
DOI: 10.1111/jdv.14542 JEADV
ORIGINAL ARTICLE
Long-term efficacy and safety of sonidegib in patients with
locally advanced and metastatic basal cell carcinoma:
30-month analysis of the randomized phase 2 BOLT study
J.T. Lear,
1,
* M.R. Migden,
2
K.D. Lewis,
3
A.L.S. Chang,
4
A. Guminski,
5
R. Gutzmer,
6
L. Dirix,
7
P. Combemale,
8
A. Stratigos,
9
R. Plummer,
10
H. Castro,
11,†
T. Yi,
12,†
M. Mone,
12
J. Zhou,
12
U. Trefzer,
13
M. Kaatz,
14
C. Loquai,
15
R. Kudchadkar,
16
D. Sellami,
12
R. Dummer
17
1
Manchester Academic Health Science Centre, University of Manchester, Manchester, UK
2
Departments of Dermatology and Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
3
Division of Medical Oncology, University of Colorado School of Medicine, Aurora, CO, USA
4
Stanford University School of Medicine, Redwood City, CA, USA
5
Royal North Shore Hospital, St Leonards, NSW, Australia
6
Medizinische Hochschule Hannover, Hannover, Germany
7
Sint-Augustinus Ziekenhuis, Antwerp, Belgium
8
Anti Cancer Institute, , Lyon, France
9
Andreas Syggros Hospital, University of Athens, Athens, Greece
10
Northern Centre for Cancer Care, Freeman Hospital, Newcastle upon Tyne, UK
11
Novartis Pharma AG, Basel, Switzerland
12
Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
13
Dermatologikum Berlin, Berlin, Germany
14
University Hospital Jena, Jena, Germany
15
University Medical Center Mainz, Mainz, Germany
16
Winship Cancer Institute of Emory University, Atlanta, GA, USA
17
Universit
€
urich-Skin Cancer Center, University Hospital, Z
€
atsSpital Z
€
urich, Switzerland
*Correspondence: J.T. Lear. E-mail: john.lear@cmft.nhs.uk
Abstract
Background Patients with locally advanced basal cell carcinoma (laBCC) or metastatic BCC (mBCC), two difficult-to-
treat populations, have had limited treatment options. Sonidegib, a hedgehog pathway inhibitor (HPI), was approved in
laBCC based on results from the BOLT trial.
Objective To evaluate long-term efficacy and safety of sonidegib in laBCC and mBCC in the BOLT 18- and 30-month
analyses.
Methods BOLT (NCT01327053, ClinicalTrials.gov), a double-blind phase 2 study, enrolled patients from July 2011 until
January 2013. Eligible HPI-treatment–na
€
ıve patients with laBCC not amenable to curative surgery/radiotherapy or mBCC
were randomized 1 : 2 to sonidegib 200 mg (laBCC, n = 66; mBCC, n = 13) or 800 mg (laBCC, n = 128; mBCC,
n = 23). Tumour response was assessed per central and investigator review.
Results With 30 months of follow-up, among patients treated with sonidegib 200 mg (approved dose), objective
response rates were 56.1% (central) and 71.2% (investigator) in laBCC and 7.7% (central) and 23.1% (investigator) in
mBCC. Tumour responses were durable as follows: median duration of response was 26.1 months (central) and
15.7 months (investigator) in laBCC and 24.0 months (central) and 18.1 months (investigator) in mBCC. Five patients
with laBCC and three with mBCC in the 200-mg arm died. Median overall survival was not reached in either population;
2-year overall survival rates were 93.2% (laBCC) and 69.3% (mBCC). In laBCC, efficacy was similar regardless of
aggressive or non-aggressive histology. Sonidegib 200 mg continued to have a better safety profile than 800 mg, with
lower rates of grade 3/4 adverse events (43.0% vs. 64.0%) and adverse events leading to discontinuation (30.4% vs.
40.0%).
†Affiliation at the time the study was conducted. HC and TY are no longer
affiliated with Novartis Pharmaceuticals.
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use,
distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Leon
Berar
d

373 Sonidegib for advanced BCC
Conclusion Sonidegib continued to demonstrate long-term efficacy and safety in these populations. These data sup-
port the use of sonidegib 200 mg per local treatment guidelines.
Received: 14 March 2017; Accepted: 7 August 2017
Conflicts of interest
Dr Lear has served as a consultant or speaker for and received honoraria from Novartis Pharmaceuticals
Corporation. Dr Migden has participated on advisory boards and received honoraria from Genentech, Inc.;
Novartis Pharmaceuticals Corporation; and Eli Lilly and Company. Dr Lewis has received research funding paid
to the institution from Novartis Pharmaceuticals Corporation. Dr Chang is a primary investigator for and has
served as a consultant for and received research grant/funding paid to the institution from Novartis
Pharmaceuticals Corporation. Dr Guminski has participated on advisory boards for Bristol-Myers Squibb, Pfizer
Inc. and Sanofi; received honoraria from Novartis Pharmaceuticals Corporation; and received travel support from
Astellas and Bristol-Myers Squibb. Dr Gutzmer has received research grants paid to the institution from Roche,
Novartis Pharmaceuticals Corporation, Johnson & Johnson and Pfizer Inc.; received honoraria from Roche,
Bristol-Myers Squibb, MSD, GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Merck Serono, Almirall,
Janssen, Amgen Inc., Galderma and Boehringer Ingelheim; and served as a consultant for Roche, Bristol-Myers
Squibb, MSD, GlaxoSmithKline, Novartis Pharmaceuticals Corporation, Almirall, LEO Pharma Inc., Amgen Inc.
and Pfizer Inc. Dr Dirix has no conflict of interest to declare. Dr Combemale has no conflict of interest to declare.
Dr Stratigos has received a research grant from Roche and Novartis Pharmaceuticals Corporation and received
honoraria from LEO Pharma Inc., Meda Pharmaceuticals Inc. and Janssen-Cilag. Dr. Plummer has participated
on an advisory board and received honoraria from Astex, Roche, Bristol-Myers Squibb, Vertex, Bayer, Pierre
Faber, Novartis Pharmaceuticals Corporation and Clovis Oncology. Dr Castro was an employee of Novartis
during the development of this manuscript and is currently an employee of Bristol-Myers Squibb. Dr Yi was an
employee of Novartis during the development of this manuscript. Dr Mone is an employee and stockholder of
Novartis Pharmaceuticals Corporation. Dr Zhou is an employee of Novartis Pharmaceuticals Corporation. Dr
Trefzer has been an advisor for and received honoraria from F. Hoffmann-La Roche Ltd, participated on an
advisory board and received honoraria from MSD, and has been a speaker and received honoraria from Novartis
Pharmaceuticals Corporation. Dr Kaatz has participated on advisory boards and received honoraria from Roche,
MSD, Novartis Pharmaceuticals Corporation, Bristol-Myers Squibb and Janssen. Dr Loquai has served as a
consultant for Bristol-Myers Squibb, Roche, Novartis Pharmaceuticals Corporation, Amgen, BioNTech and MSD.
Dr Kudchadkar has participated on an advisory board and received honoraria from Bristol-Myers Squibb and
Genentech, Inc. Dr Sellami is an employee and stockholder of Novartis Pharmaceuticals Corporation. Dr Dummer
has received research funding from Novartis Pharmaceuticals Corporation, MSD, Bristol-Myers Squibb, Roche
and GlaxoSmithKline and has served as a consultant or participated on an advisory board for Novartis
Pharmaceuticals Corporation, MSD, Bristol-Myers Squibb, Roche, GlaxoSmithKline, Amgen and Takeda.
Funding sources
This study was funded by Novartis Pharmaceuticals Corporation. Novartis Pharmaceuticals Corporation was
involved in design and conduct of the study; collection, management, analysis and interpretation of data;
preparation, review and approval of the manuscript; and decision to submit the manuscript for publication.
Introduction
Patients with locally advanced basal cell carcinoma (laBCC) or
metastatic BCC (mBCC) have historically had limited treatment
options.
1
Surgery, the primary course of treatment for most
BCCs, and radiotherapy are often not viable options in advanced
BCC.
1–3
In recent years, hedgehog (Hh) pathway inhibitors
(HPIs) were developed to block aberrant Hh signalling that is
found in most sporadic BCCs; HPIs have demonstrated efficacy
in patients with laBCC and those with mBCC.
4–13
Sonidegib (LDE225), which inhibits Hh signalling by target-
ing the Hh pathway component smoothened,
6
was approved
(200 mg once daily) in the United States and Europe for the
treatment of laBCC, in Australia for the treatment of laBCC and
mBCC and in Switzerland for the treatment of advanced BCC
that cannot be treated with curative surgery or radiotherapy.
14–17
These approvals were based on results from the pivotal phase 2
Basal Cell Carcinoma Outcomes With LDE225 Treatment
(BOLT) study (NCT01327053), in which the primary analysis
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

374 Lear et al.
was performed using data based on 6 months of follow-up.
8,9
Here, we report long-term efficacy and safety results from BOLT,
with up to 30 months of follow-up after the last patient was ran-
domized.
Materials and methods
BOLT study design and patients
BOLT is a randomized, double-blind, phase 2 study conducted
in 58 centres across 12 countries that assessed the efficacy and
safety of sonidegib 200 and 800 mg once daily, as described pre-
viously.
8
Patients ≥18 years of age with histologically confirmed
disease, either laBCC not amenable to curative surgery or radio-
therapy or mBCC, were eligible. Patients were required to have
adequate organ function and a World Health Organization
(WHO)
18
performance status ≤2. Patients with previous HPI
treatment were not eligible. Full inclusion and exclusion criteria
were reported previously.
8
Patients were randomized 1 : 2 to 200 or 800 mg based on
previous data suggesting that 800 mg would show better effi-
cacy.
7
Randomization was performed using the central Interac-
tive Response Technology system (Cenduit, Allentown, PA).
Stratification of patients was based on disease type (laBCC vs.
mBCC), histological subtype (aggressive vs. non-aggressive) and
geographic region. All patients and study personnel were blinded
until the time of the primary analysis.
The study protocol was approved at each centre by indepen-
dent ethics committee or institutional review board, and all
patients provided written informed consent prior to enrolment.
Sonidegib treatment and assessments
Sonidegib capsules were taken orally on a once-daily continuous
schedule until disease progression, intolerable toxicity, with-
drawal of patient consent, discontinuation at the discretion of
the investigator, death or study termination. Tumour evalua-
tions were conducted at baseline, during treatment and post-
treatment follow-up (weeks 5 and 9, then every 8 weeks during
year 1 and every 12 weeks thereafter), and at discontinuation
using BCC-modified Response Evaluation Criteria In Solid
Tumors (mRECIST)
8,9
for laBCC and RECIST v1.1
19
for mBCC.
mRECIST
8,9
is a stringent multimodal assessment tool that
incorporates magnetic resonance imaging (MRI) per RECIST
v1.1
19
[≥30% reduction in the sum of the longest diameters of
target lesions required for a partial response (PR)], standard and
annotated color photography per bidimensional WHO guideli-
nes
18
(≥50% reduction in the sum of the products of perpendic-
ular diameters of target lesions required for a PR), and histology
in multiple biopsies surveying the lesion area to determine a
composite overall response. Complete responses (CRs) and PRs
had to be confirmed on repeat assessments separated by
≥4 weeks. Fresh tumour biopsies were used to confirm CRs in
the presence of confounding ulceration, cyst formation and/or
scarring/fibrosis. An independent review committee re-evaluated
all central assessments for patients with laBCC.
In a prespecified analysis, responses were re-evaluated in
patients with laBCC using BCC-RECIST-like, less-stringent cri-
teria similar to those used in ERIVANCE, a phase 2 study of vis-
modegib.
11
Both mRECIST and BCC-RECIST-like integrate
MRI per RECIST v1.1, standard and annotated color photogra-
phy per WHO guidelines, and histology; however, the algorithm
used to determine overall response for each is different: multiple
scenarios exist for which the response is lower with mRECIST
vs. BCC-RECIST-like (Table S1, Supporting Information).
Blood samples for pharmacokinetic (PK) assessments were
collected from all patients through week 69; trough plasma con-
centrations were analysed at predose on weeks 1, 3, 5 and 9, then
every 4 weeks through week 21 and every 12 weeks thereafter.
Adverse events (AEs) were monitored throughout the treat-
ment period until 30 days following the last sonidegib dose
according to Common Terminology Criteria for Adverse Events
v4.03.
20
BOLT study outcomes
The primary study endpoint was objective response rate (ORR;
proportion of patients with a best overall response of CR or PR)
per central review. Secondary endpoints included ORR per
investigator review; CR rate, duration of response (DOR) and
progression-free survival (PFS) per central and investigator
review; overall survival (OS); and safety.
Statistical methods
Results were analysed based on two database cut-offs: 11 July
2014 and 10 July 2015 (18 and 30 months after the last patient
was randomized, respectively). ORR and CR rates were esti-
mated with 95% CIs. Kaplan–Meier nonparametric maximum
likelihood estimates of median time and 95% CIs were estimated
for DOR and PFS for each arm and by disease. Statistical com-
parisons of the 200- and 800-mg arms were not planned. Addi-
tional details on the statistical methods used in the study were
published previously.
8,9
Results
Patient demographics and disposition
Two-hundred and thirty patients with laBCC (n = 194) or mBCC
(n = 36) were enrolled between 20 July 2011 and 10 January
2013. Patients were randomized to sonidegib 200 mg (laBCC,
n = 66; mBCC, n = 13) or 800 mg (laBCC, n = 128; mBCC,
n = 23; Fig. S1, Supporting Information). Baseline demographics
were well balanced between arms.
8,9
Most patients with laBCC
(200 mg, 56.1%; 800 mg, 58.6%) had aggressive tumour histol-
ogy. By the data cut-off for the 18-month analysis (median fol-
low-up, 26.3 months), 87.0% of patients (200 mg, 86.1%;
800 mg, 87.4%) had discontinued treatment; by the data cut-off
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

375 Sonidegib for advanced BCC
for the 30-month analysis (median follow-up, 38.2 months),
93.0% (200 mg, 92.4%; 800 mg, 93.4%) had discontinued treat-
ment (Fig. S1, Supporting Information). The most common
reasons for treatment discontinuation were AEs [30-month anal-
ysis (200 vs. 800 mg), 29.1% vs. 37.7%], progressive disease
(36.7% vs. 14.6%) and patient decision (10.1% vs. 21.9%).
Efficacy in patients with laBCC
ORRs in patients with laBCC increased compared with the pri-
mary analysis (Table 1; Table S2, Supporting Information).
Tumour shrinkage was observed in most patients (Fig. S2, Sup-
porting Information). In the 30-month analysis, ORRs in the
200-mg arm were 56.1% (central review) and 71.2% (investiga-
tor review); in the 800-mg arm, ORRs were 45.3% and 58.6%,
respectively. Per central review, 26 of 37 patients with laBCC in
the 200-mg arm who achieved an objective response by the 30-
month data cut-off maintained the response (Fig. 1a; Fig. S3a,
Supporting Information), and the estimated median DOR in the
200-mg arm was 26.1 months (15.7 months per investigator
review). Among patients with laBCC who responded to treat-
ment in the 800-mg arm, 38 of 58 maintained an objective
response per central review (Fig. S3b, Fig. S4a, Supporting Infor-
mation), and the estimated median DOR was 23.7 months
(26.0 months per investigator review). At the 30-month data
cut-off, the median durations of PFS among patients with laBCC
were 22.1 months (central review) and 19.4 months (investiga-
tor review) in the 200-mg arm (Fig. 1b) and 22.0 and
28.0 months, respectively, in the 800-mg arm (Fig. S4b, Sup-
porting Information).
Response in patients with laBCC was also assessed using
BCC-RECIST–like criteria. Although ORRs and DORs were sim-
ilar with both sets of criteria, CR rates were higher with BCC-
RECIST-like criteria (Table S3, Supporting Information). In the
200-mg arm, CR rates in the 30-month analysis per central
review were 21.2% (BCC-RECIST-like) vs. 4.5% (mRECIST).
Efficacy was similar among patients with laBCC with aggres-
sive or non-aggressive histological subtypes. In the 30-month
analysis, ORRs in patients with aggressive vs. non-aggressive his-
tology in the 200-mg arm were 59.5% vs. 51.7% per central
review and 70.3% vs. 72.4% per investigator review (Table 1);
respective ORRs in the 800-mg arm were 44.0% vs. 47.2% per
central review and 54.7% vs. 64.2% per investigator review
(Table S2, Supporting Information). Tumour responses were
durable regardless of tumour aggressiveness.
At the time of the 18-month analysis, efficacy in patients with
laBCC was also assessed based on tumour burden at baseline.
Responses were found to be durable regardless of tumour bur-
den with both doses, with >58% of patients in each group hav-
ing responses lasting >6 months (Table S4, Supporting
Information).
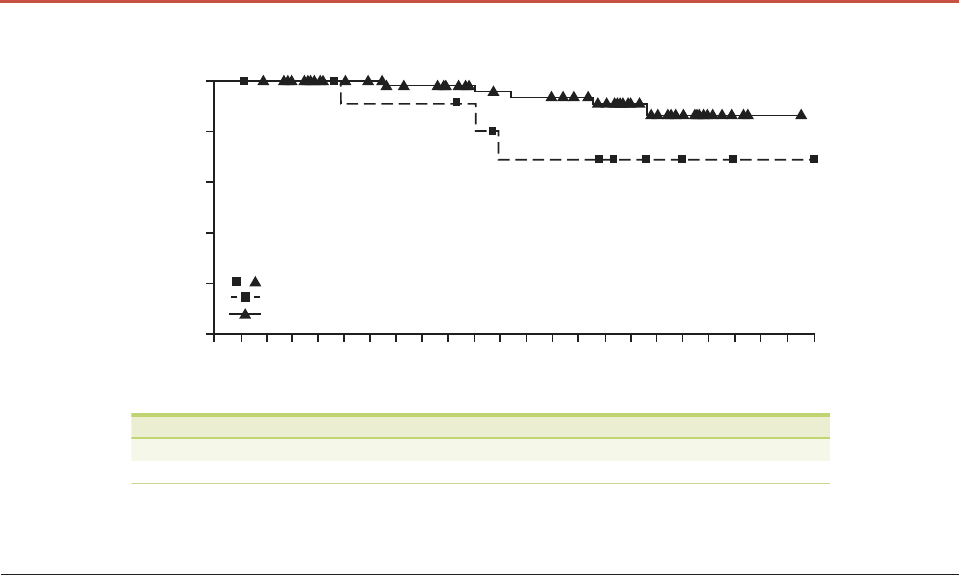
Five patients with laBCC in the 200-mg arm died by the 30-
month analysis; median OS was not yet reached and the
estimated 2-year OS was 93.2% (Table 1; Fig. 2). In the 800-mg
arm, 11 patients with laBCC died; median OS was not reached
and the estimated 2-year OS was 90.7% (Table S2, Fig. S5, Sup-
porting Information). Numerically more deaths were reported
in patients with aggressive (200 mg, 4; 800 mg, 8) vs. non-
aggressive (200 mg, 1; 800 mg, 3) histology; median OS was not
reached in either population with either dose, and 2-year OS
rates were 91.8% vs. 94.7% (200 mg) and 86.6% vs. 95.8%
(800 mg).
Efficacy in patients with mBCC
Tumour shrinkage was reported in most patients with mBCC
(Fig. S6, Supporting Information). ORRs in the 200-mg arm
were 7.7% (central review) and 23.1% (investigator review) in
the 30-month analysis, and estimated median DOR was
24.0 months (central review) and 18.1 months (investigator
review; Table 2). ORRs in the 800-mg arm were 17.4% (central
review) and 34.8% (investigator review); estimated median DOR
was not reached per central review and was 10.2 months per
investigator review (Table S5, Supporting Information). The dis-
ease control rate (CR + PR + stable disease) was high with soni-
degib 200 mg (central, 92.3%; investigator, 84.6%) and 800 mg
(central, 91.3%; investigator, 82.6%). The median PFS with
sonidegib 200 mg was 13.1 months per central and investigator
review (Fig. 1b) and with sonidegib 800 mg was 11.1 months
per central review and 14.3 months per investigator review
(Fig. S4b, Supporting Information).
In the 30-month analysis, 11 patients (200 mg, 3; 800 mg, 8)
with mBCC had died. With sonidegib 200 mg, the estimated
median OS was not reached, and the estimated 2-year OS was
69.3% (Fig. 2). With sonidegib 800 mg, the estimated median
OS was 36.7 months, and the estimated 2-year OS was 69.1%
(Fig. S5, Supporting Information).
Sonidegib PK
Sonidegib PK was evaluated based on the 18-month data cut-off
(by which time all patients had completed the scheduled PK
assessments). Mean sonidegib plasma concentrations rose until
week 13–17 with daily 200 and 800 mg dosing (Fig. S7, Sup-
porting Information). By week 17, an approximate steady state
was reached for both doses. Sonidegib demonstrated under
dose-proportional increases in plasma exposure between the two
doses tested: at week 17, the geometric mean trough concentra-
tion was 689 ng/mL with 200 mg and 1574 ng/mL with 800 mg,
corresponding to a 2.3-fold increase in trough concentration
over a fourfold dose increase.
Safety
No new safety concerns emerged with an additional 24 months
of follow-up since the primary analysis. At the 30-month data
cut-off, the median duration of exposure was 11.0 months
(range, 1.3–41.3 months) in the 200-mg arm and 6.6 months
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

376 Lear et al.
Table 1 Efficacy in patients with laBCC treated with sonidegib 200 mg by central and investigator review
Patients with laBCC Sonidegib 200 mg QD
Primary analysis* 18-month analysis† 30-month analysis‡
All patients All patients All patients Patients with Patients with
n = 66 n = 66 n = 66 aggressive non-aggressive
histology§ histology¶
n = 37 n = 29
ORR, n (%); 95% CI**
Central review 31 (47.0); 34.6–59.7 37 (56.1); 43.3–68.3 37 (56.1); 43.3–68.3 22 (59.5); 42.1–75.2 15 (51.7); 32.5–70.6
Investigator review 43 (65.2); 52.4–76.5 47 (71.2); 58.7–81.7 47 (71.2); 58.7–81.7 26 (70.3); 53.0–84.1 21 (72.4); 52.8–87.3
BOR, n (%)††
CR
Central review 2 (3.0) 3 (4.5) 3 (4.5) 2 (5.4) 1 (3.4)
Investigator review 5 (7.6) 6 (9.1) 6 (9.1) 3 (8.1) 3 (10.3)
PR
Central review 29 (43.9) 34 (51.5) 34 (51.5) 20 (54.1) 14 (48.3)
Investigator review 38 (57.6) 41 (62.1) 41 (62.1) 23 (62.2) 18 (62.1)
SD
Central review 29 (43.9) 23 (34.8) 23 (34.8) 12 (32.4) 11 (37.9)
Investigator review 16 (24.2) 14 (21.2) 13 (19.7) 8 (21.6) 5 (17.2)
PD
Central review 1 (1.5) 1 (1.5) 1 (1.5) 1 (2.7) 0
Investigator review 1 (1.5) 1 (1.5) 1 (1.5) 0 1 (3.4)
Unknown
Central review 5 (7.6) 5 (7.6) 5 (7.6) 2 (5.4) 3 (10.3)
Investigator review 6 (9.1) 4 (6.1) 5 (7.6) 3 (8.1) 2 (6.9)
DOR‡‡
Events§§/responders, n/n;
KM median (95% CI), months
Central review 4/31; NR 10/37; NR 11/37; 26.1 (NE) 7/22; 26.1 (NE) 4/15; NR
Investigator review 10/43; 20.2 (10.1–20.2) 21/47; 14.3 (12.0–20.2) 22/47; 15.7 (12.0–20.2) 9/26; 20.2 (NE) 13/21; 15.7 (11.0–20.2)
PFS¶¶
Events§§, n; KM median
(95% CI), months
Central review 7; NR 15; 22.1 (NE) 16; 22.1 (NE) 11; 22.1 (NE) 5; NR
Investigator review 15; 16.6 (13.7–22.0) 26; 19.4 (16.6–22.6) 28; 19.4 (16.6–
23.6) 12; 19.0 (NE) 16; 19.4 (9.2–22.6)
OS***
Deaths, n; KM median 1; NR 3; NR
5; NR 4; NR 1; NR
(95% CI), months
2-year OS (95% CI), % – – 93.2 (80.2–97.8) 91.8 (70.9–97.9) 94.7 (68.1–99.2)
BOR, best overall response; CR, complete response; DOR, duration of response; KM, Kaplan–Meier; laBCC, locally advanc ed basal cell carcinoma; NE, not
estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progressio n-free survival; PR, partial
response; QD, once daily; SD, stable disease; –, indicates not reported.
*Data cut-off, 28 June 2013; median follow-up (200- and 800-mg arms), 13.9 months.
†Data cut-off, 11 July 2014; median follow-up (200- and 800-mg arms), 26.3 months.
‡Data cut-off, 10 July 2015; median follow-up (200- and 800-mg arms), 38.2 months.
§Aggressive histological subtypes of BCC include micronodular, infiltrative, multifocal, basosquamous and sclerosing.
¶Non-aggressive histological subtype s of BCC include nodular and superficial.
**Proportion of patients with a BOR of CR or PR on repeat assessments ≥4 weeks apart.
††Best response recorded from the time of randomization until the earliest occurrence of disease progression, start of other antineoplastic therapy or data
cut-off date.
‡‡Time from first observed objective response (CR or PR) until disease progression or death due to any cause (responder data only).
§§Progressive disease or death due to any cause.
¶¶Time from randomization to first documented disease progression or death due to any cause.
***Time from randomization to the date of death due to any cause or the last date the patient was known to be alive.
(range, 0.3–43.5 months) in the 800-mg arm. The incidence of fatigue, appetite decreased, myalgia and vomiting (Fig. 3). The
many of the most common AEs was lower with sonidegib most common AEs reported in patients with laBCC (Fig. S8,
200 mg than 800 mg, including muscle spasms, alopecia, dys- Supporting Information) and mBCC (Fig. S9, Supporting Infor-
geusia, nausea, weight decreased, creatine kinase (CK) increased, mation) were similar. Among all patients, grade 3/4 AEs
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

377 Sonidegib for advanced BCC
(a)
0 2 4 6 8 10 12 14 16 18
Time since response (months)
Probability of remaining in
response (%)
Central review
20 22 24 26 28 30 32 34 36
100
0
0 2 4 6 8 10 12 14 16 18
Time since response (months)
Investigator review
20 22 24 26 28 30 32 34 36 38 40
20
40
60
80
100
(b)
Kaplan–Meier median: 15.67 months
Censoring times
laBCC (n/N = 22/47)
Kaplan–Meier median: 26.09 months
Censoring times
laBCC (n/N = 11/37)
37
Number of patients still at risk
laBCC 35 31 23 21 18 11 9 7 7 7 7 6 5 1 1 1 1 0 47
Number of patients still at risk
laBCC 45 42 30 26 26 23 15 12 12 79 6 5 4 3 2 2 1 1 0
Probability of remaining in
80
response (%)
60
40
20
0
Central review
Kaplan–Meier medians:
mBCC: 13.11 months
laBCC: 22.11 months
Censoring times
mBCC (
n/N = 8/13)
laBCC (n/N = 16/66)
Probability of event-free
0 2 4 6 8 10 12 14 16 18
Time since randomization (months)
Investigator review
20 22 24 26 28 30 32 34 36 38 40
Kaplan–Meier medians:
mBCC: 13.11 months
laBCC: 19.35 months
Censoring times
mBCC (
n/N= 9/13)
laBCC (n/N = 28/66)
100 100
80
Probability of event-free
80
survival (%)
survival (%)
60
40
60
40
20
0
20
0
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38
Time since randomization (months)
Number of patients still at risk Number of patients still at risk
mBCC 13 12 10 8 8 5 5 4 4 3 2 2 2 1 1 1 1 0 0 0 mBCC 13 11 11 10 10 6 6 5 5 4 2 1 1 1 1 1 1 1 0 0 0
laBCC 66 60 54 47 39 32 24 17 14 12 10 10 7 6 6 5 3 3 3 0 laBCC 66 60 56 50 39 33 28 26 23 18 14 13 8 7 7 5 3 3 3 1 0
Figure 1 Duration of response (DOR) in patients with locally advanced basal cell carcinoma (laBCC) and progression-free survival (PFS)
by central and investigator review in patients with laBCC or metastatic BCC (mBCC) treated with sonidegib 200 mg. (a) Kaplan–Meier
plots of DOR in patients with laBCC who responded to treatment with sonidegib 200 mg per central (n = 37) and investigator (n = 47)
review. (b) Kaplan–Meier plots of PFS in patients with laBCC (n = 66) and mBCC (n = 13) treated with sonidegib 200 mg per central and
investigator review.
occurred less frequently with sonidegib 200 mg (43.0%) than
with 800 mg (64.0%); similar results were reported for grade 3/4
AEs suspected to be related to treatment, with a lower incidence
in the 200-mg arm (30.4%) than the 800-mg arm (43.3%).
Increased CK was the most common grade 3/4 AE (10.9%;
200 mg, 6.3%; 800 mg, 13.3%); only one additional patient
(800 mg) had a grade 3/4 increase in CK after the primary analy-
sis cut-off. The median time to onset (range) of grade 2/3/4 CK
elevation was 12.9 (2–39) and 6.7 (2–40) weeks in the 200- and
800-mg arms, respectively; the median time to resolution (95%
CI) to grade ≤1 was 12.0 (8.0–14.0) and 15.0 (9.0–15.0) days,
respectively.
AEs requiring dose interruption and/or reduction were
reported in 43.0% and 66.7% of patients in the 200- and 800-mg
arms, respectively; the most common AEs leading to dose inter-
ruption/reduction (200 mg vs. 800 mg) were muscle spasms
(1.3% vs. 16.7%), CK increased (6.3% vs. 12.0%), and nausea
(6.3% vs. 12.0%). AEs leading to treatment discontinuation also
occurred less frequently with the 200-mg dose (30.4%) than the
800-mg dose (40.0%), with most (60.7%; 200 mg, 54.2%;
800 mg, 63.3%) being grade 1/2. The most common AEs leading
to discontinuation (200 mg vs. 800 mg) were muscle spasms
(5.1% vs. 8.0%), weight decreased (2.5% vs. 6.0%), dysgeusia
(3.8% vs. 4.7%) and alopecia (1.3% vs. 6.0%).
Serious AEs (SAEs) irrespective of cause were reported in
20.3% and 38.7% of patients treated with sonidegib 200 mg and
800 mg, respectively (Table S6, Supporting Information); SAEs
related to sonidegib treatment occurred in 3.8% and 16.0%,
respectively. Increased CK and rhabdomyolysis were the most
commonly reported SAEs among all patients (2.6% each;
200 mg, 1.3% each; 800 mg, 3.3% each); due to a lack of renal
impairment, none of the cases of rhabdomyolysis were con-
firmed by an independent review and adjudication committee of
experts on muscle toxicity.
8
On-treatment deaths were reported in eight patients
(200 mg, 1; 800 mg, 7), four of whom had laBCC (200 mg, 1;
800 mg, 3) and four had mBCC (800 mg, 4); none of these
deaths were considered treatment related. Four on-treatment
deaths [two due to progressive disease (both with mBCC) and
one each due to congestive cardiac failure (laBCC) and cardiac
death (laBCC)] were reported in the primary analysis,
8
and
four occurred following the primary analysis, including one
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.
378 Lear et al.
Probability of survival (%)
100
80
60
40
20
0
Time since randomization (months)
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46
Kaplan–Meier medians:
mBCC: NE
laBCC: NE
Censoring times
mBCC (n/N = 3/13)
laBCC (n/N = 5/66)
Number of patients still at risk
13mBCC 13 12 12 12 10 10 10 10 10 9 6 6 6 6 5 4 3 2 2 1 1 1 0
laBCC 66 66 65 63 58 55 53 49 48 45 41 39 38 37 35 32 21 18 13 6 3 1 1 0
Figure 2 Overall survival (OS) in patients with locally advanced basal cell carcinoma (laBCC) or metastatic BCC (mBCC) treated with
sonidegib 200 mg. Kaplan–Meier plot of OS in patients with laBCC (n = 66) or mBCC (n = 13) treated with sonidegib 200 mg. NE, not
estimable.
patient treated with sonidegib 200 mg (laBCC) who died of
acute respiratory distress on study day 612, and three patients
treated with 800 mg who died of cardiac arrest (laBCC), sepsis
(mBCC) and respiratory arrest (mBCC) on study days 349,
391 and 433, respectively.
Discussion
With long-term follow-up in BOLT, sonidegib 200 and 800 mg
continued to demonstrate sustained efficacy in patients with
laBCC (regardless of histology) and mBCC. Since the time of the
primary analysis, ORRs in the 200-mg arm improved in patients
with laBCC and remained similar in patients with mBCC. Higher
rates of response were observed by investigator vs. central review,
which could be due to investigators having the opportunity to
physically examine lesions that are often complicated by post-
treatment ulceration, cyst formation, scarring/fibrosis and ill-
defined borders.
8
Responses in patients with laBCC were durable
regardless of tumour burden at baseline, with most patients (cen-
tral, 70.3%; investigator, 53.2%) who responded to treatment
with sonidegib 200 mg maintaining an objective response at the
30-month data cut-off. Moreover, when responses in patients
with laBCC were scored using less-stringent response criteria
(BCC-RECIST-like criteria, similar to criteria used in ERI-
VANCE
11
), CR rates were similar to those reported with vismod-
egib in ERIVANCE.
12
This analysis underscores the efficacy of
sonidegib in patients with laBCC. However, efficacy in patients
with disease that recurred following prior HPI therapy is
unknown because these patients were excluded from BOLT.
The rate of survival in patients with BCC is extremely
high
21,22
; however, the prognosis for patients with advanced
BCC is less clear.
1
In patients with laBCC, the survival rate is
unknown due in part to a lack of reporting of these data in
patient registries. In patients with mBCC, the median survival
prior to 1990 was estimated to be 8 months and recently has
been estimated to be as high as 7 years due to improved thera-
pies, including HPIs.
23–25
In BOLT, the median OS in patients
with laBCC was not reached with either dose by the 30-month
analysis, and the estimated 2-year OS was >90% in both arms; in
patients with mBCC, the median OS was not yet reached with
sonidegib 200 mg and was 36.7 months with 800 mg, and the
estimated 2-year OS was 69% in both arms. These survival
rates are similar to those reported with vismodegib [85.5%
(laBCC) and 62.3% (mBCC) at 2 years in ERIVANCE], the only
other approved HPI.
13
The PK of sonidegib is different from that of vismodegib. Vis-
modegib exposure did not increase above the 150-mg daily
dosage due to saturated protein binding, and the half-life was
4 days after repeated dosing.
26,27
In contrast, sonidegib exposure
increased 2.3-fold between 200 and 800 mg and reached an
approximate steady state at 17 weeks. Previously, PK expo-
sure–response and exposure–safety analyses including the 200-
and 800-mg doses (based on the 18-month data cut-off) showed
no exposure–efficacy relationship but a reduced risk of grade 3/4
800 mg. Additionally, in population PK model, sonidegib
CK elevation with lower exposure, further supporting the
favourable benefit–risk profile of sonidegib 200 mg vs.
28
a
had a predicted elimination half-life of 29.6 days and an accu-
mulation ratio of 21.
29
Sonidegib 200 mg continued to be better tolerated than soni-
degib 800 mg, and no additional safety concerns were reported
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

379 Sonidegib for advanced BCC
Table 2 Efficacy in patients with mBCC treated with sonidegib 200 mg by central and investigator review
Patients with mBCC Sonidegib 200 mg QD
n = 13
Primary analysis* 18-month analysis† 30-month analysis‡
ORR, n (%); 95% CI§
Central review 2 (15.4); 1.9–45.4 1 (7.7)¶; 0.2–36.0 1 (7.7); 0.2–36.0
Investigator review 3 (23.1); 5.0–53.8 3 (23.1); 5.0–53.8 3 (23.1); 5.0–53.8
BOR, n (%)**
CR
Central review 0 0 0
Investigator review 0 0 0
PR
Central review 2 (15.4) 1 (7.7)¶ 1 (7.7)
Investigator review 3 (23.1) 3 (23.1) 3 (23.1)
SD
Central review 10 (76.9) 11 (84.6) 11 (84.6)
Investigator review 8 (61.5) 8 (61.5) 8 (61.5)
PD
Central review 0 0 0
Investigator review 2 (15.4) 2 (15.4) 2 (15.4)
Unknown
Central review 1 (7.7) 1 (7.7) 1 (7.7)
Investigator review 0 0 0
DOR††
Events/responders, n/n‡‡;
KM median (95% CI), months
Central review 0/2; NR 0/1; NR 1/1; 24.0 (NE)
Investigator review 0/3; NR 1/3; 17.7 (NE) 2/3; 18.1 (17.7–18.4)
PFS§§
Events, n; KM median (95% CI), months‡‡
Central review 4; 13.1 (5.6–13.1) 6; 13.1 (NE) 8; 13.1 (5.6–33.1)
Investigator review 7; 13.1 (9.2–16.6) 8; 13.1 (NE) 9; 13.1 (9.2–19.4)
OS¶¶
Deaths, n; KM median (95% CI), months 1; NR 3; NR 3; NR
2-year OS (95% CI), % – – 69.3 (31.2–89.1)
BOR, best overall response; CR, complete response; DOR, duration of response; KM, Kaplan–Meier; mBCC, metastatic basal cell carcinoma; NE, not estim-
able; NR, not reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response;
QD, once daily; SD, stable disease; –, indicates not reported.
*Data cut-off, 28 June 2013; median follow-up (200- and 800-mg arms), 13.9 months.
†Data cut-off, 11 July 2014; median follow-up (200- and 800-mg arms), 26.3 months.
‡Data cut-off, 10 July 2015; median follow-up (200- and 800-mg arms), 38.2 months.
§Proportion of patients with a BOR of CR or PR on repeat assessments ≥4 weeks apart.
¶BOR of one patient changed from a PR to SD due to identification of a new lesion by central rereview in a photograph received after the cut-off for the primary
analysis (28 June 2013).
**Best response recorded from the time of randomization until the earliest occurrence of disease progression, start of other antineoplastic therapy or data cut-
off date.
††Time from first observed objective response (CR or PR) until disease progression or death due to any cause (responder data only).
‡‡Progressive disease or death due to any cause.
§§Time from randomization to first documented disease progression or death due to any cause.
¶¶Time from randomization to the date of death due to any cause or the last date the patient was known to be alive.
with either dose. Lower incidences of grade 3/4 AEs, SAEs and
AEs leading to discontinuation were observed with 200 mg vs.
800 mg. The majority of the most common AEs were generally
grade 1 or 2 and were similar to those reported with other
HPIs
10–12,30–39
; muscle-related AEs and the increase in CK that
can accompany these AEs are thought to be a class effect of
HPIs.
11,12,30,31,35,39
Muscle-related AEs were effectively managed
with dose adjustments or interruptions. In the future, patients
who experience sonidegib-related AEs will be managed using this
approach in an attempt to prolong exposure and enhance
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.

380 Lear et al.
All grades (%)
24
10
20
14
21
10
26
18
12
15
12
16
15
27
31
27
41
32
35
37
37
44
3
6
5
11
11
9
11
12
9
4
3
25
9
9
4
13
11
19
13
23
13
27
8
1
1
2
4
1
2
1
9
4
6
5
1
3
1
5
3
0 102030405060708090 100
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
Grade 1
Grade 2
Grade 3
Grade 4
Muscle
spasms
Alopecia
Dysgeusia
Nausea
Diarrhoea
Weight
decreased
CK
increased
Fatigue
Appetite
decreased
Myalgia
Vomiting
54
69
49
58
44
60
39
47
32
24
30
43
30
37
30
37
23
35
19
28
11
29
AEs in ≥20% of all patients (laBCC + mBCC)
treatment benefit. In addition, future clinical trials designed to
optimize the treatment regimen of HPIs may further improve
outcomes.
Overall, these results support the use of sonidegib 200 mg as a
treatment option for patients with advanced BCC according to
local guidelines.
14–17
Acknowledgements
We thank the patients and their families, the study investigators,
their clinical teams and the study site staff, and the members of
the study committees. We also thank the Novartis BOLT clinical
study team, Yi Wu of Novartis for statistical support, the inde-
pendent data monitoring committee (Mark R. Pittelkow, J
€
urgen
C. Becker and Stephen L. George), the efficacy independent
review (Vernon K. Sondak, James Grichnik and Lawrence
Schwartz), and the muscle safety review and adjudication com-
mittee (Robert S. Rosenson, Vinay Chaudhry and Paul D.
Thompson). Medical editorial assistance was provided by Karen
Kaluza, PhD (ArticulateScience LLC) and funded by Novartis
Pharmaceuticals Corporation.
References
1 Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in
the management of advanced basal cell carcinoma: a UK perspective. Br J
Cancer 2014; 111: 1476–1481.
2 Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ 2003; 327:
794–798.
3 Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet
2010; 375: 673–685.
4 Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in can-
cer. Ther Adv Med Oncol 2010; 2: 237–250.
5 Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the
PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcino-
mas. Br J Dermatol 2005; 152:43–51.
6 Pan S, Wu X, Jiang J, et al. Discovery of NVP-LDE225, a potent and
selective smoothened antagonist. ACS Med Chem Lett 2010; 1: 130–134.
7 Rodon J, Tawbi HA, Thomas AL, et al. A phase I, multicenter, open-
label, first-in-human, dose-escalation study of the oral smoothened inhi-
bitor sonidegib (LDE225) in patients with advanced solid tumors. Clin
Cancer Res 2014; 20: 1900–1909.
8 Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different
doses of sonidegib in patients with locally advanced or metastatic basal
cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2
trial. Lancet Oncol 2015; 16: 716–728.
9 Dummer R, Guminski A, Gutzmer R, et al. The 12-month analysis
from Basal Cell Carcinoma Outcomes with LDE225 Treatment
(BOLT): a phase II, randomized, double-blind study of sonidegib in
patients with advanced basal cell carcinoma. J Am Acad Dermatol
2016; 75: 113–125.
10 Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog path-
way inhibitor GDC-0449 in patients with refractory, locally advanced or
metastatic solid tumors. Clin Cancer Res 2011; 17: 2502–2511.
11 Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismod-
egib in advanced basal-cell carcinoma. N Engl J Med 2012;
366:
2171–2179.
12 Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carci-
noma (BCC) study: 12-month update of efficacy and safety of vismodegib
in advanced BCC. J Am Acad Dermatol 2015; 72: 1021–1026. e8
13 Sekulic A, Migden M, Basset-Seguin N, et al. Long-term safety and effi-
cacy of vismodegib in patients with advanced basal cell carcinoma: final
update (30-month) of the pivotal ERIVANCE BCC study. J Clin Oncol
2014; 32: 9013.
14 Novartis Pharmaceuticals Corporation. Odomzo (sonidegib) Prescribing
Information. 2016. URL https://www.pharma.us.novartis.com/sites/www.
pharma.us.novartis.com/files/odomzo.pdf (last accessed: 28 September
2016).
15 European Medicines Agency: Committee for Medicinal Products for
Human Use. CHMP summary of opinion for Odomzo. 2015. URL http://
www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opin
ion_-_Initial_authorisation/human/002839/WC500188762.pdf (last
accessed: 28 September 2016).
16 Swissmedic authorized medicines. Swissmedic authorization of Odomzo.
2015. URL https://www.swissmedic.ch/arzneimittel/00156/00221/00222/
00230/index.html?lang=en (last accessed: 28 September 2016).
17 Australian Government. Department of Health and Ageing: Therapeutic
Goods Administration. Australian public assessment report for Odomzo,
2015.
18 World Health Organization. WHO Handbook for Reporting Results for
Cancer Treatment. World Health Organization, Geneva, 1979.
19 Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation cri-
teria in solid tumours: revised RECIST guideline (version 1.1). Eur J Can-
cer 2009; 45: 228–247.
20 US Department of Health and Human Services. National Cancer Insti-
tute. Common terminology criteria for adverse events (CTCAE) version
4.03, 2010. URL https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-
06-14_QuickReference_5x7.pdf (last accessed: 28 September 2016)
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.
24
10
20
14
21
10
26
18
12
15
12
16
15
27
31
27
41
32
35
37
37
44
3
6
5
11
11
9
11
12
9
4
3
25
9
9
4
13
11
19
13
23
13
27
8
1
1
2
4
1
2
1
9
4
6
5
1
3
1
5
3
0 102030405060708090100
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
800
200
Grade 1
Grade 2
Grade 3
Grade 4
Muscle
spasms
Alopecia
Dysgeusia
Nausea
Diarrhoea
Weight
decreased
CK
increased
Fatigue
Appetite
decreased
Myalgia
Vomiting
All grades (%)
54
69
49
58
44
60
39
47
32
24
30
43
30
37
30
37
23
35
19
28
11
29
AEs in ≥20% of all patients (laBCC + mBCC)
Patients (%)
Figure 3 Adverse events (AEs), regardless of causality, reported
in ≥20% of all patients treated with sonidegib, by treatment arm.
The most common AEs reported in patients treated with sonidegib
200 mg (n = 79) or 800 mg (n = 150) assessed using the National
Cancer Institute’s Common Terminology Criteria for Adverse
Events v4.03.
20
Reported AEs include those that occurred during
treatment and within 30 days of treatment discontinuation. For a
patient who had multiple occurrences of the same AE, the AE is
reported only once at the highest severity rating. CK, creatine
kinase; laBCC, locally advanced basal cell carcinoma; mBCC,
metastatic BCC.

381 Sonidegib for advanced BCC
21 American Cancer Society. Basal and squamous cell skin cancers, 2016.
URL https://www.cancer.org/cancer/basal-and-squamous-cell-skin-cance
r.html (last accessed: 28 September 2016).
22 National Cancer Institute. Skin cancer treatment (PDQ)–health profes-
sional version, 2016. URL https://www.canc er.gov/types/skin/hp/skin-trea
tment-pdq (last accessed: 28 September 2016).
23 von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of
five cases and review of 170 cases in the literature. J Am Acad Dermatol
1984; 10: 1043–1060.
24 Raszewski RL, Guyuron B. Long-term survival following nodal metastases
from basal cell carcinoma. Ann Plast Surg 1990; 24: 170–175.
25 Danial C, Lingala B, Balise R, et al. Markedly improved overall survival in
10 consecutive patients with metastatic basal cell carcinoma. Br J Derma-
tol 2013; 169: 673–676.
26 Lorusso PM, Jimeno A, Dy G, et al. Pharmacokinetic dose-scheduling
study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients
with locally advanced or metastatic solid tumors. Clin Cancer Res 2011;
17: 5774–5782.
27 Genentech, Inc. Erivedge (vismodegib) prescribing information, 2012.
URL https://www.gene.com/download/pdf/erivedge_prescribing.pdf (last
accessed: 28 September 2016).
28 Zhou J, Quinlan M, Hurh E, Sellami D. Exposure-response analysis of
sonidegib (LDE225), an oral inhibitor of the hedgehog signaling pathway,
for effectiveness and safety in patients with advanced solid tumors. J Clin
Pharmacol 2016; 56: 1406–1415.
29 Goel V, Hurh E, Stein A, et al. Population pharmacokinetics of sonidegib
(LDE225), an oral inhibitor of hedgehog pat hway signaling, in healthy
subjects and in patients with advanced solid tumors. Cancer Chemother
Phramacol 2016; 77: 745–755.
30 Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients
with advanced basal cell carcinoma (STEVIE): a pre-planned interim
analysis of an international, open-label trial. Lancet Oncol 2015; 16:
729–736.
31 Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study
of patients with advanced basal cell carcinoma treated with the Hedge-
hog pathway inhibitor, vismodegib. J Am Acad Dermatol 2013; 70:60–
69.
32 Gajjar AJ, Gururangan S, Qaddoumi IA, et al. A prospective phase II
study to determine the efficacy of GDC 0449 (vismodegib) in adults
with recurrent medulloblastoma (MB): a Pediatric Brain Tumor Con-
sortium study (PBTC 25B) [abstract]. J Clin Oncol 2013; 31: 2035.
33 Goldman J, Eckhardt SG, Borad MJ, et al. Phase 1 Dose-escalation trial of
the oral investigational hedgehog signaling pathway inhibitor TAK-441 in
patients with advanced solid tumors. Clin Cancer Res 2014; 21: 1002–
1009.
34 Italiano A, Le Cesne A, Bellera C, et al. GDC-0449 in patients with
advanced chondrosarcomas: a French Sarcoma Group/US and French
National Cancer Institute Single-Arm Phase II Collaborative Study. Ann
Oncol 2013; 24: 2922–2926.
35 Jimeno A, Weiss GJ, Miller WH Jr, et al. Phase I study of the hedgehog
pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Can-
cer Res 2013; 19: 2766–2774.
36 Siu LL, Papadopoulos K, Alberts SR, et al. A first-in-human, phase I study
of an oral hedgehog (HH) pathway antagonist, BMS-833923 (XL139), in
subjects with advanced or metastatic solid tumors [abstract]. J Clin Oncol
2010; 28: 2501.
37 Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedge-
hog pathway in patients with the basal-cell nevus syndrome. N Engl J Med
2012; 366: 2180–2188.
38 Wagner AJ, Messersmith WA, Shaik MN, et al. A phase I study of PF-
04449913, an oral hedgehog inhibitor, in patients with advanced solid
tumors. Clin Cancer Res 2015; 21: 1044–1051.
39 Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label
clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal
cell carcinoma. J Am Acad Dermatol 2014; 71: 904–911.
Supporting information
Additional Supporting Information may be found in the online
version of this article:
Table S1. Composite overall response in locally advanced basal
cell carcinoma (laBCC) determined by mRECIST and BCC-
RECIST-like criteria
Table S2. Efficacy in patients with locally advanced basal cell
carcinoma (laBCC) treated with sonidegib 800 mg by central
and investigator review
Table S3. Efficacy in patients with locally advanced basal cell
carcinoma (laBCC) by central and investigator review per BCC-
recist-like criteria (30-month analysis)
Table S4. Efficacy in patients with locally advanced basal cell
carcinoma (laBCC) by baseline tumor burden by treatment arm
(18-month analysis)
Table S5. Efficacy in patients with metastatic basal cell carci-
noma (mBCC) treated With Sonidegib 800 mg by central and
investigator review
Table S6. Serious adverse events reported in ≥1% of patients in
either arm, regardless of causality (30-month analysis)
Figure S1. BOLT trial profile.
Figure S2. Waterfall plots of best change from baseline in the
size of target lesions in patients with locally advanced basal cell
carcinoma (laBCC).
Figure S3. Duration of response (DOR) in patients with locally
advanced basal cell carcinoma (laBCC).
Figure S4. Kaplan–Meier plots of duration of response (DOR)
in patients with locally advanced basal cell carcinoma (laBCC)
and of progression-free survival (PFS) in patients with laBCC
and metastatic BCC (mBCC) treated with sonidegib 800 mg by
central and investigator review.
Figure S5. Kaplan–Meier plot of overall survival (OS) in patients
with locally advanced basal cell carcinoma (laBCC) and meta-
static BCC (mBCC) treated with sonidegib 800 mg.
Figure S6. Waterfall plots of best change from baseline in the
size of target lesions in patients with metastatic basal cell carci-
noma (mBCC).
Figure S7. Mean trough-concentration time profiles for sonide-
gib 200 or 800 mg.
Figure S8. Adverse events (AEs), regardless of causality, reported
in ≥20% of patients with locally advanced basal cell carcinoma
(laBCC) treated with sonidegib, by treatment arm.
Figure S9. Adverse events (AEs), regardless of causality, reported
in ≥20% of patients with metastatic basal cell carcinoma
(mBCC) treated with sonidegib, by treatment arm.
JEADV 2018, 32, 372–381 © 2017 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd
on behalf of European Academy of Dermatology and Venereology.
