. "Supplemental Sampling
in
the
PPI
Pharmaceuticals
Index." Producer
Price Indexes Detailed
Price
Report (January):
8-10.
Katon, Wayne, and others. 1992. "Adequacy
and Duration of Antidepressant
Treatment
in
Primary Care." Medical Care
30
(January):
67-76.
Keith, Alison M., and Ernst R. Berndt. 1994.
"A
Primer
on Issues
in
the
Measurement of Price Change
and
Price Impact:
An
Application
to Phar-
maceuticals." Working Paper
25-94.
MIT
Program
on
the Pharmaceutical
Industry, Cambridge, Mass. September.
Masson, Alison, and Robert
L.
Steiner.
1985. Generic Substitution and Pre-
scription
Prices: Economic
Effects of
State
Drug
Product
Selection Laws.
Washington, D.C.: Federal Trade Commission,
Bureau of Economics.
National Association of Chain Drug Stores. 1995. "NACDS
PRIME
Index
Reports Increases in Prices
for
Top
500
Prescriptions
Dispensed
in
Com-
munity Pharmacies." News release, Alexandria, Va., December
7.
Nolen-Hoeksema, Susan. 1990. Sex Differences
in
Depression.
Stanford Uni-
versity Press.
Pakes, Ariel. 1996. "Hedonic
Bounds
to
Exact
Price
Indexes." Unpublished
draft
manuscript.
Yale
University, Department
of
Economics.
May.
Papolos, Demitri F.,
and Janic
Papolos.
1992.
Overcoming
Depression, rev.
ed.
Harper
Collins
Publishers.
Physicians' Desk Reference
Generics. 1996.
2d
ed. Montvale, N.J.: Medical
Economics
Company.
Potter, William Z.,
Matthew V.
Rudorfer,
and Husseini Manji. 1991. "Drug
Therapy:
The
Pharmacologic
Treatment
of
Depression."
New
England
Jour-
nal
of
Medicine
325
(9, August 29):
633-42.
Regier, Darrel A.,
and others.
1988. "The
NIMH
Depression
Awareness,
Recognition,
and Treatment
Program: Structure, Aims,
and Scientific Ba-
sis.
"
American Journal
of Psychiatry
145
(November):
1351-57.
Rothbarth, Erwin. 1940-41.
"The Measurement
of
Change
in
Real Income
under Conditions
of
Rationing."
Review
of
Economic Studies
8
(2):
100-7.

188
Brookings Papers. Microeconomics 1996
Suslow, Valerie Y.
1996. "Measuring Quality
Change
in
the
Market for
Anti-
Ulcer Drugs."
In
Competitive Strategies
in
the Pharmaceutical
Industry,
edited
by
Robert
B.
Helms.
Washington,
D.C.: American
Enterprise
Insti-
tute.
Triplett, Jack E.
1983. "Concepts of Quality
in
Input
and
Output Price Mea-
sures: A
Resolution of the User-Value Resource-Cost Debate."
In
The
U.S.
National
Income and
Product Accounts:
Selected
Topics,
edited
by Murray
F. Foss.
University of Chicago Press for the National
Bureau
of
Economic
Research.
Turkington, Carol
Ann, and Eliot
F.
Kaplan. 1994.
Making the Prozac Deci-
sion:
Your
Guide
To
Antidepressants.
Los
Angeles:
Lowell
House.
U.
S. General
Accounting Office.
1996. Consumer
Price
Index:
Cost-of-Living
Concepts
and the
Housing
and Medical
Care
Components. Report
to
the
Ranking
Minority Member, Committee
on
Banking
and
Finanical Services,
House of
Representatives, GAO/GGD-96-166.
Washington, D.C. August.

Comments
Comment
by
Theodore E. Keeler:
This
is
an
interesting paper
with
important and
worthwhile contributions.
Many complain about
rapidly
rising costs in
the
health
care sector, yet
because
of
the complexity of
the product and
changes
in
technology
and
product quality,
it is
espe-
cially difficult
to
determine whether these
rapid
cost increases
stem
from higher
prices, higher quantities,
or
quality improvements.
The
present paper
adds valuable insight into this area.
The results
are
of
special interest
in that
they indicate the
importance
of
understanding
the value
of
improved products
and
of
reduced
costs
from generic
products.
The
results
show
also
the importance
of
quality
change and represent innovative use
of
hedonic
price indexes, and
they
represent a clear
improvement
over
conventional
BLS
price indexes.
Overall, then,
I
find
much
to
agree
with
in
this
paper.
Nevertheless,
I
do have
an
area
of
concern:
specifically,
all
the index
number theory
employed by
the
authors of this
paper
assumes that
prices
of each
drug
represent
a
consumer's
marginal willingness
to
pay
for
those
drugs.
For
many people, however,
prescription drugs
are
paid
for
through
health
insurance,
and the existence
of
moral hazard
combined
with
reasonably generous health insurance
polices
can call into
strong
question
the
validity
of the
simple proposition
that
prices represent
consumers'
marginal willingness
to
pay
for the relevant
products.
It is
easy
to see
why
a
study
such as
this
would
abstract from
the matter of
moral hazard with
insurance, because
the
likely
effects are
complicated,
and the
present
study
innovates
in other
directions.
Nevertheless,
I
believe
it
is worthwhile
to
try
to
understand
some-
thing about these
effects,
because
they
are
potentially important. Any-
189

190
Brookings Papers:
Microeconomics 1996
thing
near a
complete
or
rigorous
discussion of
these
effects are
well
beyond
the
scope
of these comments and would be a
paper
in
itself; my
goal, instead,
is to sketch
very
crudely
and
intuitively
some
likely
effects
of
moral hazard
for this
analysis, suggesting,
I
hope,
both
the
importance
of
the issue
and
the need
for
further
research.
Moral Hazard and Pharmaceutical Price Indexes
Health insurance
will
likely
exaggerate
a consumer's
apparent
mar-
ginal willingness
to
pay
for newer or more expensive drugs. Take the
case
that the authors make
of
the
introduction of
a generic substitute
for an
existing
branded
drug.
Because
the two
drugs are therapeutically
the
same,
one would
expect patients
to
move in
large
numbers to
the
generic
if
the
branded
drug
costs much more. In
fact,
that
does not
always happen.
The
authors of this
paper and Griliches
and
Cockburn
in
an
earlier
(1994)
article are
puzzled
slightly by the
fact
that
even if
the
price
of a
branded
drug
is much more than
that of the
generic,
the
branded
drug
maintains much more market share than one
might expect.
I
strongly
believe that
health
insurance
should
explain
at least
some of
that:
if the
patient pays only
10-20
percent
of
the difference
in
prices
(or
even
less),
then
the
patient
might
well
prefer
the
branded
product,
if
it is thought to be
even
modestly
superior.
A
study
that
ignores
this
fact
may
tend to overstate consumers'
true
willingness
to
pay
for
more
expensive products
of
any type.
To
see this
point
from a
slightly
different
perspective,
consider
the
following grossly simplified
case. Recall
from
the
paper
that
one rela-
tively
accurate
way
to
incorporate
a
new
product
into
a
price
index
is
to
include the
reservation price
of a
"typical" consumer
for
the
product
just
before it is introduced. BLS indexes are asserted
to
overstate in-
creases
in
these
prices
in
part
because
they
fail to
incorporate
these
relatively high
reservation
prices
at
the earliest
time of
introduction of
the
product.
Moral hazard
may
cause
this
estimated reservation
price
to
be over-
stated,
however. To see
this,
consider
the effects
of the
introduction of
a
new
(expensive) drug
with the
simplest (but
most
expensive) likely
form
of
health
insurance,
that which
simply
reimburses the
provider
(doctor, drug store, hospital,
and so
on)
a
fixed
percentage (often
75 to

Ernst R. Berndt, lain M. Cockburn, and Zvi Griliches 191
80 percent) of total expenses (with no overall limitation on them), so
that the patient makes a copayment of, say, 20 percent and the insurance
company pays the rest. If, in fact, the consumer's willingness to pay
per dose is $1, then, with the
introduction
of
insurance, the observed
reservation price (including that observed in econometric studies such
as are included in this paper)
will
be $1.00/0.2
=
$5-much higher
than the real reservation price.
Any discussion
of
biases
in
an
estimated
index
would have
to
deal
with relative changes in prices over time, and real-world insurance
is
also
more complicated-involving
issues that
go beyond my
comments
here. But it would seem quite possible
that moral
hazard
could
easily
bias
upward the estimate
of a
person's reservation price
for a new
drug,
which could clearly affect calculations of price indexes.
To make our analysis more realistic, consider
the
case
of two sub-
stitute drugs, a branded one and
a
new generic. Assume also that res-
ervation
price differentials
are distributed
among patients,
so that some
will
switch to the generic at a
low
price differential, but others require
a
higher differential. This realistic situation
is the
one on
which
the
authors have built up a new
Paasche
index
of
pharmaceutical prices.
Once again, however,
moral hazard could
easily
render the use
of
direct
comparison
of
market prices
inaccurate.
Suppose
that
one
particular
consumer is observed to be willing
to switch
from
the brand to the
generic at a price difference
of
$1 per daily
dose
(that is,
if
the brand
costs $2, the consumer
will
buy
the
generic only
if
its
price
falls
to
$1
a
dose).
In
reality, though,
if
insurance
is
covering
80
percent
of the
difference, the real reservation price
differential
is
only
20
cents:
ob-
served market
data will
exaggerate
the
apparent willingness
of the con-
sumer to pay for the more expensive drug.
If
this
simplified
view
of
reality
is
correct,
it has
clear
implications:
consumer preferences
could
easily
be closer
to the FDA
approach
of
considering
branded and
generic drugs equivalent
than the observed
data
would
suggest.
This view
also
implies
that the
coefficients
in the
hedonic price equations might
well
also
overestimate
the
consumer's
marginal willingness to pay
to obtain the
positive aspects
of a
drug,
or
to
avoid the negative effects,
relative to the consumer's true
prefer-
ences.
Why
not
buy expensive drugs
with fewer side effects
if insurance
is
paying for
it?

192 Brookings Papers:
Microeconomics 1996
Theoretical and Empirical Validity of Moral Hazard
in
Pharmaceuticals Consumption
Most health economists are convinced
that moral
hazard
is
rampant
in the fee-for-service part
of
the
U.S. health care
sector,
but
not
every-
one might agree.
After
all, people
seem to want
to
buy
health insur-
ance-in some meaningful sense, maybe
what their insurance
pays
is
a
measure
of
the value they
attach to medical care. Where
is
the market
failure? There are
strong
reasons
to
question
that.
First,
the
tax
benefits
accorded
to
employer-provided
health care have
historically
tended
to
cause overprovision
of
it; second, during
the 1960s and 1970s, Blue
Cross and
Blue Shield had
a
largely
artificial
monopoly
on U.S. health
insurance, and they represented the interests
of the
providers
(doctors
and hospitals) who controlled
them.
'
For various reasons they endea-
vored to continue the existence of
this
type
of
fee-for-service
indemnity
insurance. The growth of managed
care in the 1980s and 1990s is a
sign that over the very long pull,
the
marketplace
will not
support
this
moral hazard (more about
that
below),
and
it is
likely
to
be less
impor-
tant today than
it
was in 1980,
the first
year
of the
study.
In health care as
a
whole,
there is
ample
evidence
that
American
patients
are indeed
prone
to moral hazard
in a
fee-for-service
setting.
This evidence
is
based not only
on
econometric studies,
but also
on
a
large, expensive, controlled experiment conducted by the
RAND
Cor-
poration,
in which
a
carefully
selected
sample
of the
population
was
given varying amounts
of
insurance,
and
the
amount of health
care
then
consumed was observed.2
There
is, however,
less evidence
as
to
whether
expenditures
on
phar-
maceuticals (typically covered by insurance) are prone
to moral
hazard.
One
study,
that
of
Sean Sullivan
(1992),
based
on
the
elderly
with and
without
pharmaceutical supplemental
insurance to
Medicare,
provides
strong evidence
that
patients
are indeed
responsive
to
pharmaceutical
prices, and
that moral hazard does exist
in
this
area.3
Specifically,
the
1. For a discussion of these forces
in health
insurance, see,
for
example,
Feldstein
(1993, pp. 149-69).
2. For a survey
of
the effects
of moral hazard
in health
insurance
on
the demand for
health care in general, see any good textbook
in
health economics, such as Feldstein
(1993, pp. 74-105). For results of the RAND experiment, see Manning and others
(1987).
3. Sullivan (1992).

Ernst R. Berndt, lain
M. Cockburn, and Zvi
Griliches
193
study
found that
controlling
for
many
other
variables, patients with
drug insurance spend
12.5 percent a year more on
drugs
than
do patients
without such insurance. Another recent
study, however, found that
insurance status of
patients does not
significantly affect
whether the
patient
is
prescribed
a
generic
or brand
drug, except
that
HMO
patients
are
more
likely
to be
prescribed generic
drugs (see below).4
The
data
set,
however,
did not
include basic variables
such as
drug prices.
Rika
Mortimer,
a Ph.D.
student
at
Berkeley,
is
currently
investigating
this
very issue but has no
results
as
yet.
Managed Care
Given the costs of moral
hazard,
it
is
reasonable
to
expect
that
the
market would provide
forms
of insurance that
avoid
it.
Health
mainte-
nance
organizations
(HMOs) clearly
do have an incentive to
use less
expensive
drugs
and to avoid moral hazard. That
is
because
they
collect
a
fixed
(capitation)
fee
per patient per
unit of
time,
and
the
less
they
spend
on health
care,
the
more
money
they
make.
Empirical
evidence
indeed
shows
that
patients
in
HMOs
do
indeed use
generic
drugs
more
than
do
patients using
more conventional
indemnity
insurance with fee-
for-service reimbursement.
This
fact has
implications for the authors'
empirical
work.
Specifi-
cally,
it
implies
that
in
years
in which HMOs
had
a
greater
market
penetration,
the effects
of
moral hazard on their hedonic
coefficients
are
likely
to
be weaker. The market
share
of HMOs has
indeed
grown
from
1980
to the
present.5 That means that the
observed
willingness
to
pay
for
various attributes
(and
hence
the
coefficients)
may
well
have
shifted
substantially
over
time.
It would seem to make
sense
to
test for
that
possibility,
but
to
keep
in
mind
that this
change may
represent
not
changes
in
consumer
preferences,
but
changes
in insurance
reimburse-
ment.
4. Hellerstein
(1994).
5. According to
Oberlander (forthcoming), as of
1995,
58
percent
of
insured em-
ployees of firms with
more than ten employees belonged to
indemnity or PPO plans,
whereas fully 42
percent belonged to HMOs or point-of-service
plans.
In
1994, 62
percent belonged
to
indemnity plans
or PPOs. In
contrast,
in
1990
HMO
penetration
rates were only 15
percent or so. HMO penetration of Medicare
is
currently
still mini-
mal-only 10 percent
of Medicare enrollees are enrolled
in
any
form of
managed care.

194
Brookings
Papers:
Microeconomics
1996
An
Opposite
Bias
with
Fee-for-Service
Insurance
In the
specific
case of
antidepressants,
it is
possible
to find
an ex-
ample with
fee-for-service
reimbursement
in which
the
techniques used
by the authors
might possibly
underestimate
the
marginal
willingness
of
consumers
to pay
for
drugs.
To see this,
recall that
talk
therapy is
often viewed as a substitute
for
drugs.
Furthermore,
talk
therapy
is
quite expensive relative
to
many drugs (a
once-a-week
visit to a
psy-
chiatrist can
average out
to
$15 to $20 a
day-more
than the cost of
the daily dose
of many drugs).
If
talk
therapy were
covered
by insur-
ance, patients
might prefer
a
talk-intensive,
drug-free (or
drug-exten-
sive) treatment.
Conversely,
consumers
who have to
pay
out of
their
own
pockets
might
instead
prefer
a
drug-intensive
treatment,
with
little
talk
therapy,
even at relatively
high drug prices.
So elimination
of
moral
hazard in
mental health treatment
could
actually raise the
willingness
to
pay for
drugs relative to what is
happening
with
insurance
today.
This
might seem
probable, given
that,
as the
authors
point
out,
HMOs
often prefer
to
substitute drugs
for
talk
therapy
if
they can,
and
HMOs
may
in some
ways
mimic health
care consumers would choose in
the
absence
of moral hazard. Even ten
years ago,
however,
most
indemnity
and
PPO
(preferred provider
option)
insurance
companies,
even if
they
would
reimburse
most
things
on a
fee-for-service
basis, nevertheless
tended
to
put
tight
restrictions on
reimbursement
for
psychotherapy,
because
it was known to be
especially prone
to moral hazard.
This
means that the amount
of
moral
hazard
connected to talk
therapy
is
likely to be
limited in most
standard
insurance
policies.
Obviously
these comments
only
sketch a
rough
outline
of
some
of
the
effects
of
health
insurance on
the
authors'
results.
Nevertheless,
I
hope it is clear now
why
I
believe
that
if
we are to
understand and
interpret those
results
accurately,
we need
to
better
understand the
implications of health insurance on
pharmaceutical
consumption and
why further research
in this
direction
is
justified.
Comment
by
Martin Neil
Baily:
The
general
discussion of
depression
and
its
treatments
in a
historical
setting
I found
very
interesting.
It
is
unusual to see
such a section
in
an
economics
paper,
but as a
sometime
macroeconomist,
I
found
it
refreshing
to see human
beings
described
with normal frailties and
not
just
as
maximizing
robots.

Ernst R. Berndt, lain M. Cockburn,
and
Zvi
Griliches
195
Moving to the meat
of
the
paper,
I
had trouble at the outset because
the interpretation of a producer
price
index
(PPI)
for the
ethical
drug
industry is problematic. The
issue
surfaces almost immediately when
it
is discovered that Prozac,
a principal innovation in recent years,
is
not
included in the sample because
it
is
not manufactured in the United
States. The true value
added in the actual manufacture
of
drugs
is small
for
most drugs, and
the final price
is
determined by research
and
de-
velopment costs, marketing,
and profit. The ex-factory price
of the
drug
is set by the company based
in part on taxes and does not accurately
reflect the contribution
to value by
the
manufacturing process.
Prozac
and other
drugs may
be
manufactured
in
Puerto
Rico,
but their value
was created by research and
development and marketing carried
out
in
the
United States or, possibly,
overseas.
PPIs are often used in the
calculation of real manufacturing
output
and hence productivity,
which can create significant distortions.
In
the
case of "foreign" manufacture,
the value may be incorrectly
attributed
by country,
but even when
all
of
the value-added
is
created
within
the
United
States,
there is misattribution
by industry.
This
paper
does
not
use
the PPIs for that
purpose,
so I
am
not
criticizing
what
they
have
done. But
thinking
about
this
problem
made me realize
how
difficult
it
is to use PPIs for real output
computations
in a world where the non-
manufacturing input
to
production
is
growing.
Ethical
drugs
are
at one
end of a
spectrum,
but the same
problem
arises elsewhere. The value-
added created by an
auto or a machine
tool
plant depends
heavily
on
the design and process engineering
that is done elsewhere.
In
this
paper,
the
authors are
essentially treating
the
PPI for antide-
pressants as
an
input
into
the
consumer
price
index.
The
discussion
of
generics
and the
use of hedonic
regressions
all
go
in
this
direction,
so
I will
simply accept
that framework without
further
comment. The
interesting
issue then
is how the
BLS
deals
with
generics
and new
products and
how the results
of what
they
do
compare
with
approaches
suggested by
index number theory.
The authors
deserve
a lot
of
credit,
as indeed
they
do for their
previous work in
this
area,
for pointing
out
some
of the
problems
that
exist in BLS
procedures-in
particular,
the
fixed market basket that
delays
the introduction of
major
new
products
and that
delays recog-
nition of
changes
in
market shares.
The authors
also
point
to some odd
quirks
of the BLS
sample,
which
apparently
includes
drugs
that are not

196
Brookings
Papers: Microeconornics 1996
antidepressants.
They make some important points and add
significantly
to their earlier
work.
Previous
research, including work by Caves,
Whinston, and Hurwitz
reported in this
journal, has shown that the
prices
of
branded drugs
increase when
generics are introduced.6
Together with the fact that
traditionally the
BLS has handled generics by
making
them
distinct new
products, that
implies that rapid entry of generics
will
result
in
an
increase in drug
prices. The Berndt, Cockburn,
and Griliches paper is
valuable in
showing
the inflation
implications
of
the
pattern
of
market
behavior
and index
number
methodology.
The fact that the
BLS has
now changed its
approach and
is
treating generics
as
identical products
to the branded
drugs makes
the
Berndt,
Cockburn, and Griliches story
less dramatic but is
very welcome in terms
of
the accuracy of price
indexes going forward. The authors' results
showing
how much
differ-
ence the alternative
approach
would have made
in
the
past
are
dramatic
enough.
The results for new
drugs
led
me
to think about
an
argument
that is
made concerning
the extent to
which
existing index number methods
may capture
innovation and new
products.
The
argument
holds that
existing products have to
compete
with the new
products
and that in a
perfectly competitive
market, therefore,
the
price
of
the old
products
has to go down to
reflect
the entrance
of the
new
products.
In
practice
there are differentiated
products
in
imperfect
competition,
and the
entry
of
new
products
may change
the
elasticity
of
demand and cause the
price
of
the
existing products
to
rise. For
example,
CDs and CD
players
have largely driven
out
LPs
and
turntables. But
there
is still
a niche
market
of buyers who believe
in the old
products.
Perhaps
the
prices
of
these
products
have
gone up
and not
down.
Discount
clothing
stores
sell
copies
of the latest
fashions-perhaps
that drives
up
the
price
of
the
high
fashion items. In
general,
if
the
pricing
behavior that is
seen
in
drugs
also
applies
to other
products,
then
existing price
indexes will
miss
much of
the
impact
of new
products
and
quality change.
We
desperately
need
more data on these issues.
The issue
of how
to value the
generics
raises
interesting questions
of
consumer
sovereignty.
The traditional
BLS method
did
assume a
full
6. Caves,
Whinston,
and Hurwitz
(1991).

Ernst R. Berndt, lain M. Cockburn, and Zvi Griliches 197
consumer sovereignty notion.
The BLS
assumed
that a
branded
anti-
depressant was twice as valuable
as
the generic
that
is
next
to it.
A counterargument is that the price differentials reflect inefficiency
in the market. People do not realize that a generic drug is chemically
identical to an existing branded drug.
In this
case,
as the share of
generic
drugs increases, we should
count that
legitimately
as a
price
decrease.
This is the new BLS approach.
My own reaction
is that
the
new BLS
approach
is
correct.
I
would
count fully the generic
as a reduction in
price. But, nevertheless,
one
of
the procedures that
is
suggested
in this
paper,
the
procedure
of
splitting the difference,
is
a
reasonable, practical
alternative. It
is
a
reasonable compromise between
the two
alternatives
of
saying
that
people feel
better because
they
are
buying
a
branded
drug
rather than a
generic
one
or, conversely, saying
that
people
are foolish or
lack in-
formation
when they buy
a
branded,
rather
than a
generic, product.
A
reason that
I
prefer the new BLS method
of
counting
the
full price
decline
associated
with
the introduction
of a
generic
is
that there
is
a
private market incentive
for
companies
to disseminate what
is, accord-
ing to the FDA, false information, namely that the branded drug is
superior
to
the generic.
If the
FDA is
correct
that
the
two
are
equivalent,
then it is
still
in the interest
of the manufacturers
of
the branded
product
to
persuade customers
and doctors
that is not the
case.
Turning to the hedonic approach that
is
used
in this
paper,
I
had
trouble
understanding
what
the coefficients
on the different
drug
char-
acteristics
in
their regressions actually represent.
The
right
model
for
antidepressants may
be a
matching model,
a model such as
those used
in
job search.
A
standard view
of
hedonics
is
that
consumers
trade
off
price against
some side-effect characteristics.
To
put
it
bluntly, you
would
pay
a
little bit more
for a
drug
in
order
to
have a little bit less
constipation
or
a little bit less
of
some
other side effect.
My understanding
of
the treatment
for
depression
is
that a
doctor
will
suggest
a
particular antidepressant
based
on
the
patient's history
and
the nature
of the
depression
and other
information. There is
then,
essentially,
a
trial for some
period
of
time to see
if
that
drug
works.
The information about the effect
of a
given drug
on a
patient
is
costly
to
acquire.
Doctor and
patient may
not
know for
some
period
of months

198 Brookings Papers: Microeconomics 1996
whether a drug is going to be effective
or
the nature
of the
side effects.
If the drug works, then the patient stays on it.
If it
does not, then the
doctor gives the patient a prescription
for a
different antidepressant.
Some people have to cycle through
various
drugs
for
as
long
as a
couple
of
years, trying
to find
an
appropriate
match between
a
drug
and their
particular problems.
Such a
matching
model
helps explain
in
part why
the
demands are
relatively inelastic, because a patient
who has
finally
found a
drug
that
works will
develop a great allegiance
to
it.
There
is
resistance to
chang-
ing the drug just because a new one comes
on the
market
or
just because
there
is
a price change.
How
are price differentials among drugs established? Perhaps the
simple reduced
form
hedonic regression
works
fine,
but that is not
obvious
beforehand. Presumably,
all else
being equal,
the doctor
and
patient
will start out with a
cheaper drug
first.
(All
else
being equal
presumably means where drugs
have the same initial
probability
of
creating a successful match.)
There would be some
price elasticity
in
that process.
A
further complication
is
created by third-party payment,
however. Many patients
have
part
or
all of their
drug
cost
covered
by
insurance. This point
is
stressed extensively
in Ted
Keeler's comments.
Limits set by insurance providers
mean
that many people are
on
drug
plans that specify a certain
list of
antidepressants, and
so
they
have to
choose one
of
those
or
face
a
sharp
increase
in
the incremental cost to
move off that list.
HMOs, which account
for an
increasing
share of medical
care,
if
they
are
providing drugs
as
part
of their
package,
would have an incen-
tive
to
have
their
doctors
start
with
the
cheaper drugs-assuming equiv-
alent
probability
of success.
In
general
this
is not
a market that
resembles
a
simple
textbook
utility
tradeoff.
There
are third-party payments.
Decisions
are
made when
patients
do
not
have
full information. And the
particular
market
equi-
librium
may
look more like
a
matching
model. It would
have been
helpful
if
the authors had discussed
how
we should
actually interpret
their coefficients
and
whether
they
would be the
right
ones for use
in
a
price
index
given
these
market characteristics.
My
concerns about
the
hedonics
would
have
been more
muted
except
for the fact that
the
results seem
surprising
to
me. It
may
be the method
used,
or
the
data,
or the time
period,
but somehow
the
impact
on
welfare

Ernst R.
Berndt, lain M. Cockburn, and
Zvi
Griliches
199
of the
introduction of the new
classes of
antidepressants may not
be
fully captured.
The results
reported in the paper are that when
you adjust
for
quality,
the impact is
not all that great. I would like more
explanation
of how
much of the
quality improvement is
being captured. There
is
the shift
from the old
class of drugs
to
the
new class of
drugs.
And then
there
are new entrants
with
slightly
different
profiles.
The
impact
of the
first
should be
huge. And the impact
of the second
should
also
be
fairly
large because
the class of drugs
becomes usable
for
a broader
group of
patients-more
drugs,
even when
they
look
similar
in
average
perfor-
mance, allow
for more successful matches.
In
conclusion,
this is a
paper
that is in
progress, and
I
want to
applaud
the
work
these
authors
have
done in
this
paper and
in
previous ones on
price indexes. It is
just
terrific as a set of
work, and
I
congratulate them
on it
and look
forward
to further
progress
ahead.
Commentators'
References
Caves,
Richard E., Michael
D.
Whinston,
and Mark A.
Hurwitz. 1991.
"Pat-
ent
Expiration, Entry,
and
Competition
in
the
U.S.
Pharmaceutical
Indus-
try." Brookings Papers
on Economic
Activity, Microeconomics: 1: 1-48.
Feldstein, Paul. 1993. Health Care Economics,
4th
ed. Albany: Delmar
Pub-
lishers.
Griliches, Zvi, and Ian Cockburn. 1994. "Generics
and
New Goods
in
Phar-
maceutical Price Indexes." American Economic Review 84
(December):
1213-32.
Hellerstein, Judith K. 1994. "The Demand for Post-patent Prescription
Phar-
maceuticals." NBER Working Paper 4981.
National
Bureau of Economic
Research, Cambridge, Mass. December.
Manning,
Willard
G.,
and
others. 1987.
"Health Insurance and
the Demand
for Medical Care: Evidence from
a
Randomized Experiment."
American
Economic Review 77 (June): 251-78.
Oberlander, Jonathan. forthcoming. "Managed
Care
and
Medicare
Reform."
Journal of Health Politics, Policy,
and Law.
Sullivan, Sean. 1992. "The Demand
for
Prescription Drugs among
Elderly
Americans." Ph.D. dissertation, University
of
California.
